Clarithromycin might attenuate the airway inflammation of smoke-exposed asthmatic mice via affecting HDAC2
Introduction
Asthma is one of the most common respiratory diseases characterized by chronic airway inflammation and hyperresponsiveness which lead to episodes of variable airway obstruction, wheezing and coughing (1). There are tons of millions of patients suffering from this disease and the number of sufferers is still incredibly increasing. Since the first generation of inhaled corticosteroids (ICS) proved to be effective and was widely accepted, ICS has been the mainstay of the asthma management. Even though there is a bright future for asthma patients, the morbidity and mortality of asthma are still high because of those who are not sensitive to glucocorticoid treatment (2).
Smoking has been regarded as the primary factor affecting the effectiveness of ICS, and this theory has been proved by many researches (3,4). Smoking causes oxidative reaction leading to impaired histone deacelytase2 (HDAC2) function which plays a major role in switching off the activated inflammatory genes. Smoking may also leads to the transformation of airway inflammation, which may be one of the underlying mechanisms responsible for decreased response to glucocorticoid treatment. Macrolides have been demonstrated to be endowed with anti-inflammatory and immune modulatory effects besides of their antimicrobial effects (5). They are effective auxiliary treatment against some pulmonary diseases such as cystic fibrosis, bronchiectasis (6). They exert different effects according to their given dosage. Practically, they would show antimicrobial effect when administered at high dosage, while anti-inflammatory and immunomodulatory effect can be demonstrated when given at lower dosage. Collectively, these effects would ameliorate tissue damage, decrease mucus viscosity, and support the rational application of macrolides for the treatment of chronic inflammatory airways diseases (7). Even though, several clinical trials have evaluated the effectiveness of macrolides as adjunct approach for asthma treatment, its effectiveness is still debatable. Most of the reported clinical trials on the one hand are not randomized controlled trials, or the samples are too small to be representative; on the other hand, these clinical trials are mainly focused either on the outcomes of clinical examination and tests, such as physical signs, pulmonary function, or on patients’ subjective feelings such as symptoms, etc. there is no article exploring the underlying mechanisms of how macrolides effect. Therefore, this article was dedicated to find out the effectiveness of clarithromycin, a member of the macrolides family, on the airway inflammation of smoke-exposed asthmatic mice.
Materials and methods
Mice
A total of 40 specific pathogen-free female BALB/c mice, 6 weeks old, weight (16±2) g, were purchased from Beijing HFK Bio-technology Co. Ltd. Mice were maintained under standard environment with a room temperature of 23 °C. All animal welfare protocols and experimental procedures were performed according to institutional guidelines and conformed to the requirements of the authority for animal research conduct.
Experimental protocol
Mice were divided into four groups according to random principle, and each group had ten mice: control group, asthmatic group (OVA group), smoke-exposed asthmatic group (SEA group), and clarithromycin group (CAM group) respectively. The experimental protocol of establishing asthma model was performed according to literature data (8) as follows. Mice were sensitized and challenged by ovalbumin (OVA, Sigma, Germany) as follows. For initial sensitization (on day 0 and 14th), each mouse was intraperitoneally injected with 20 μg OVA emulsified in 0.2 mL of sterile phosphate buffered saline (PBS, Solarbio, China) containing 2 mg of aluminum hydroxide (Sigma, Germany) or with 2 mg aluminum hydroxide in 0.2 mL PBS as control. From day 21st to 45th, mice in OVA group, SEA group and CAM group were challenged with nebulized 1% OVA, or PBS alone as in control group for 30 minutes per day. From day 21st to 45th, mice in SEA and CAM group were also exposed to four commercial filtered cigarettes (12 mg of tar oil, 0.9 mg of nicotine, and 14 mg of carbon monoxide per cigarette, Daqiamen Factory, China) for 30 minutes once per day except the control group and OVA group. For drug intervention, mice in CAM group were given clarithromycin (CAM, Sigma, Germany) 0.2 mg emulsified in 0.2 mL carboxymethlcellulose (CMC, Sigma, Germany) orally once per day for 20 days from 21st to 45th, the other groups were given 0.2 mL CMC only.
Determination of airway hyperresponsiveness (AHR) to methacholine
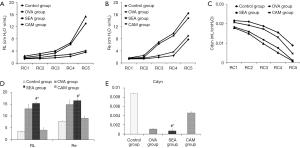
The protocol of invasive AHR measurement was also finished as previously described (8,9). Weigh mice and administer 1% (w/v) pentobarbital sodium 0.1 mL/10 g for anesthesia (Merck, Germany). Sterilized neck skin with 75% ethanol, incised neck skin and separated neck muscle and expose trachea. Incised the trachea and insert the tracheal tube and fixed the tube with suture. Placed mice in the body plethysmograph chamber and connected the inserted tracheal tube to the ventilator with a tidal volume of 6 mL/kg and respiratory rate of 90 strokes/min (Bestlab, China). A needle with heparin-saline filled catheter was injected into venae jugularis interna to administrate methacholine (0 mg/kg, 0.025 mg/kg, 0.05 mg/kg, 0.1 mg/kg and 0.2 mg/kg, intervals of 5 minutes every each concentration) (8). Lung function was assessed by the AniRes 2005 lung function system (Bestlab, AniRes 2005, version 3.0, China) according to the manufacturer’s instructions. Lung resistance of expiration (Re), lung resistance of inspiration (RL) and lung compliance (Cdyn) were used to evaluate the airway responsiveness.
Bronchoalveolar lavage fluid (BALF)
After AHR measurement, cut the ribcage to expose heart and lungs, and punctured the heart with 1 mL syringe to collect blood (8). Collect serum from the obtained blood and stored at −80 °C. Keep the left side of lungs from being lavaged for histopathological analysis by knotting the left bronchus. Take out the tracheal tube and insert a cannula instead and tie a suture to fix the cannula. Applied 1 mL syringe to gently press 0.2 mL PBS into the lung and withdrew the fluid. Repeat this process for another 2 times. The BALF were centrifuged for 7 minutes, 2,000 rpm, 4 °C to collect the whole cells in pellet. Collect supernatant and store at −80 °C for subsequent analysis. The pallet of cells were resuspended in 100 μL PBS and used for total and differential cells counts. Dilute cells if necessary. Load a standard hemacytometer with cell suspension and count the cells number. For cytospins, withdraw 20 μL the cell suspension after being filtered and allow the slides to dry naturally at room temperature and then stain the slides by using Wright-Giemsa stain (Baso Diagnostics Inc, China).
Measurement of cytokines in BALF
IL-4 and IL-8 were selected to detect the airway inflammation using Enzyme linked immunosorbent assay (ELISA) method with human IL-4 and IL-8 Duoset ELISA (Neobioscience technology, China). The procedure was performed according to the manufacturer’s instructions.
Histopathological analysis of the lungs
After the BALF, the lungs were removed and then infiltrated in 10% formalin at least 72 hours. A lung block from the lobe was paraffin-embedded, and 3 μm sections were cut and stained routinely with hematoxylin and eosin (10). The morphological changes were observed with microscope at magnification of ×100 and ×200.
Measurement of activity of HDAC2 in lung tissue
First of all, prepare the nuclear extracts with the EpiQuickTM Nuclear Extraction kit I (Epigenteck, USA), and operate the whole procedure following the protocol instructions. First, nuclear extracts need to be prepared using nuclear extraction kit (Epigenteck, USA). Nuclear extracts can be used immediately to detect the activity of HDAC2 or be frozen at −80 °C for future use. EpiQuickTM HDAC2 activity assay kit (Epigenteck, USA) was applied to analyze the HDAC2 activity for each sample. The assay kit contains seven different contents from HB1 to HB7. Dilute HB1 with distilled water to working concentration. Adjust protein concentration to 0.5 μg/μL with HB2 and add 5 μg of the protein solution into the central area of each well and incubate the strip wells at 37 °C for 90 minutes. For the control add 5 μL of HB2. Add 150 μL of HB3 and incubate at 37 °C for 40 minutes. Aspirated and wash each well with diluted HB1 and add diluted HB4 and incubate at room temperature for 60 minutes. Wash each well and add diluted HB5 and incubate at room temperature for 30 minutes. Wash each well and add 100 μL of HB6 and incubate at room temperature for 5 minutes. Add 50 μL of HB7 to each well and read absorbance on microplate reader at 450 nm.
Western blot analysis
Lung tissues were lysed in RIPA buffer supplemented with protease inhibitor cocktail (Solarbio, China). Protein estimation was performed by the bicinchoninic acid (BCA) method as described by the manufacturer (Biomed, China). Thirty micrograms of protein from the lung tissue was electrophoresed on SDS-8% polyacrylamide gel, transferred onto PVDF membranes and immunochemically stained with monoclonal rabbit anti-HDAC2 (Abcam, USA) antibody. β-actin (Jackson, USA) was used for loading control. The membranes were incubated with HRP conjugated anti-mouse and anti-rabbit secondary antibodies. Immobilion Western chemiluminescent HRP substrate (Millipore, USA) was used to for expose the blot. The density of the bands was processed with the Gel pro analysis software.
Statistical analysis
All data were analyzed using the SPSS statistical package, version 19.0 for windows. Values were presented as means ± standard error. One-way ANOVA Dunnett test was applied for statistical analysis between groups. P values <0.05 were considered to be statistically significant.
Results
Assessment of airway responsiveness
In response to different concentrations of intravenously administered methacholine, airway responsiveness showed concentration-dependent increase both in Re and RL and concentration-dependent decrease in Cdyn (Figure 1). Airway responsiveness increased in OVA and SEA group when compared to control group, and Re in SEA group seemed to be higher than those in OVA group even though there was no significant difference. After clarithromycin intervention, those three indicators were significantly improved in CAM group compared to SEA group.
Histopathological observations of lung histology
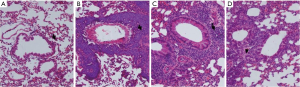
Histopathological changes were exhibited in Figure 2. In control group, there was no obvious sign of inflammatory cell infiltration, small bronchi, bronchioles and lung alveoli were structurally intact, mucosal epithelia were normal. Both of OVA group and SEA group showed significant airway inflammation in lung tissues. Eosinophils in OVA group accounted for a large part of inflammatory cells besides mononuclear cells. In SEA group, cilia were severely destroyed. In contrast to OVA group, the SEA group had prevailed neutrophils rather than eosinophils; cilia were also injured. In CAM group, the inflammatory cell infiltration was attenuated, and cilia were less injured than SEA group.
Cell counts in BALF
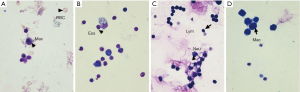
Total cell counts in OVA group, SEA group and CAM group dramatically increased compared to control group, while total cell counts in CAM group reduced significantly compared to SEA group (Figure 3). In control group, macrophage accounted for a large part in the whole cells, while eosinophils in OVA group was the highest compared to the other three groups, and neutrophils in SEA group was extremely high compared to the other three groups (P<0.05). Eosinophils and neutrophils in CAM group were both reduced compared to SEA group (P<0.05). Figure 4 showed different cell type stained with Wright-Giemsa staining method.

IL-4 and CXCL1 levels in BALF
To determine the type of airway inflammation, IL-4 and CXCL1 levels were chosen to represent the Th2 and Th1 cytokines. As shown in Figure 5, the level of IL-4 increased in OVA group, while CXCL1 level elevated dramatically in SEA group and CAM group compared to control group (P<0.05), but the level of IL-4 in CAM group showed significant reduction compared to SEA group (P<0.05). CXCL1 in CAM group decreased dramatically when compared to SEA group (P<0.05).

HDAC2 activity in lung tissue
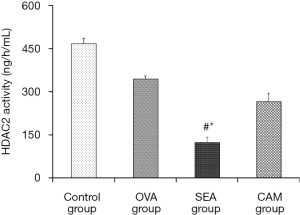
HDAC2 activity in the lung tissues were tested by an assay kit and the results were showed in the Figure 6. Control group had the best HDAC2 activity, HDAC2 activity in OVA group was decreased compared to control group; while the SEA group showed the lowest HDAC2 activity, and the difference between SEA group and control group was significant (P<0.05). After clarithromycin intervention, HDAC2 activity in CAM group was improved significantly in comparison to SEA group (P<0.05).
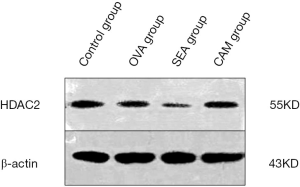
HDAC2 expression in lung tissue
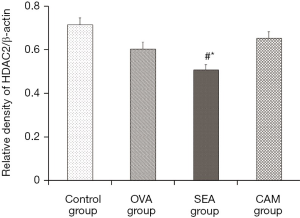
To determine whether CAM influences the expression of HDAC2, western blotting method had been adopted to test HDAC2 expression in lung tissue. HDAC2 expression decreased dramatically in the SEA group compared to control group, while its expression in CAM group improved compared to the SEA group, which suggested that CAM might have a positive effective on HDAC2 expression (Figure 7). To quantify the bands of HDAC2 and β-actin in the four groups, the images were processed with the Gel pro analysis system, and the results showed in the Figure 8. The relative density of HDAC2/β-actin showed that the ratio in SEA group significantly lower than control group (P<0.05), while the ration in CAM group improved dramatically in comparison to SEA group (P<0.05), which supported the theory that clarithromycin might attenuate airway inflammation by affecting the expression and activity of HDAC2.
Discussion
AHR is a major criterion in the demonstration of asthma model. Airway responsiveness to methacholine can be applied to identify if the model is successful. The parameters most commonly chosen for assessment of the airway reactivity were mainly focused on lung resistance and lung compliance (11). RL, Re and Cdyn were adopted as indicators to represent airway resistance. RL represents total airway resistance of inspiration, Re represents expiratory resistance of the airway and Cdyn stands for the dynamic lung compliance. As the results showed, asthma model fit the criteria, indicators above changed accordingly to the different concentrations of methacholine. IL-4 has been proved to be dramatically elevated in asthma patients and rodents (12,13), and it also is a candidate marker to demonstrate asthma model. The total cell counts, especially eosinophils in BALF were dramatically high, and these can also be adjunctive indicators to establish asthma model. Still, histopathological slices exhibited a large amount of inflammatory cells infiltration as the picture showed.
Smoke is one of the most notorious factors that affecting the process of airway inflammation and the effectiveness of ICS treatments (14,15). Smoke can cause immune response shifting from Th2-dominant inflammation to Th1-dominant inflammation, and inflammatory cytokines may also transform from IL-4 to CXCL1 (16). CXCL1 level had elevated both in serum and BALF in SEA group, and the airway responsiveness were much more changeable. Total cell counts and eosinophils and neutrophils in BALF were higher even than OVA group. In the histopathological analysis showed ambient infiltration of inflammatory cells in the lung tissues. These results fit the discovery proposed and proved by former researchers (15). This study also compared the difference of HDAC2 activity between each group, the results were overwhelming. Control group had the highest HDAC2 activity, OVA group was lower, while SEA group had the lowest HDAC2 activity; this suggested that smoke might be the primary factor leading to the decreased HDAC2 activity. While after clarithromycin intervention, the HDAC2 activity was improved significantly, which implies that clarithromycin may be used as an adjunctive treatment to those patients who are glucocorticoid insensitive caused by decreased HDAC2 activity.
Macrolides can exert its anti-inflammatory and immunomodulatory effects beyond antimicrobial effects (7). The underlying mechanism might be as follows: (I) decreasing mucus production by inhibiting neutrophil elastase; (II) attenuating the production of pro-inflammatory mediators; (III) stimulating phagocytosis of apoptotic cells by alveolar macrophages (7). Several trials had already done to explore if macrolides can benefit smoke-exposed asthma patients, but the results were controversial when it comes to clinical treatment (17,18). In this article, low-dosage and long-term clarithromycin was administrated to the smoke-exposed asthmatic mice, and the results were better than those without clarithromycin treatment mice. Lung resistance, RE and dynamic lung compliance were all improved in clarithromycin group. And the airway inflammation of lung tissue and BALF was also attenuated significantly, such as dramatically decreased CXCL1 level, and cell counts in BALF. These findings support the conclusion that clarithromycin can suppress AHR and inflammation, and the results were in line with previous study (19,20), though the experimental protocols were different. The potential mechanism was postulated to be related with the transcription factors such as nuclear factor kappa B (21) and HDAC2 (22,23), which are believed to play an important part in the process of inflammation and anti-inflammation. In this study, the HDAC2 activity was also tested to find the difference between each group, and the results still was positive. Clarithromycin intervention improved the HDAC2 activity in CAM group compared to SEA group. The HDAC2 expression also dramatically decreased in the SEA group, while clarithromycin attenuated the expression of HDAC2 and this might be one of those potential mechanisms of how macrolides work.
In this research, clarithromycin inhibited the airway inflammation and AHR in smoke-exposed asthmatic mice. The results suggest that clarithromycin might be effective as adjunctive therapy in treating asthmatic patients who are smokers and/or second smokers.
Acknowledgements
Min Hao is grateful to researchers Qin Kong and Meng Wang for their generosity and technical support. Min Hao also appreciates Zhencui Ren, Zhe Cai, Lili Wang for their help and backup.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Brusselle GG, Joos G. Is there a role for macrolides in severe asthma? Curr Opin Pulm Med 2014;20:95-102. [PubMed]
- Louis R, Schleich F, Barnes PJ. Corticosteroids: still at the frontline in asthma treatment? Clin Chest Med 2012;33:531-41. [PubMed]
- Lazarus SC, Chinchilli VM, Rollings NJ, et al. Smoking affects response to inhaled corticosteroids or leukotriene receptor antagonists in asthma. Am J Respir Crit Care Med 2007;175:783-90. [PubMed]
- Spears M, Donnelly I, Jolly L, et al. Effect of low-dose theophylline plus beclometasone on lung function in smokers with asthma: a pilot study. Eur Respir J 2009;33:1010-7. [PubMed]
- Spagnolo P, Fabbri LM, Bush A. Long-term macrolide treatment for chronic respiratory disease. Eur Respir J 2013;42:239-51. [PubMed]
- Reiter J, Demirel N, Mendy A, et al. Macrolides for the long-term management of asthma--a meta-analysis of randomized clinical trials. Allergy 2013;68:1040-9. [PubMed]
- Friedlander AL, Albert RK. Chronic macrolide therapy in inflammatory airways diseases. Chest 2010;138:1202-12. [PubMed]
- Reddy AT, Lakshmi SP, Reddy RC. Murine model of allergen induced asthma. J Vis Exp 2012;e3771. [PubMed]
- Qiao Y, Li B, Yang G, et al. Irritant and adjuvant effects of gaseous formaldehyde on the ovalbumin-induced hyperresponsiveness and inflammation in a rat model. Inhal Toxicol 2009;21:1200-7. [PubMed]
- Underwood S, Foster M, Raeburn D, et al. Time-course of antigen-induced airway inflammation in the guinea-pig and its relationship to airway hyperresponsiveness. Eur Respir J 1995;8:2104-13. [PubMed]
- Gong PH, Gao ZC, Hu P, et al. Investigation of the measurement of murine airway hyperresponsiveness and the therapeutic effects of budesonide on ovalbumin sensitized and challenged mice. Chin Med J (Engl) 2005;118:1959-64. [PubMed]
- Mojtabavi N, Dekan G, Stingl G, et al. Long-lived Th2 memory in experimental allergic asthma. J Immunol 2002;169:4788-96. [PubMed]
- Chen X, Gao YD, Yang J. Elevated interferon regulatory factor 4 levels in patients with allergic asthma. J Asthma 2012;49:441-9. [PubMed]
- Lanckacker EA, Tournoy KG, Hammad H, et al. Short cigarette smoke exposure facilitates sensitisation and asthma development in mice. Eur Respir J 2013;41:1189-99. [PubMed]
- Tamimi A, Serdarevic D, Hanania NA. The effects of cigarette smoke on airway inflammation in asthma and COPD: therapeutic implications. Respir Med 2012;106:319-28. [PubMed]
- St-Laurent J, Bergeron C, Pagé N, et al. Influence of smoking on airway inflammation and remodelling in asthma. Clin Exp Allergy 2008;38:1582-9. [PubMed]
- Richeldi L, Ferrara G, Fabbri LM, et al. Macrolides for chronic asthma. Cochrane Database Syst Rev 2005;CD002997. [PubMed]
- Brusselle GG, Vanderstichele C, Jordens P, et al. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicentre randomised double-blind placebo-controlled trial. Thorax 2013;68:322-9. [PubMed]
- Hrvacić B, Bosnjak B, Bosnar M, et al. Clarithromycin suppresses airway hyperresponsiveness and inflammation in mouse models of asthma. Eur J Pharmacol 2009;616:236-43. [PubMed]
- Lin CC, Liaw SF, Wu KM, et al. Effect of erythromycin on bronchial hyperreactivity and inflammation in ovalbumin-sensitized brown Norway rats. Respir Physiol Neurobiol 2008;161:267-72. [PubMed]
- Lorentz A, Klopp I, Gebhardt T, et al. Role of activator protein 1, nuclear factor-kappaB, and nuclear factor of activated T cells in IgE receptor-mediated cytokine expression in mature human mast cells. J Allergy Clin Immunol 2003;111:1062-8. [PubMed]
- Ito K, Ito M, Elliott WM, et al. Decreased histone deacetylase activity in chronic obstructive pulmonary disease. N Engl J Med 2005;352:1967-76. [PubMed]
- Ito K, Caramori G, Lim S, et al. Expression and activity of histone deacetylases in human asthmatic airways. Am J Respir Crit Care Med 2002;166:392-6. [PubMed]


