Severe aortic regurgitation and partial anomalous pulmonary venous connection in a Turner syndrome patient
Introduction
Turner syndrome (TS) is one of the most common sex chromosome diseases, affecting 1 in 2,500 live-born females. Short stature (if untreated) and ovarian dysgenesis (streak ovary) are two typical clinical manifestations of these patients. Other clinical features include lymphedema (webbed neck, cystic hygroma), hypothyroidism, vision and hearing problems (1). It is estimated that 22-70% of TS patients have a form of congenital heart defects. Also, a variety of cardiovascular abnormalities has been found associated with TS, the most common of which are bicuspid aortic valve (BAV, appearing in 15-30% of TS patients) and coarctation of the aorta (CoA, appearing in up to 17% of TS patients) (2). Here we report a TS patient presented with severe aortic regurgitation, BAV and partial anomalous pulmonary venous connection (PAPVC).
Case presentation
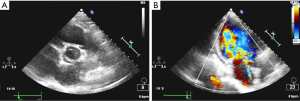
A 29-year-old Chinese woman presented to cardiac surgery outpatient clinic for assessment of palpitation and exertion dyspnea, which lasted more than 1 year and became more severe in recent months. Her medical history was otherwise unremarkable except that she never had a menstrual cycle. Physical examination revealed a short, thin woman: 140 cm in height and 45 kg in weight. Blood pressure was 110/60 mmHg. Bilateral lower extremity pitting edema was observed. Cardiovascular examination found a grade 3 diastolic decrescendo murmur that was heard at the right sternal border of the second intercostal space. Peripheral arterial pulses were palpable. Electrocardiography was unremarkable. Chest X-ray showed left atrium and left ventricle enlargement, and bilateral lung congestion. Transthoracic echocardiography (TTE) demonstrated BAV (Figure 1A), severe aortic valve regurgitation (Figure 1B), moderate pulmonary hypertension and a suspected PAPVC. The aortic root diameter was 31 mm. TS were suspected due to her short stature and amenorrhea. Chromosome analysis revealed a 45X karyotype, which confirmed the diagnosis. Computed tomography venography (CTV) was arranged to further study the pulmonary veins. It revealed the left upper pulmonary vein was dilated and drained to the left branchiocephalic vein (Figure 2A); there were two right upper pulmonary veins, one of which was drained to the dilated left upper pulmonary vein (Figure 2B,C). The other one was drained to the left atrium normally.
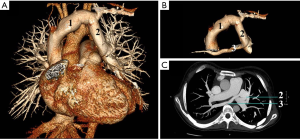
The patient was diagnosed as (I) TS; (II) severe aortic regurgitation; (III) BAV; (IV) PAPVC, supracardiac type; (V) heart failure, NYHA Class IV. Drug therapy was provided to improve her cardiac function, which included diuretics, beta-blockers and angiotensin-converting enzyme inhibitors (ACEI). Surgical treatment was recommended to the patient even though she refused it. Unfortunately, the patient died one month later because of the irreversible heart failure.
Discussion
Although it is not common for cardiologists or cardiac surgeons to make a first diagnosis of TS, it is still necessary to pay attention to female cardiac patients who have short stature, amenorrhea, lymphedema and/or other clinical features. Chromosome analysis can be helpful. Since cardiovascular defects are considered as the most serious complication of TS, the Turner Syndrome Consensus Study Group guideline recommends arranging a comprehensive cardiovascular evaluation for each TS patient at the time of diagnosis (1). Our patient had a severe aortic regurgitation and PAPVC, both of which contributed to her heart failure. Therefore, surgical treatment was suggested. Given that TS has relatively complex genetic heterogeneity, the surgical risk should be carefully evaluated. Madriago et al. (3) showed that cardiac operation outcomes were generally comparable between patients with TS and those with non-TS. Specifically, for aortic valve surgery, there was no difference in the length of hospital stay and mortality. The operation of PAPVC resulted in higher surgical mortality in patients with TS compared with those with non-TS (14.3% vs. 1.9%). With regard to this, our patient may still benefit more from surgical treatment than from drug therapy alone.
For the surgical strategy, we considered performing the aortic valve replacement following the correction of PAPVC. As this patient had concomitant valve problems, standard median sternotomy will be adopted. Cardiopulmonary bypass will be established with ascending aortic and bicaval cannulation. After the junction between the left upper pulmonary vein (i.e., left vertical vein) and the left branchiocephalic vein is exposed, the left upper pulmonary vein will be transected and anastomosed to the left atrial appendage. Aortic valve replacement with a prosthetic valve will then be performed. For the ascending aorta, we believed that there was no need to replace it at this point of time. Firstly, this patient’s imaging findings showed there was no aneurysmal dilation. Secondly, for patients with genetic disease, the 2010 ACCF/AHA Guideline for Thoracic Aortic Disease (4) recommends that the ratio of maximal ascending or aortic root area (πr2) in cm2 divided by the patient’s height in meters exceeding 10 is considered as a surgical indication. Our patient’s ratio was 5.39 (r=1.55 cm, height =1.4 m) which was less than 10. Thirdly, according to two large-scale clinical studies (5,6), TS patients with ascending aortic size index (ASI) over 2.5 cm/m2 should be considered as high risk and prophylactic aortic operation is required. However, our patient’s ASI was 2.38. Therefore, we would prefer to closely monitor her aortic diameter after discharge.
Recently, the association of aortic dissection and TS has been reported. Aortic dissection is six times more common in TS than in general population, and most commonly affects TS patients at their mid-30 s (7). It is also important to carefully evaluate the risk of aortic dissection for TS patients. As TS patients usually have short stature and low body weight, the predictive value of aortic diameter is relatively low in these patients. As was mentioned above, ASI, which is normalized by body surface area, has been proposed to evaluate the risk of aortic dissection in TS patients (5,6).
Aortic dissection is also another devastating complication of pregnant TS patients. TS patient can achieve pregnancy with either oocyte donation (majority) or spontaneous pregnancy (very rare, 2-8%) (8). However, the estimated risk of death during pregnancy from aortic dissection or rupture is 2% or higher among this group of patients. Moreover, pregnancy-related changes to the aorta may further increase the risk of aortic dissection, aortic rupture, and premature death in subsequent years (9,10). The Practice Committee of the American Society for Reproductive Medicine recommended in 2012 (10) that cardiac MRI revealing any significant abnormality and/or ASI over 2 cm/m2 represents an absolute contraindication for attempting pregnancy in a TS patient. In addition, for a TS patient with normal cardiac MRI and evaluation, she is still considered at much higher risk for associated morbidity and mortality, and requires careful observation and frequent reevaluation throughout the pregnancy.
Acknowledgements
The authors thank Ms. Anqi Wang (Columbia University) and Mr. Can Wang (Fudan University) for their critical reading of the manuscript. The authors also thank Mr. Chen Huang (DRAWING FUN Studio) for his assistance in preparing the illustrations.
Funding: This study was supported by a grant from Key Projects in the National Science & Technology Pillar Program during the Twelfth Five-Year Plan Period (No. 2011BAI11B20).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Bondy CA; Turner Syndrome Study Group. Care of girls and women with Turner syndrome: a guideline of the Turner Syndrome Study Group. J Clin Endocrinol Metab 2007;92:10-25. [PubMed]
- Mortensen KH, Andersen NH, Gravholt CH. Cardiovascular phenotype in Turner syndrome--integrating cardiology, genetics, and endocrinology. Endocr Rev 2012;33:677-714. [PubMed]
- Madriago E, Nguyen T, McFerson M, et al. Frequency and outcomes of cardiac operations and catheter interventions in Turner syndrome. Am J Cardiol 2012;110:580-5. [PubMed]
- Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation 2010;121:e266-369. [PubMed]
- Matura LA, Ho VB, Rosing DR, et al. Aortic dilatation and dissection in Turner syndrome. Circulation 2007;116:1663-70. [PubMed]
- Carlson M, Airhart N, Lopez L, et al. Moderate aortic enlargement and bicuspid aortic valve are associated with aortic dissection in Turner syndrome: report of the international turner syndrome aortic dissection registry. Circulation 2012;126:2220-6. [PubMed]
- Turtle EJ, Sule AA, Webb DJ, et al. Aortic dissection in children and adolescents with Turner syndrome: risk factors and management recommendations. Arch Dis Child 2015;100:662-6. [PubMed]
- Bondy C. Pregnancy and cardiovascular risk for women with Turner syndrome. Womens Health (Lond Engl) 2014;10:469-76. [PubMed]
- Hagman A, Källén K, Barrenäs ML, et al. Obstetric outcomes in women with Turner karyotype. J Clin Endocrinol Metab 2011;96:3475-82. [PubMed]
- Practice Committee of American Society For Reproductive Medicine. Increased maternal cardiovascular mortality associated with pregnancy in women with Turner syndrome. Fertil Steril 2012;97:282-4. [PubMed]


