A case of chronic expanding hematoma resulting in fatal hemoptysis
Abstract
An 80-year-old woman presented with a huge intrathoracic mass which had increased in size over 4 years. Computed tomography showed a thick calcified capsule and early-enhanced streaks inside the mass. Needle biopsy aspirated pure blood and fibrous connective tissue. F-18 fluorodeoxyglucose positron-emission tomography showed moderate FDG uptake at the periphery with central photon defects. Gallium-67 scintigraphy showed no abnormal uptake. On suspicion of chronic expanding hematoma, we recommended surgical resection, but the patient requested only follow-up. One year later, she was hospitalized with cardiac tamponade and subsequent massive hemoptysis. Repeated embolization was ineffective, and the patient soon succumbed.
Key words: Chronic expanding hematoma; fluorodeoxyglucose F18 positron-emission tomography; gallium-67 scintigraphy; pyothorax associated lymphoma; trans-arterial embolization
Introduction
Chronic expanding hematoma (CEH) presents as a progressively enlarging mass in patients with histories of trauma or surgery. It can occur in different locations in the body, often simulating neoplasia (1). In Japan and Korea, CEH in patients with a history of tuberculous pleurisy has been reported (2-6). It is considered a specific form of chronic empyema (7,8), and is sometimes designated chronic hemorrhagic empyema (9,10). In patients with CEH arising from chronic empyema, differential diagnosis needs to distinguish between CEH and malignancies such as pyothorax-associated lymphoma (PAL) (11-14). CEH in the thorax can be life-threatening, leading to respiratory failure or hemoptysis. Whereas total surgical removal should be considered, this may be too invasive for elderly patients, with the risk of massive bleeding with extensive resections such as pleuropneumonectomy (5). Here we report an 80-year-old patient with CEH, whose F-18 fluorodeoxyglucose positron-emission tomography (FDG-PET) and Ga scintigraphy images were helpful in excluding lymphoma. She requested follow-up without surgery, and had an unfavorable prognosis even with repeated trans-arterial embolization (TAE).
Case report
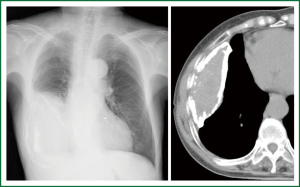
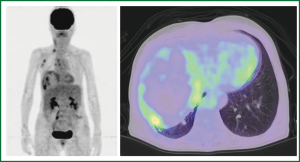
An 80-year-old woman presented with chest oppression and an intrathoracic mass which had been increasing in size over a period of 4 years (Figure 1). She had had tuberculous pleurisy 50 years previously. Chest CT showed a large heterogeneous mass with a thick wall containing flecks of calcification, in which early-enhanced streaks originating from the thick capsule could be seen extending into the mass on dynamic enhancement imaging (Figure 2). Although CEH was suspected, differentiation from tumors such as PAL was required. On needle biopsy, pure blood with a hemoglobin concentration of 11.2 mg/dL was aspirated from a subcapsular lesion, and a biopsy revealed fibrous connective tissue with hyalinization, and no specific findings of malignancy or infection. FDG-PET showed moderate FDG uptake only at the periphery of the mass with central photon defects (Figure 3). The maximum standardized uptake value (SUVmax) of the lesion was 3.8. Abnormal FDG uptake was also observed in the mediastinal, neck and axillary lymph nodes. Open cervical and axillary lymph node biopsy revealed follicular hyperplasia. Gallium-67 scintigraphy showed no abnormal uptake, helping to exclude PAL. With a clinical diagnosis of CEH, we recommended surgical resection with preoperative TAE, but the patient and her family requested follow-up without surgical intervention. She was therefore followed-up as an out-patient for one year, with a gradually enlarging mass and occasional hemosputum.
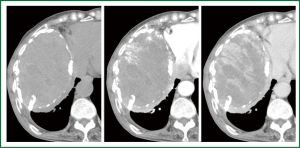
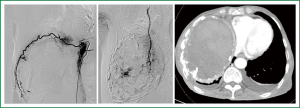
After one year follow-up, she was hospitalized with cardiac tamponade. Pericardial drainage yielded 570 mL of bloody fluid with a hemoglobin concentration of 5.9 mg/dL. For optimal symptomatic palliation, TAE was undertaken for the right intercostal and intramammary arteries. Additional pericardial sclerotherapy was successful without re-retention of bloody pericardial effusion. Six days later, massive repetitive hemoptysis occurred. Although repeated TAE for recanalized intercostal arteries did reduce staining intensity of contrast-enhanced CT (Figure 4), massive hemoptysis persisted and the patient succumbed. Autopsy was not permitted.
Discussion
Reid et al. (1) proposed the existence of CEH as a separate entity based on their pathological studies of 6 cases of CEH in different locations other than the thorax. These entities had a common structure with a central mass of blood, a wall of granulation tissue, and dense, fibrous tissue at the periphery. They presented clinically as slowly expanding space-occupying masses months or years after the initial injury. These findings are the same in thoracic CEH, sometimes arising from chronic empyema. To distinguish CEH from malignant disease, CT-guided needle biopsy is useful for exclusion of possible malignant cells or pathogenic bacteria. However, care needs to be exercised to avoid the risk of massive bleeding. Pure blood can be continuously aspirated from CEH, sometimes leading to massive bleeding and hemorrhagic shock after needle aspiration (2,9,15). In the present case, FDG-PET revealed moderate FDG uptake only at the periphery of the mass with central photon defects, with an SUVmax of 3.8. This is consistent with previous reports of FDG-PET imaging of CEH with discrete spots of moderate FDG uptake at the rim attributed to inflammation, and an SUVmax of 3.7 to 5.5, seen in pelvic CEH (16) and in intrathoracic CEH (6,7). This type of FDG-PET finding is probably characteristic of CEH, suggesting chronic inflammation or granulation tissue in the fibrous wall. The presence of a mass-like lesion arising from chronic empyema could result in a differential diagnosis of PAL (11-14). However, PAL shows strong FDG accumulation in FDG-PET (12,13) and marked uptake in Ga scintigraphy (14). Thus, the results of FDG-PET and Ga scintigraphy excluded lymphoma in the present case.
CEH shows progressive expansion over a long period of time and may grow to a very large mass occupying the whole hemithorax, distorting the mediastinum, and resulting in respiratory distress (2,9,15). Total surgical removal, including the capsule, should be the treatment of choice (10), but curettage of the contents could result in massive bleeding from the hypervascular subcapsular lesion. Incomplete resection carries a risk of hemorrhagic death (2) or recurrence of CEH (3,10), while removal of the inner substance (3,9) or thoracoscopic irrigation (17) has been reported to be successful on occasion. The risk of bleeding may depend on the vascularity of the subcapsular area. In the present case, dynamic CT revealed prominent early-enhanced streaks inside the mass, suggesting a risk of massive bleeding from the hypervascular subcapsular lesion. Regarding the outside of the capsule, strong adhesion to the surrounding tissue and abundant newly-developing arteries may be present, especially in patients with a history of tuberculous pleurisy. In these cases, combined lobectomy or pleuropneumonectomy is often required (2). Intraoperative bleeding >2,000 mL is associated with most surgical cases, and more massive bleeding >20,000 mL has been reported when separating the adhesive capsule (15) or as originating from inside the capsule (18). Presurgical TAE (3,4,9,19) or intraoperative balloon occlusion of the subclavian artery (20) have been reported to result in reduction of intraoperative blood loss.
CEH after tuberculous pleurisy tends to occur in elderly patients, who may appear too frail to withstand the invasiveness of surgery with massive bleeding or extensive resection. Palliative therapy other than surgery has seldom been reported (2,5), although one case of successful TAE resulted in diminution of the chest wall mass in an 82-year-old patient (5). In the present case, the patient and her family requested follow-up without surgical resection. After one year, whereas cardiac tamponade could initially be controlled with TAE and pericardial sclerotherapy, rapidly occurring subsequent massive hemoptysis persisted in spite of repeated TAE. Thus, TAE can be helpful as a preoperative preparation or treatment in a stable patient, but its effects are likely to be transient and insufficient as a definitive treatment modality for critical hemoptysis.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Reid JD, Kommareddi S, Lankerani M, et al. Chronic expanding hematomas. A clinicopathologic entity. JAMA 1980;244:2441-2.
- Harada K, Taniki T, Yoshizawa K, et al. Clinical studies of chronic tuberculous pleurisy with persisting hemorrhagic lesion. Nihon Kyobu Geka Gakkai Zasshi 1983;31:2152-8.
- Matsuge S, Hosokawa Y, Yamazaki S, et al. Five cases of surgically resected chronic expanding hematoma in the chest. Kyobu Geka 2000;53:768-73.
- Tsubochi H, Sato N, Imai T. Chronic expanding hematoma with bronchopleural fistula and empyema space. Ann Thorac Cardiovasc Surg 2009;15:171-3.
- Muramatsu T, Shimamura M, Furuichi M, et al. Treatment strategies for chronic expanding hematomas of the thorax. Surg Today 2011;41:1207-10.
- Kwon YS, Koh WJ, Kim TS, et al. Chronic expanding hematoma of the thorax. Yonsei Med J 2007;48:337-40.
- Takahama M, Yamamoto R, Nakajima R, et al. Extrathoracic protrusion of a chronic expanding hematoma in the chest mimicking a soft tissue tumor. Gen Thorac Cardiovasc Surg 2010;58:202-4.
- Kuronuma K, Ootake S, Ikeda K, et al. Chronic expanding hematoma in the chest. Intern Med 2008;47:1411-4.
- Fukuhara K, Nakagawa K, Fujiwara K, et al. Successful surgery for hemorrhagic empyema accompanied by an asymptomatic bronchial fistula. The Japanese Association for Chest Surgery 2002;16:554-8.
- Iuchi K, Tanaka H, Nakamura K. Pathogenesis and treatment of chronic empyema. Nihon Geka Gakkai Zasshi 2004;105:751-6.
- Iuchi K, Ichimiya A, Akashi A, et al. Non-Hodgkin’s lymphoma of the pleural cavity developing from long-standing pyothorax. Cancer 1987;60:1771-5.
- Asakura H, Togami T, Mitani M, et al. Usefulness of FDG-PET imaging for the radiotherapy treatment planning of pyothorax-associated lymphoma. Ann Nucl Med 2005;19:725-8.
- Ito K, Kubota K, Morooka M, et al. F-18 FDG PET/CT findings in two patients with pyothorax-associated lymphoma. Clin Nucl Med 2010;35:802-5.
- Ueda T, Andreas C, Itami J, et al. Pyothorax-associated lymphoma: imaging findings. AJR Am J Roentgenol 2010;194:76-84.
- Iuchi K, Tanaka Y, Tanaka H, et al. A case of chronic hemorrhagic empyema for twenty five years after the pulmonary lobectomy. Nihon Kyobu Rinsyo 1994;53:869-74.
- Hamada K, Myoui A, Ueda T, et al. FDG-PET imaging for chronic expanding hematoma in pelvis with massive bone destruction. Skeletal Radiol 2005;34:807-11.
- Roper CL, Cooper JD. Chronic expanding hematoma of the thorax. J Thorac Cardiovasc Surg 2001;122:1046-8.
- Shinada J, Yoshimura H, Hirai S, et al. Chronic hemorrhagic empyema developed in thirty three years after the right pneumonectomy--a case report. Nihon Kyobu Geka Gakkai Zasshi 1991;39:1204-7.
- Tezuka N, Fujino S, Inoue S, et al. A case of chronic hemorrhagic pyothorax treated by multi-stage operation including omentopexy. The Japanese Association for Chest Surgery 1999;13:679-84.
- Suzuki S, Handa M, Kobayashi S, et al. A case of remove of foreign body granuloma after extraperiosteal sponge plombage 20 years ago. Nihon Kyobu Geka Gakkai Zasshi 1988;36:2332-5.


