Effect of airway Pseudomonas aeruginosa isolation and infection on steady-state bronchiectasis in Guangzhou, China
Introduction
Bronchiectasis is a chronic airway disease associated with the vicious cycle in which bacteria play pivotal roles (1,2). Haemophilus influenzae and Pseudomonas aeruginosa (PA) have been the most common potentially pathogenic bacteria in bronchiectasis (3,4). The age of 14 years or less, FEV1 80% predicted or less and cystic bronchiectasis were factors associated with bacterial infection (5). Bacterial infection triggers airway inflammation (6-9) and predisposes to persistent infection (10), which in turn stimulates secretion of inflammatory mediators (11) leading to oxidative stress (12,13). These could be reflected by the increase in sputum, serum or exhaled gas inflammatory markers (11,14). Bacterial infection, despite clinically stable, elicits pronounced airway inflammation (11) that is associated with mucus hypersecretion, airway obstruction and bronchial wall thickening leading to airflow limitation (15) and reduced exercise and diffusing capacity (16). Furthermore, sputum microbiota compositions (particularly the ‘core microbiota’) significantly correlate with clinical measures of bronchiectasis, including lung function (17). Bacterial infection has also been associated with secretion of enzymes into the airways which could be reflected by the sputum purulence (18). These collectively indicated that airway bacterial infection may be responsible for airway destruction and loss of lung function.
It should be noted that PA exhibited a unique feature in bronchiectasis characterized by prolonged respiratory symptoms and rapid decline in lung function (19) that might be partially associated with increased airway cyanides levels (20). The newly developed metrics for measuring disease severity, the bronchiectasis severity index (21) and FACED score (22), have consistently taken PA chronic colonization into account, indicating the critical importance of identifying potential infection with PA in bronchiectasis patients. Despite the high rate of eradication (23), it would be challenging to eliminate PA biofilms that yielded permanent airway inflammation and destruction and may dramatically temper the efficacy of antibiotics (24), despite that these might be alleviated by long-term administration of macrolides (25). However, these phenomena had been less common in airways harboring miscellaneous bacteria (10,26).
Here, we aimed to investigate: (I) the differences in inflammatory indices and lung function between patients harboring PA and other bacteria; (II) correlation between the bacterial density of PA and inflammatory and lung function indices; and (III) factors associated with PA isolation or infection.
In our study, sputum culture results in the preceding year were also taken into account. Bacterial colonization (referred to as infection for PA only) was defined as sputum culture positive of the identical bacteria for at least two occasions within 1 year, at least 3 months apart (21). Bacterial isolation denoted sputum culture positive of pathogenic bacteria at baseline. We specifically defined PA isolation as baseline sputum culture positive of PA, with at least two occasions (at least 3 months apart) of culture negative or positive of other pathogenic bacteria in the preceding year.
Methods
Subjects
Between September 2012 and November 2013, we recruited consecutive patients with steady-state bronchiectasis and healthy subjects aged 18 to 70 years. Diagnosis of bronchiectasis was based on chest high-resolution computed tomography (HRCT) within 12 months. Eligible patients had to remain exacerbation-free for 4 weeks. Bronchiectasis exacerbation was defined as three or more of following items lasting for over 2 days: (I) considerably increased sputum purulence or volume; (II) worsened tachypnea or dyspnea; (III) increased coughing; (IV) fever; (V) fatigue or exercise intolerance; (VI) wheezing; (VII) increased pulmonary crackles; (VIII) compatible radiologic findings (21,27-31). Patients with malignancy, upper respiratory tract infections or antibiotic use within 4 weeks were excluded.
Healthy subjects with baseline FEV1 80% predicted or greater and normal chest radiograph and had no upper respiratory tract infection for 4 weeks were recruited. Healthy subjects were included in our study because we aimed to: (I) highlight the impacts of PA isolation or infection on clinical parameters as compared with healthy subjects; (II) derive the z-scores of inflammatory markers for performing correlation analyses, which have not been previously reported.
The study protocol was approved by Ethic Committee of First Affiliated Hospital of Guangzhou Medical University. Subjects gave written informed consent prior to the study.
Sputum and blood sampling
Sputum was sampled during hospital visits, between 9:00 a.m. and 12:00 a.m. Following removal of contents in oral cavity by thorough rinsing with distilled water, patients were instructed to expectorate and collect sputum in a 60 mL sterile plastic container, for bacterial culture and preparation of sol phase. Hypertonic saline (3-5%) was employed for induction if sputum yield was insufficient. Sputum samples with 25 leukocytes or greater and 10 epithelial cells or lower under microscopic field (×100) were deemed eligible. Two random aliquots of sputum plugs were pipetted within 2 hours for culture and ultracentrifugation (50,000 g) for 90 min, without pre-treatment with albumin or dithiothreitol, to harvest sputum sol subsequently stored in −80 °C freezers. Bacterial culture and measurements of inflammatory markers were done on an identical sputum sample, during the initial visits for all patients.
Following phlebotomy, peripheral blood samples were sent for blood routine tests and centrifugation for storage in −80 °C freezers.
Assessment of sputum sol and serum inflammatory mediators
Serum IL-8 and tumor necrosis factor-α (TNF-α), and sputum sol IL-1β, IL-8 and TNF-α, were measured by using Liquid chips (Biorad Inc., USA), based on manufacturer’s instructions. Briefly, samples were pipetted to 96-well plate coated with monoclonal antibodies for incubation for 1 hr under room temperature. Following plate rinsing with buffer solution, the solution containing magnetic microbeads was added for further incubation. This was followed by thorough rinsing with buffer solution, and streptavidin-phycoerythrin was subsequently added to incubate for 15 min. Stop solution was added to terminate reactions. Concentrations of inflammatory mediators were assessed using Bioplex reader (Biorad Inc., USA) and recorded as pg/mL or ng/mL as appropriate.
Sputum bacterial load
Blood agar and chocolate agar plates (Biomeurix Inc., France) were adopted as culture media. Fresh sputum was homogenized with SPUTASOL (Oxoid SR089A) and serially diluted with natural saline [10-4, 10-5 and 10-6]. This was followed by addition of 10 μL respective diluent to the plates with a micropipette tube and the inoculation using 10 μL standardized rings. The plates were positioned in a thermostatic box containing 5% carbon dioxide at 37 °C for overnight incubation (24-48 hours). Negative plates were re-incubated and re-examined on a daily basis for 4 days prior to disposal (32).
Potentially pathogenic microorganisms (PPMs) were categorized as PA, Haemophilus spp and miscellaneous bacteria (Stenotrophomonas maltophilia, Staphylococcus aureus, Escherichia coli, Sphingomonas paucimobilis, Klebsiella spp, Alcaligenes faecalis subsp faecalis, Pseudomonas pseudoalcaligenes and Serratia marcescens). Non-PPMs (commensals) comprised Neisseria, α-Streptococcus hemolyticus, and coagulase-negative staphylococcus. Bacterial load, measured as colony forming unit per milliliter (cfu/mL), was reported for PPMs only. Only the plates with 30 to 300 cfu were counted for bacterial load.
Lung function tests
Spirometry was conducted by using spirometers (QUARK PFT, COSMED Inc., Italy). Between-maneuver variation was <5% or 200 mL in FVC and FEV1 (33), with the maximal values being reported. Maximal mid-expiratory flow (MMEF) was chosen from the best maneuver. Predicted values were selected by using the reference model by Zheng and coworkers (34). Short-acting bronchodilators were withdrawn for at least 6 hours and long-acting bronchodilators 24 hours prior to spirometry.
Diffusing capacity was measured with gas analyzers (QUARK PFT, COSMED Inc., Milan, Italy) by using single-breath carbon monoxide washout technique. Ingestion of food, exercising, smoking and alcohol drinking were withheld for 24 hours. Between-test interval was 4 min. Measurements were deemed acceptable if coefficient of variation for DLCO was within 10% or 3 mL/(min × mmHg). DLCO was reported as the mean of two technically acceptable maneuvers.
Statistical analysis
Statistical analysis was performed using SPSS 16.0 version package (SPSS Inc., Chicago, IL, USA). Dot plots were depicted using Graphpad Prism 5.0 (Graphpad Co. Ltd, San Diego, USA). Data were expressed as mean ± standard deviation or median (interquartile range) as appropriate. Categorical data were presented as No (%) and compared using chi-square tests. Between-group comparisons were made using independent t-test or Mann-Whitney test as indicated, and among-group comparisons were performed using one-way analysis-of-variance or Kruskal-Wallis test as appropriate. Dot plots were applied to demonstrate the distribution of bacterial load and inflammatory indices. Because the normal values for inflammatory markers are lacking, the means and standard deviations in healthy subjects were imputed to calculate the z-score. The correlation between bacterial density and inflammatory indices and lung function parameters was analyzed based on Pearson or Spearman’s model. Univariate Logistic analysis was conducted to explore the factors of PA isolation and infection, and results were reported as OR and 95% confidence interval (95% CI). Data with P value of 0.10 or less were entered into multivariate analysis as covariates using backward selection technique. For multiple comparisons, we applied Bonferroni correction for adjustment, with a statistical significance being defined as P<0.015. P<0.05 was taken as statistically significant for other comparisons unless otherwise stated.
Results
Subject recruitment
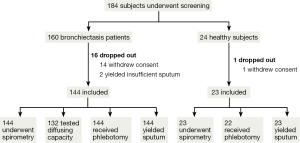
See Figure 1.
Baseline levels
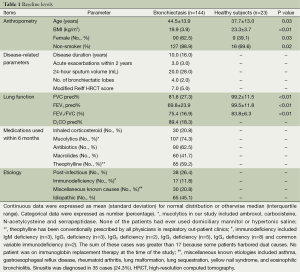
Bronchiectasis patients differed significantly from healthy subjects in anthropometry and spirometry (all P<0.05) except for the height (P=0.11). Of the 144 bronchiectasis patients, 90 (62.5%) were females, had a median disease duration of 10.0 years and acute exacerbations of 3.0 per patient within 2 years. The common medications used within 6 months were mucolytics (74.3%), antibiotics (62.5%) and theophylline (59.2%). The underlying causes of bronchiectasis included idiopathic (42.4%), post-infectious (26.4%) and immunodeficiency (11.8%) (Table 1).
Full table
Sputum bacteriology
A total of 56 patients (38.9%) with 24-hour sputum volume of 5 mL or lower failed to produce sufficient spontaneous sputum for bacterial culture at baseline visit and required sputum induction and chest physiotherapy to aid sputum collection. All healthy subjects underwent induction for deriving sputum samples for inflammatory biomarker measurements.
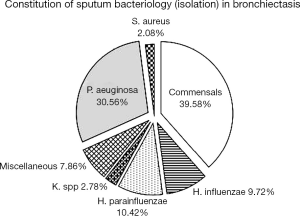
Of 144 bronchiectasis patients, 57 (39.58%) isolated commensals. Of 87 patients with positive sputum culture, 44 (30.56%) isolated PA, 15 (10.42%) Haemophilus parainfluenzae and 14 (9.72%) Haemophilus influenzae. The bacterial density of PA did not, apart from Haemophilus parainfluenzae (P<0.01), differ statistically from that of other PPMs (all P>0.05). A total of 39 patients had PA infection (27.08%) and 6 (4.17%) Haemophilus influenzae colonization when clinically stable (Figure 2).
Of 44 patients isolated and 39 infected with PA, 32 (72.7%) and 28 (71.8%) had mucoid strains. Of the 44 cases isolated with PA, the bacterial loads were 106 cfu/mL or less in 2 cases (4.5%), greater than 106 and less than 107 cfu/mL in 10 cases (22.7%), greater than 107 and less than 108 cfu/mL in 13 cases (29.5%), and 108 cfu/mL or greater in 19 cases (43.2%).
Inflammatory markers and lung function in bronchiectasis patients isolated or infected with PA and healthy subjects
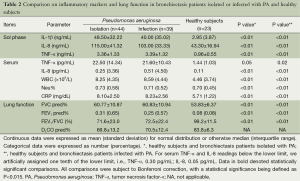
Bronchiectasis patients isolated or infected with PA yielded significantly higher levels of sputum sol and serum biomarkers (all P<0.05) except for serum TNF-α (P=0.05 and 0.02, respectively) than healthy subjects. Significantly impaired lung function was found in bronchiectasis patients isolated or infected with PA (all P<0.05). Diffusing capacity was not compared because we did not measure in healthy subjects (Table 2).
Full table
Inflammatory markers and lung function in bronchiectasis patients isolated or infected with PA and other PPMs
Patients isolated with PA yielded higher levels of sputum sol IL-1β, but not IL-8 and TNF-α. However, in patients infected with PA, the level of sputum IL-1β did not differ statistically from that of other PPMs.
Patients isolated with PA yielded significantly higher levels of serum TNF-α than the non-PA counterparts (data not shown). However, the difference in serum TNF-α between patients isolated with PA and other PPMs was statistically insignificant. Significantly higher WBC was also noted in patients infected with PA as compared with the non-PA counterparts.
Spirometric indices and DLCO were numerically but not statistically lower in patients isolated with PA (all P<0.05). Similar findings also applied in patients infected with PA as compared with the non-PA counterparts (Table 3).
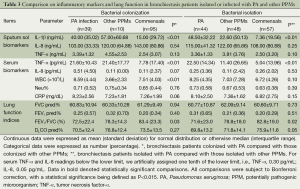
Full table
Correlation between bacterial density of PA and clinical indices
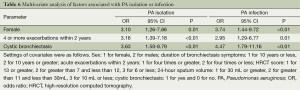
In bronchiectasis patients isolated with PA, the bacterial density was positively correlated with the z-scores of sputum sol IL-1β and TNF-α (all P<0.05), but not serum biomarkers or lung function indices (all P>0.05) (Table 4).
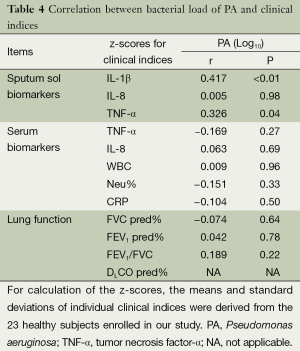
Full table
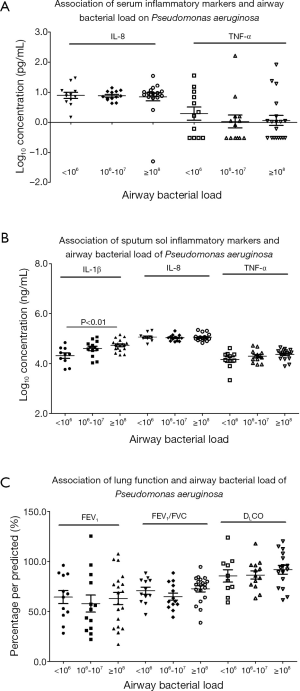
The bacterial density of PA and inflammatory markers and lung function are also plotted in Figure 3. Because the distribution of bacterial density of PA did not assume normal distribution, we performed logarithmic transformation before further analyses. Sputum sol biomarkers (in particular IL-1β), but not serum biomarkers or lung function indices (P>0.05), tended to be higher as with increased bacterial density.
Factors associated with PA isolation and infection
In univariate analysis (Table 5), factors associated with PA isolation or infection consisted of being females, having bronchiectasis symptoms for 10 years or greater, four or more exacerbations within 2 years, HRCT score of 7 or greater, 24-hour sputum of 30 mL or greater and cystic bronchiectasis (all P<0.05).
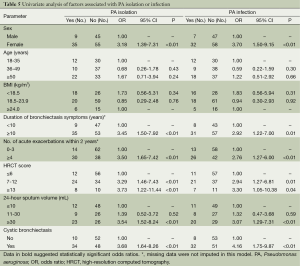
Full table
In multivariate analysis (Table 6), factors associated with PA isolation or infection consisted of female gender (PA isolation: OR =3.10, 95% CI: 1.26-7.66, P=0.01; PA infection: OR =3.74, 95% CI: 1.44-9.72, P<0.01), four or more acute exacerbations within 2 years (PA isolation: OR =3.16, 95% CI: 1.39-7.18, P<0.01; PA infection: OR =2.95, 95% CI: 1.29-6.77, P=0.01) and cystic bronchiectasis (PA isolation: OR =3.63, 95% CI: 1.50-8.79, P<0.01; PA infection: OR =4.47, 95% CI: 1.79-11.16, P<0.01).
Full table
Discussion
Our data suggested that 30% of patients with steady-state bronchiectasis in Guangzhou, China harbored PA in airways. The rate of PA isolation was significantly higher than that of Haemophilus influenzae as compared with reports from western countries. PA yielded airway and systemic inflammatory responses and lung function impairment. Intriguingly, PA was not associated with poorer diffusing capacity, and the numerically but not statistically impaired lung function in patients with PA isolation or infection might have been attributed to the relatively small sample size of our study. Apart from sputum sol IL-1β and TNF-α, inflammatory and lung function indices did not correlate significantly with the bacterial density of PA. Female gender, frequent exacerbations and cystic bronchiectasis were consistently associated with PA isolation or infection.
Similar with our overall findings, Hartl et al. (35) documented that patients harboring PA presented with markedly higher levels of bronchoalveolar lavage IL-1β, IL-8 and TNF-α, as compared with cystic fibrosis patients harboring other PPMs and healthy subjects. Chalmers et al. (36) found that airways harboring PA elicited significantly higher levels of IL-8, IL-1β and TNF-α in stable bronchiectasis. The fact that systemic antibiotic therapy resulted in marked reduction in airway and systemic inflammation as a consequence of elimination or suppression of bacteria (36) in turn validated the hypothesis that higher bacterial density was likely to account for heightened airway inflammation.
Of all PPMs, PA elicited prolonged respiratory symptoms, higher mortality rate (36) and poorer lung function (5,37) in bronchiectasis. Apart from heightened airway inflammation, higher density of PA was associated with higher levels of sputum sol IL-1β and TNF-α. Intriguingly, these findings were not associated with miscellaneous PPMs, pointing to the unique roles of PA in eliciting airway inflammation. PA has a tendency of forming biofilms in airways (23), which makes it challenging to eliminate by antibiotics or the immune system. In the present study, of 44 and 39 cases isolated and infected with PA, mucoid strains were found in 32 (72.7%) and 28 cases (71.8%), indicating the formation of biofilms leading to heightened airway inflammation. Notably, airway inflammation did not correlate with systemic inflammation, as evidenced by the fact that the density of PA did not correlate with serum biomarkers.
Effects of PA on airway destruction could be indirectly reflected by lung function impairment (38). In the present study, patients isolated or infected with PA had poorer spirometry. This was partially concordant with the findings of Davies et al. (5), who found that patients chronically colonized with PA had poorer spirometry and diffusing capacity and higher residual volume than patients intermittently or never isolated with PA. The discrepancy regarding the diffusing capacity could be explained by the fact that children (mean: 12 years) were the dominant cohort in the study by Davies et al. (5). To our knowledge, the underlying pathogenesis of bronchiectasis differed significantly in children and adults, therefore findings might not be cross-validated. Or it could be that patients with comparatively severe bronchiectasis (mean FEV1: 33%, FVC: 50%) in Davies’ study (5) might have increased the likelihood of positive findings.
We also showed that being females, frequent exacerbations and cystic bronchiectasis were factors associated with PA infection, which was consistent with previous literature reports. By conducting a 2-year follow-up study on 77 bronchiectasis patients (mean: 58 years, 66% females) from tertiary hospitals, Angrill et al. (4) documented the risk factors of airways harboring PPMs included: diagnosis of bronchiectasis at the age of 14 years or less (OR: 3.92); FEV1 <80% predicted (OR: 3.91); cystic or varicose bronchiectasis (OR: 4.80). The observed discrepancy with our study was likely to arise from different study designs, since the difference in the rate of PA isolation was insignificant (26-32% vs. 30.6%).
Intriguingly, the disparity of the prevalence of PA and Haemophilus influenzae in our cohort as compared with that of western countries might be associated with extensive application of antibiotics in China, the growing prevalence of multi-drug resistant PA, ethnicity and the severity of bronchiectasis. Consistent with the study in Hong Kong by Tsang et al. (10,26), our sister study (27) also showed that PA has been the predominant pathogenic microorganism in mainland China.
Our study has several clinical implications. PA plays a unique role in aggravating airway inflammation and lung function decline, suggesting that early administration of anti-inflammatory medications (i.e., nebulized antibiotics, corticosteroids) may alleviate airway inflammation and reduce bacterial density leading to suppression of the vicious cycle and the risk of future exacerbations. Clinicians should also raise alert of PA isolation or infection to female patients and those with low FEV1, and frequent exacerbations. The systematic follow-up scheme should be implemented to identify patients with systemic inflammation and rapid decline in lung function who need to be actively treated for improving the prognosis.
Some limitations should be considered. First, the lack of long-term follow-up visits has rendered it impossible to determine the relation between bacterial density and the rate of lung function decline, aggravation of airway inflammation or the prognosis. Second, routine sputum culture might have underestimated the effects of miscellaneous microbiota taxa on PA. Novel techniques, including 16srRNA pyrosequencing, might offer further insights. Third, the correlation analysis inherently could not confirm the casual relations between bacterial infection and airway inflammation, but we have reinforced that PA infection was associated with persistent airway inflammation. Fourth, it should be acknowledged that PA infection could have been underestimated, since sputum culture results in the previous year were not available in some patients, particularly those residing in rural areas where medical healthcare resource and services were limited. Fifth, the relatively small sample size would have been underpowered for analyses of different subgroups (i.e., never infected, free from infection, intermittent colonization and chronic colonization of PA). Studies with larger sample sizes might be warranted to resolve this issue. Furthermore, bronchiectasis patients and healthy subjects were not matched for age, gender and smoking status, which would limit the generalizability of our findings. Finally, the addition of enzymology assays, as previously proven (18), may offer a global assessment of airway inflammation.
In conclusion, PA elicits significant airway and systemic inflammation and is responsible for poor lung function. The bacterial density of PA correlates with sputum sol IL-1β and TNF-α, but not other inflammatory or spirometric indices. Female gender, frequent exacerbations and cystic bronchiectasis are indicators of PA isolation or infection.
Acknowledgements
We wholeheartedly thank Prof. Kenneth Wah-Tak Tsang and June Sun (Hong Kong University, Hong Kong SAR, China) and Wen-Ming Liu [Biorad Inc. (Guangzhou branch)] for their technical assistance.
Funding: Changjiang Scholars and Innovative Research Team in University ITR0961, The National Key Technology R&D Program of the 12th National Five-year Development Plan 2012BAI05B01 and National Key Scientific & Technology Support Program: Collaborative innovation of Clinical Research for chronic obstructive pulmonary disease and lung cancer No. 2013BAI09B09 (to Profs. NS Zhong and RC Chen), National Natural Science Foundation No. 81400010 and 2014 Scientific Research Projects for Medical Doctors and Researchers from Overseas, Guangzhou Medical University No. 2014C21 (to Dr. WJ Guan).
Disclosure: Profs. NS Zhong and RC Chen declared that they had received Changjiang Scholars and Innovative Research Team in University ITR0961, The National Key Technology R&D Program of the 12th National Five-year Development Plan 2012BAI05B01 and National Key Scientific & Technology Support Program: Collaborative innovation of Clinical Research for chronic obstructive pulmonary disease and lung cancer No. 2013BAI09B09. Dr. WJ Guan declared that he has received National Natural Science Foundation No. 81400010 and 2014 Scientific Research Projects for Medical Doctors and Researchers from Overseas, Guangzhou Medical University No. 2014C21. All other authors declared no potential conflict of interest. None of the funding sources had any role on the study.
References
- Barker AF. Bronchiectasis. N Engl J Med 2002;346:1383-93. [PubMed]
- Goeminne PC, Nawrot TS, Ruttens D, et al. Mortality in non-cystic fibrosis bronchiectasis: a prospective cohort analysis. Respir Med 2014;108:287-96. [PubMed]
- Pasteur MC, Helliwell SM, Houghton SJ, et al. An investigation into causative factors in patients with bronchiectasis. Am J Respir Crit Care Med 2000;162:1277-84. [PubMed]
- Angrill J, Agustí C, de Celis R, et al. Bacterial colonisation in patients with bronchiectasis: microbiological pattern and risk factors. Thorax 2002;57:15-9. [PubMed]
- Davies G, Wells AU, Doffman S, et al. The effect of Pseudomonas aeruginosa on pulmonary function in patients with bronchiectasis. Eur Respir J 2006;28:974-9. [PubMed]
- Chmiel JF, Davis PB. State of the art: why do the lungs of patients with cystic fibrosis become infected and why can't they clear the infection? Respir Res 2003;4:8. [PubMed]
- King PT, Hutchinson PE, Johnson PD, et al. Adaptive immunity to nontypeable Haemophilus influenzae. Am J Respir Crit Care Med 2003;167:587-92. [PubMed]
- Sadikot RT, Blackwell TS, Christman JW, et al. Pathogen-host interactions in Pseudomonas aeruginosa pneumonia. Am J Respir Crit Care Med 2005;171:1209-23. [PubMed]
- Starner TD, Zhang N, Kim G, et al. Haemophilus influenzae forms biofilms on airway epithelia: implications in cystic fibrosis. Am J Respir Crit Care Med 2006;174:213-20. [PubMed]
- Tsang KW, Ho PL, Lam WK, et al. Inhaled fluticasone reduces sputum inflammatory indices in severe bronchiectasis. Am J Respir Crit Care Med 1998;158:723-7. [PubMed]
- Loukides S, Horvath I, Wodehouse T, et al. Elevated levels of expired breath hydrogen peroxide in bronchiectasis. Am J Respir Crit Care Med 1998;158:991-4. [PubMed]
- Horvath I, Loukides S, Wodehouse T, et al. Increased levels of exhaled carbon monoxide in bronchiectasis: a new marker of oxidative stress. Thorax 1998;53:867-70. [PubMed]
- Angrill J, Agustí C, De Celis R, et al. Bronchial inflammation and colonization in patients with clinically stable bronchiectasis. Am J Respir Crit Care Med 2001;164:1628-32. [PubMed]
- Roberts HR, Wells AU, Milne DG, et al. Airflow obstruction in bronchiectasis: correlation between computed tomography features and pulmonary function tests. Thorax 2000;55:198-204. [PubMed]
- Koulouris NG, Retsou S, Kosmas E, et al. Tidal expiratory flow limitation, dyspnoea and exercise capacity in patients with bilateral bronchiectasis. Eur Respir J 2003;21:743-8. [PubMed]
- Loubeyre P, Paret M, Revel D, et al. Thin-section CT detection of emphysema associated with bronchiectasis and correlation with pulmonary function tests. Chest 1996;109:360-5. [PubMed]
- Rogers GB, van der Gast CJ, Cuthbertson L, et al. Clinical measures of disease in adult non-CF bronchiectasis correlate with airway microbiota composition. Thorax 2013;68:731-7. [PubMed]
- Goeminne PC, Vandooren J, Moelants EA, et al. The Sputum Colour Chart as a predictor of lung inflammation, proteolysis and damage in non-cystic fibrosis bronchiectasis: a case-control analysis. Respirology 2014;19:203-10. [PubMed]
- Evans SA, Turner SM, Bosch BJ, et al. Lung function in bronchiectasis: the influence of Pseudomonas aeruginosa. Eur Respir J 1996;9:1601-4. [PubMed]
- Ryall B, Davies JC, Wilson R, et al. Pseudomonas aeruginosa, cyanide accumulation and lung function in CF and non-CF bronchiectasis patients. Eur Respir J 2008;32:740-7. [PubMed]
- Chalmers JD, Goeminne P, Aliberti S, et al. The bronchiectasis severity index. An international derivation and validation study. Am J Respir Crit Care Med 2014;189:576-85. [PubMed]
- Martínez-García MÁ, de Gracia J, Vendrell Relat M, et al. Multidimensional approach to non-cystic fibrosis bronchiectasis: the FACED score. Eur Respir J 2014;43:1357-67. [PubMed]
- White L, Mirrani G, Grover M, et al. Outcomes of Pseudomonas eradication therapy in patients with non-cystic fibrosis bronchiectasis. Respir Med 2012;106:356-60. [PubMed]
- Palanisamy NK, Ferina N, Amirulhusni AN, et al. Antibiofilm properties of chemically synthesized silver nanoparticles found against Pseudomonas aeruginosa. J Nanobiotechnology 2014;12:2. [PubMed]
- Serisier DJ, Martin ML, McGuckin MA, et al. Effect of long-term, low-dose erythromycin on pulmonary exacerbations among patients with non-cystic fibrosis bronchiectasis: the BLESS randomized controlled trial. JAMA 2013;309:1260-7. [PubMed]
- Tsang KW, Chan K, Ho P, et al. Sputum elastase in steady-state bronchiectasis. Chest 2000;117:420-6. [PubMed]
- Guan WJ, Gao YH, Xu G, et al. Characterization of lung function impairment in adults with bronchiectasis. PLoS One 2014;9:e113373. [PubMed]
- Guan WJ, Gao YH, Xu G, et al. Capsaicin cough sensitivity and the association with clinical parameters in bronchiectasis. PLoS One 2014;9:e113057. [PubMed]
- Guan WJ, Gao YH, Xu G, et al. Impulse oscillometry in adults with bronchiectasis. Ann Am Thorac Soc 2015; [PubMed]
- Gao YH, Guan WJ, Xu G, et al. Validation of the Mandarin Chinese version of the Leicester Cough Questionnaire in bronchiectasis. Int J Tuberc Lung Dis 2014;18:1431-7. [PubMed]
- Gao Y, Guan W, Xu G, et al. The role of viral infection in pulmonary exacerbations of bronchiectasis in adults: A prospective study. Chest 2014; [PubMed]
- Ho PL, Chan KN, Ip MS, et al. The effect of Pseudomonas aeruginosa infection on clinical parameters in steady-state bronchiectasis. Chest 1998;114:1594-8. [PubMed]
- Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005;26:319-38. [PubMed]
- Zheng J, Zhong N. Normative values of pulmonary function testing in Chinese adults. Chin Med J (Engl) 2002;115:50-4. [PubMed]
- Hartl D, Griese M, Kappler M, et al. Pulmonary T(H)2 response in Pseudomonas aeruginosa-infected patients with cystic fibrosis. J Allergy Clin Immunol 2006;117:204-11. [PubMed]
- Chalmers JD, Smith MP, McHugh BJ, et al. Short- and long-term antibiotic treatment reduces airway and systemic inflammation in non-cystic fibrosis bronchiectasis. Am J Respir Crit Care Med 2012;186:657-65. [PubMed]
- Loebinger MR, Wells AU, Hansell DM, et al. Mortality in bronchiectasis: a long-term study assessing the factors influencing survival. Eur Respir J 2009;34:843-9. [PubMed]
- Martínez-García MA, Soler-Cataluña JJ, Perpiñá-Tordera M, et al. Factors associated with lung function decline in adult patients with stable non-cystic fibrosis bronchiectasis. Chest 2007;132:1565-72. [PubMed]