Prognostic value of stromal decorin expression in patients with breast cancer: a meta-analysis
Introduction
Breast cancer is generally regarded as the most common malignant disease in female patients. It is nearly the No. 1 cancer diagnosed among Chinese women especially those in urban areas, and the second leading cause of cancer-related deaths in women around the world (1,2). On the basis of the statistical records in the last decade, the incidence and mortality of breast cancer have gradually increased (1,3). According to authoritative estimation, approximately 1.7 million newly diagnosed cases and 0.5 million deaths per year were caused by breast cancer around the world in recent years (4). To deal with such a great challenge to women health, systematic neo-adjuvant or adjuvant therapies, such as chemotherapy, radiotherapy and hormonal therapy, are developed and have largely improved the prognosis of breast cancer (5,6). However, the survival outcomes of breast cancer patients are still not optimistic, especially in high-risk patients, such as the elderly and those with long-term use of oral contraceptives (7,8). Therefore, it has been increasingly necessary to identify an effective biomarker for accurately predicting the prognosis of breast cancer patients.
In recent years, oncologists have increasingly paid attention to the potential biological functions of Decorin (DCN), the most extensively studied representative of small leucinerich proteoglycans (SLRPs) in extracellular matrix (ECM), in many common malignances including breast cancer (9). A large number of studies have investigated its role in oncogenesis, tumor progression, angiogenesis and metastasis. Many laboratorial investigations have identified the potential molecular mechanisms mediating the impacts of stromal DCN expression on the biological characteristics of malignances (10). However, far fewer clinical studies addressing the association between stromal DCN expression and disease prognosis are reported up to now. The prognostic significance of DCN expression varies in many cancers including lung cancer (11), glioblastoma (12), spindle cell sarcomas (13) and breast cancer (14-17). Although most of these available evidences aim to highlight the prognostic value of stromal DCN expression in breast cancer, some controversial results still exist and a consensus has not been reached until now.
Based on applying the evidence-based methods to a larger number of pooled samples from eligible studies, the pooled outcomes may help oncologists to clarify the prognostic value of DCN in breast cancer. Therefore, we conducted this meta-analysis to evaluate the prognostic value of stromal DCN expression in breast cancer patients with detailed subdivision and comprehensive assessment.
Materials and methods
A systematic review and meta-analysis does not require necessary patients’ consent or ethical approval. We carried out this meta-analysis in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (18). The additional PRISMA 2009 checklist is given in the Supplementary data 1.
Searching strategies
No language limitations were applied in this meta-analysis. Four electronic databases including PubMed, EMBASE, the Web of Science and China National Knowledge Infrastructure (CNKI) were selected for identification of the eligible literatures published up to June 2015. Four searching strings were combined with several key words and the Boolean operators “AND” and “OR”. The key words are listed as follows: (I) “decorin or DCN”; (II) “breast cancer or breast carcinoma or breast neoplasm or breast tumor”; (III) “mammary cancer or mammary carcinoma or mammary neoplasm or mammary tumor”. The full search details using these terms were summarized in the Supplementary data 2. Additionally, we also manually searched the reference lists of relevant papers to identify any one included study with no duplication.
Inclusion and exclusion criteria
The following inclusion and exclusion criteria were established to determine the eligible literatures for our meta-analysis.
Inclusion criteria: (I) the target disease is breast cancer, benign diseases in the mammary glands and ducts are not considered; (II) the expression level of stromal DCN is evaluated independently rather than in company with other markers; (III) demographic data or survival curves are available in original literatures, and the endpoints prefer to be the cancer-specific survival (CSS) and disease-free survival (DFS); (IV) the associated statistical results including hazard ratio (HR), relative risk (RR) and odds ratio (OR) from multivariate analysis and/or univariate analysis, are directly reported in original literatures.
Exclusion criteria: (I) the specific types of literatures including reviews, preclinical experiments, letters, conference abstracts and comments are excluded; (II) the survival outcomes are not associated with DCN expression in breast cancer cases; (III) DCN expression in the malignant epithelium of breast cancer are not considered.
Quality assessment
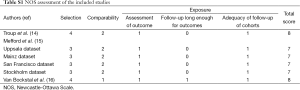
Newcastle-Ottawa Scale (NOS) was applied to estimate the quality of original non-randomized studies (19). Three perspectives involving selection, comparability and exposure were considered for a semi-quantitative estimation. The “star system” with a maximum of 9 stars was applied as the assessment tool. After grading all of the included studies, we regarded 8-9 stars as a good quality, 6-7 stars as a fair quality, and lower than 6 stars as a poor quality.
Data collection
We designed an Excel sheet to collect the following details: (I) publication data including authors, publication year and nations; (II) experimental data including study design, study period, detecting materials, detecting methods, cut-off values and follow-ups; (III) demographic data including enrolled samples, ages, receptor status and the number of patients with positive expression and negative expression of DCN; (IV) statistical data including survival analyses, published statistics with 95% confidence interval (CI) based on multivariate analysis and/or univariate analysis, and the sources of these conducted outcomes.
Statistical analysis
In this meta-analysis, we determined to apply the HR with 95% CI as the appropriate summarized statistics. HR is generally regarded as the only statistical parameter compatible for both censoring and time-to-events (20). However, there were many papers just displaying the survival rate with P value from log-rank test or Kaplan-Meier (K-M) survival curves, to reflect the survival outcomes. Tierney et al. (21) have reported a practical method to extract the HR with 95% CI using the published survival data and K-M curves, and incorporate them into meta-analysis. Therefore, if the HRs were not reported in original literatures, we determined to calculate them using the survival rates, analyzed events and P value from log-rank test in accordance with the described instructions. The referred formulas are displayed as follows:

In which O-E is the log rank Observed minus Expected events and V is the log rank Variance (21). Then we extracted the survival details by Engauge Digitizer 4.1 (http://sourceforge.net) from the K-M curves to measure the accuracy of estimated HRs. Moreover, the RRs conducted from multivariate analysis could be directly considered as HRs and incorporated into our meta-analysis (22). As for the ORs reported in some studies, we transformed them into RRs using the following formula:

Where the P value is the incidence of the outcome of interest in the non-exposed group (23).
However, these calculated survival outcomes are based on univariate analysis instead of multivariate analysis, which means that other possible confounders cannot be adequately eliminated (21). Therefore, to correctly interpret the final conclusion, we pooled the individual outcomes from univariate analysis and multivariate analysis, respectively. Then, we determined the prognostic value of DCN in breast cancer according to the comprehensive assessment of the summarized outcomes based on both univariate analysis and multivariate analysis.
Q-test and I2 statistic were employed to estimate the level of heterogeneity across the included studies. Fine heterogeneity was defined as I2<40% and P>0.1, and a fixed-effect model test was determined at the same time. On the contrary, the random-effect model test would be performed if the significant heterogeneity was revealed within this meta-analysis (I2≥40% or P≤0.1) (24). Sensitivity analysis was carried out to further identify the possible origins of heterogeneity. Then, the identified study which possibly contributed to the high heterogeneity would be excluded and a repeated meta-analysis of the remaining studies was applied for adjustments. The strong robustness of our meta-analysis would be confirmed if there were no substantial varies between the adjusted outcomes and primary outcomes (25).
Finally, the potential publication bias in this meta-analysis was assessed by both Begg’s test and Egger’s test. Its presence was suggested by the symmetry of funnel plot conducted by Begg’s test, and in which log HR was plotted against their corresponding standard errors (SEs) (26). The significant bias would be confirmed if P value<0.05. All the procedures of statistical analysis were accomplished by STATA 12.0 (STATA Corporation, College Station, TX, USA).
Results
The selection of included studies
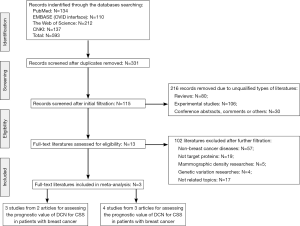
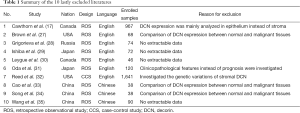
In accordance with the described searching strategies, a total of 593 citations were identified by searching through the selected four electronic databases, including 134 citations in PubMed, 110 citations in EMBASE (via Ovid interface), 212 citations in the Web of Science (via the campus network of Sichuan University) and 137 citations in CNKI. After excluding the duplicated records, 331 literatures entered into the initial filtration, which was based on screening the titles and abstracts. Then, after excluding 216 of them due to the unqualified literature types, including 80 reviews, 106 laboratory experiments and 30 conference abstracts, letters or comments, the further filtration was conducted by reading through the full-text of remaining literatures. After that, there were 13 full-text literatures identified for possible eligibility in our meta-analysis. The associated details of the 102 excluded literatures were briefly summarized in Figure 1. Finally only three English literatures were determined to be included in this meta-analysis, which contained 6 studies assessing the prognostic value of DCN for CSS or DFS (14-16). The other 10 literatures were excluded for the final quantitative analysis (17,27-35), and the reasons for exclusion were briefly summarized in Table 1.
Full table
The characteristics of included studies
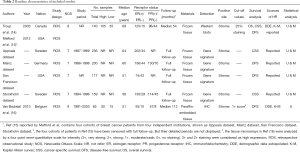
The basic characteristics of the three eligible literatures (14-16) are listed in Table 2. Mefford et al. (15) reported four cohort studies based on four independent datasets from different institutions, including two studies focusing on the CSS outcomes and the other two studies investigating the prognostic significance of stromal DCN expression for DFS in breast cancer patients. Thus, the three eligible literatures actually contained 6 included studies and all of them were retrospective observational studies. A total of 917 breast cancer cases were enrolled in the present meta-analysis, with 65 to 236 patients in each included study. These studies were published between 2003 and 2013, and their enrolled samples ranged from 1987 to 2003. Positive DCN expression was targeted in the stromal tissues of breast. The samples used by most researchers were prepared from frozen tissues except that paraffin-embedded tissues were used in one study (16). The detecting methods and their corresponding defined cut-offs varied in different investigations. The four cohort studies reported by Mefford et al. (15) were based on four independent datasets including Uppsala dataset (Sweden), Mainz dataset (Germany), San Francisco dataset (USA) and Stockholm dataset (Sweden). All of these studies published the HRs with 95% CI conducted from both univariate analysis and multivariate analysis. Troup et al. (14) reported the analyzed events, K-M curves and corresponding P values for overall survival (OS), CSS and DFS. However, only the results related to OS and DFS from both univariate analysis and multivariate analysis were published, and the decisive factor was OR instead of HR. The last included study was conducted by Van Bockstal et al. (16). It only analyzed the prognostic significance of stromal DCN expression for DFS but showed no associated results from multivariate analysis. The other characteristics of these 3 literatures are summarized in Table 2, including the expression of estrogen-receptor (ER) and progesterone-receptor (PR), follow-ups and statistical details. In addition, the quality level of each included study was represented by the number of stars (Table 2). Their details are listed in Table S1 (see Supplementary data 3).
Full table
Full table
Assessment of the association between stromal DCN expression and CSS
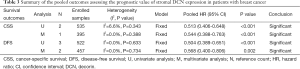
Three included studies (from two literatures) (14,15) focused on the prognostic significance of stromal DCN expression for CSS in breast cancer, which conducted three survival results from univariate analysis (14,15) and two results from multivariate analysis (15). On the one hand, the pooled HR based on univariate analysis was 0.513 (95% CI: 0.406-0.648; P<0.001) (Table 3 and Figure 2A), indicating that stromal DCN expression might be a strong predictor of higher CSS in breast cancer patients. Meanwhile, the fixed-effect model was determined by the low heterogeneity (I2=6.6%, P=0.343). On the other hand, the pooled HR based on multivariate analysis also revealed the significantly higher CSS in patients with positive expression of DCN compared to those with negative expression of DCN (HR: 0.544; 95% CI: 0.388-0.763; P<0.001) (Table 3 and Figure 2B) with a fixed-effect model (I2=0.0%, P=0.388). These two pooled analyses both indicated that stromal DCN expression might promise the good prognosis for CSS in patients with breast cancer.
Full table
Assessment of the association between stromal DCN expression and DFS
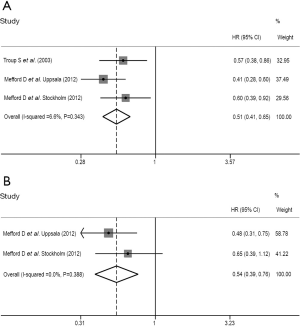
We pooled four DFS outcomes based on univariate analysis from three literatures (14-16) and three DFS outcomes based on multivariate analysis from two literatures (14,15), respectively. The summarized HR of univariate analysis was 0.504 (95% CI: 0.389-0.651; P<0.001) (Table 3 and Figure 3A), revealing that stromal DCN expression was positively associated with high DFS of patients with breast cancer. Meanwhile, the summarized outcomes of multivariate analysis also showed the statistically significant relationship between stromal DCN expression and better DFS of breast cancer (HR: 0.568; 95% CI: 0.400-0.806; P=0.002) (Table 3 and Figure 3B). Additionally, the low heterogeneity was observed among these included studies and a fixed-effect model was performed (univariate analysis: I2=0.0%, P=0.633; multivariate analysis: I2=0.0%, P=0.734).
Sensitivity analysis
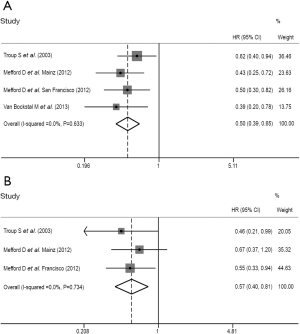
We performed a further sensitivity analysis and additional adjustments in both assessments of the prognostic value of stromal DCN expression for CSS and DFS in breast cancer patients. All of the forest plots conducted from sensitivity analysis were shown as Figure 4. We identified none of the independent outcomes from included studies was out of the estimated ranges by visually inspecting these forest plots. Therefore, the leave-one-out method and repeated analysis of the rest studies were no more necessary. The strong robustness of our meta-analysis was thus clarified.

Publication bias
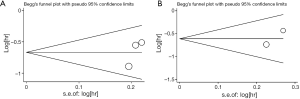
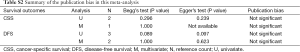
There was no evidence for publication bias observed within this meta-analysis, which were examined by both Begg’s test and Egger’s test. The funnel plots conducted from Begg’s test and corresponding P values were shown in the Supplementary data 3 (Figures S1,S2 and Table S2). Moreover, we must warn about the poor efficacy of both Begg’s test and Egger’s test when far fewer than 20 studies met the inclusion of meta-analysis. The poor sensitivity of both Begg’s test and Egger’s test should be seriously concerned due to the lack of enough number of included studies in our meta-analysis.

Full table
Discussion
To the best of our knowledge, our investigation is the first comprehensive and detailed meta-analysis to evaluate the prognostic value of stromal DCN expression in patients with breast cancer, although only three eligible literatures are available at present. We determined to apply the CSS and DFS as the summarized endpoints in our meta-analysis. Compared to OS, the patients’ death caused by non-malignant diseases or accidents will be eliminated adequately for extrapolating the CSS and DFS. Thus, CSS and DFS may better reflect the relationship between stromal DCN expression and cancer-related survival. After pooled analysis of both CSS and DFS, we demonstrate that high expression level of stromal DCN can predict the good prognosis in patients with breast cancer.
DCN is firstly separated and purified in 1978 (36). As a SLRP in stromal tissues, DCN is identified to be a key factor for some specific procedures of oncogenesis and tumor progression by latest investigations. Of course, the assistant functions of other SLRPs cannot be ignored. A laboratory study reported by Csordás et al. (37) shows that stable expression of DCN can suppress the functions of epidermal growth factor receptor (EGFR) by directly down-regulating the activity of EGFR and EGFR kinase in vivo and inhibiting the EGFR-mediated mobilization of intracellular calcium. Similarly, Santra et al. (38) also discover the potential anti-oncogenic role of DCN in down-regulating the activity of ErbB family to suppress mammary carcinoma cell growth and affect their differentiation grades. DCN has also been proved as a significant suppressor of intracellular β-catenin to inhibit cell growth and migration (10). Given these discoveries, many oncologists recommend that DCN may be utilized as an effective anti-malignance agent because its antagonism for multiple tyrosine kinase receptors can reduce oncogenesis and tumor progression.
When focusing on the relationship between malignant angiogenesis and DCN expression level, some fundamental researches also reveal the impacts of DCN on tumor progression. The most notable mechanism is the binding of DCN on transforming growth factor-β (TGF-β) receptors to compete with their primary ligands and thus to prevent tumor angiogenesis. When comparing DCN-treated fibroblasts and control fibroblasts, Zhang et al. (39) identify that DCN has a down-regulating effect on TGF-β1 production. It is supported by the discovery from Huijun et al. (40) in evaluating the feasibility of transferring DCN to anti-thymocyte serum (ATS) in a rat model. They find that the injection of DCN can significantly decrease the expression of TGF-β1 in rats. Both of the above investigations have suggested the anti-cancer functions of DCN. However, they are still in laboratory stage until now (9). The clinical trials based on a large size of samples are highly expected to confirm its efficacies in the future.
Meanwhile, some other researchers postulate the potential association between DCN expression and cancer metastasis based on clarifying the biological functions of DCN in stromal tissues. The majority of relevant investigations have mentioned the potential impacts of DCN expression on the loss of E-cadherin and β-catenin in cancer cells (41,42). E-cadherin plays a key role in regulating cellular adhesion, epithelium-mesenchymal transition and metastasis in solid carcinomas (43,44). The direct evidences from a DCN knockout mouse model demonstrate that DCN expression regulates the robustness of E-cadherin and thus significantly inhibits cancer cell growth and metastasis (42). In addition, DCN can also dramatically affect the down-regulation of β-catenin and the E-cadherin binding partner in vivo to maintain cell maturation (41). However, examining the statistical significance of these discoveries still needs more explorations. The involvement of stromal DCN in ca ncer metastasis remains a debate according to the present few investigations.
On the basis of these laboratorial discoveries, clinicians increasingly pay attention to the possible biological characteristics of stromal DCN in the prognoses of some common malignances, including breast cancer (11-17). The present evidences for the prognostic significance of stromal DCN expression in breast cancer can only be found in four investigations. In our meta-analysis, the cohort study conducted by Cawthorn et al. (17) was finally excluded because of the following two reasons. First, the major objective of this study was to assess the prognostic significance of epithelial DCN expression rather than stromal DCN expression in breast cancer. Second, the endpoints in this study were OS and DFS but the DFS outcomes from multivariate analysis were not shown. Notably, one issue exciting our interests is the complete conflict between the conducted outcomes from this study and the pooled outcomes of our meta-analysis. Cawthorn et al. (17) conclude that high DCN expression in malignant epithelium is significantly associated with worse prognosis in breast cancer patients. But the DCN expression in stromal tissues of breast cancer seems to predict higher survival rates in most of the relevant studies, which is also supported by our meta-analysis. DCN is generally considered as a component of ECM but it also exists in epithelial cells. However, far fewer researches provide eligible evidences revealing the differences in the biological functions of DCN expression between epithelium and stromal cells. Current investigations suggest that the dysregulation of angiogenesis and abnormal epithelial cell responses may be directly correlated with the impacts of DCN which was mainly expressed surrounding the epithelial cells (45). However, the relevant molecular mechanisms remain unclear until now, possibly due to the complicated interactions between DCN and the multiple components of ECM with cell surface receptors. The involvement of DCN expression in epithelial cells is still open to more investigations in the future.
Although the pooled analyses indicate that positive expression of stromal DCN can significantly predict the good prognosis of breast cancer, few of available evidences cannot be ignored for the accurate interpretation of this conclusion. On the one hand, the summarized outcomes may be not so convincing due to the limited availability of included studies. On the other hand, the publication bias may be not efficiently identified, although it may be not so necessary to test publication bias when less than 10 studies included in meta-analysis.
To resolve this issue and draw conclusions as accurate as possible based on the present evidences, we applied the following two strategies during the literatures retrieval and statistical analysis. First, the key words included in the searching strategies covered all of the possible descriptive forms of “breast cancer” and “decorin” (see Supplementary data 2). Besides, we searched four universal electronic databases and allowed the literatures in non-English languages. Second, the previous meta-analyses addressing the prognostic value of cancer biomarkers usually combine the results from univariate analysis and multivariate analysis together. In our meta-analysis, we classified the available data according to their statistical sources and pooled them separately. The final conclusion describing the prognostic value of DCN in breast cancer would be drawn, only when consistent pooled outcomes were obtained from both univariate analysis and multivariate analysis. Therefore, we recognize that the great homogeneity of included studies in this meta-analysis may reveal the feasibility and rationality of these two strategies, which contribute to accurately confirm the relationship between stromal DCN expression and the prognosis of breast cancer. Even so, the validity of our summarized outcomes is still urgently required to be further evaluated in the updated systematic reviews pooling more included studies in the future.
Limitations
Finally, some limitations exist in our meta-analysis and they should be acknowledged. First, the pooled analysis were based on only 917 enrolled samples from six included observational studies. Lack of enough evidences may cause adverse effects on the validity of summarized outcomes. Second, uniform cut-off definitions and detecting methods may be the major confounding factors affecting the final results. Third, we only searched one Chinese native database except the other three universal English databases, although no limitations of language were applied. The possible included studies from the native databases in other languages such as French, Spanish or Russian may be missed for our meta-analysis. Finally, we found the included studies in our meta-analysis all came from the non-Asian nations. Thus, oncologists should judiciously evaluate the generality of our summary outcomes in the clinical settings of China.
Conclusions
In conclusion, the integrated outcomes of our meta-analysis indicate that high stromal DCN expression can significantly predict the good prognosis in patients with breast cancer. The small number of the present evidences may cause few influences on the validity of this conclusion. Therefore, the discoveries from our meta-analysis should better be confirmed in the updated review pooling more associated studies in the future.
Acknowledgements
We thank the assistance of the staff in the Department of Thoracic Surgery, West China Hospital, Sichuan University.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Supplementary data 1
Full table
Supplementary data 2
Summary of electronic literature search

Full table

Full table

Full table
The search strategy in CNKI database is not displayed here because of the Chinese words using in the retrieval details.
References
- DeSantis C, Siegel R, Bandi P, et al. Breast cancer statistics, 2011. CA Cancer J Clin 2011;61:409-18. [PubMed]
- Jia M, Zheng R, Zhang S, et al. Female breast cancer incidence and mortality in 2011, China. J Thorac Dis 2015;7:1221-6. [PubMed]
- Westlake S, Cooper N. Cancer incidence and mortality: trends in the United Kingdom and constituent countries, 1993 to 2004. Health Stat Q 2008;38:33-46. [PubMed]
- Stewart BW, Wild CP, editors. World Cancer Report 2014. Lyon: IARC, 2014:517-19.
- Papadimitriou K, Ardavanis A, Kountourakis P. Neoadjuvant therapy for locally advanced breast cancer: Focus on chemotherapy and biological targeted treatments’ armamentarium. J Thorac Dis 2010;2:160-70. [PubMed]
- Christinat A, Di Lascio S, Pagani O. Hormonal therapies in young breast cancer patients: when, what and for how long? J Thorac Dis 2013;5 Suppl 1:S36-46. [PubMed]
- Assi HA, Khoury KE, Dbouk H, et al. Epidemiology and prognosis of breast cancer in young women. J Thorac Dis 2013;5 Suppl 1:S2-8. [PubMed]
- Pinto AC. Sexuality and breast cancer: prime time for young patients. J Thorac Dis 2013;5 Suppl 1:S81-6. [PubMed]
- Schaefer L, Iozzo RV. Biological functions of the small leucine-rich proteoglycans: from genetics to signal transduction. J Biol Chem 2008;283:21305-9. [PubMed]
- Goldoni S, Humphries A, Nyström A, et al. Decorin is a novel antagonistic ligand of the Met receptor. J Cell Biol 2009;185:743-54. [PubMed]
- Biaoxue R, Xiguang C, Hua L, et al. Decreased expression of decorin and p57(KIP2) correlates with poor survival and lymphatic metastasis in lung cancer patients. Int J Biol Markers 2011;26:9-21. [PubMed]
- Pope WB, Mirsadraei L, Lai A, et al. Differential gene expression in glioblastoma defined by ADC histogram analysis: relationship to extracellular matrix molecules and survival. AJNR Am J Neuroradiol 2012;33:1059-64. [PubMed]
- Matsumine A, Shintani K, Kusuzaki K, et al. Expression of decorin, a small leucine-rich proteoglycan, as a prognostic factor in soft tissue tumors. J Surg Oncol 2007;96:411-8. [PubMed]
- Troup S, Njue C, Kliewer EV, et al. Reduced expression of the small leucine-rich proteoglycans, lumican, and decorin is associated with poor outcome in node-negative invasive breast cancer. Clin Cancer Res 2003;9:207-14. [PubMed]
- Mefford D, Mefford J. Stromal genes add prognostic information to proliferation and histoclinical markers: a basis for the next generation of breast cancer gene signatures. PLoS One 2012;7:e37646. [PubMed]
- Van Bockstal M, Lambein K, Gevaert O, et al. Stromal architecture and periductal decorin are potential prognostic markers for ipsilateral locoregional recurrence in ductal carcinoma in situ of the breast. Histopathology 2013;63:520-33. [PubMed]
- Cawthorn TR, Moreno JC, Dharsee M, et al. Proteomic analyses reveal high expression of decorin and endoplasmin (HSP90B1) are associated with breast cancer metastasis and decreased survival. PLoS One 2012;7:e30992. [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [PubMed]
- Wells GA, Shea B, O’Connell D. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa Health Research Institute. Available online: www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998;17:2815-34. [PubMed]
- Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [PubMed]
- Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available online: www.cochrane-handbook.org
- Zhang J, Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA 1998;280:1690-1. [PubMed]
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539-58. [PubMed]
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [PubMed]
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088-101. [PubMed]
- Brown LF, Guidi AJ, Schnitt SJ, et al. Vascular stroma formation in carcinoma in situ, invasive carcinoma, and metastatic carcinoma of the breast. Clin Cancer Res 1999;5:1041-56. [PubMed]
- Grigorieva E, Eshchenko T, Rykova VI, et al. Decreased expression of human D-glucuronyl C5-epimerase in breast cancer. Int J Cancer 2008;122:1172-6. [PubMed]
- Ishiba T, Nagahara M, Nakagawa T, et al. Periostin suppression induces decorin secretion leading to reduced breast cancer cell motility and invasion. Sci Rep 2014;4:7069. [PubMed]
- Leygue E, Snell L, Dotzlaw H, et al. Lumican and decorin are differentially expressed in human breast carcinoma. J Pathol 2000;192:313-20. [PubMed]
- Oda G, Sato T, Ishikawa T, et al. Significance of stromal decorin expression during the progression of breast cancer. Oncol Rep 2012;28:2003-8. [PubMed]
- Reed CC, Waterhouse A, Kirby S, et al. Decorin prevents metastatic spreading of breast cancer. Oncogene 2005;24:1104-10. [PubMed]
- Cao H, Shu Z, Wang W, et al. The Expression of Decorin in Breast Cancer. Chin J Oncol Clin 2006;33:1105-08.
- Song Y, Cao H, Zheng D, et al. The expression of decorin mRNA in breast cancer. J Changchun Univ Tradit Chin Med 2007;23:23-4.
- Wang B, Li Y, Huang B, et al. The expression of Decorin, p16 and PCNA in breast cancer and the relationship between them and lymph nodes metastasis. J Dalian Med Univ 2001;23:256-8.
- Fisher LW, Termine JD, Young MF. Deduced protein sequence of bone small proteoglycan I (biglycan) shows homology with proteoglycan II (decorin) and several nonconnective tissue proteins in a variety of species. J Biol Chem 1989;264:4571-6. [PubMed]
- Csordás G, Santra M, Reed CC, et al. Sustained down-regulation of the epidermal growth factor receptor by decorin. A mechanism for controlling tumor growth in vivo. J Biol Chem 2000;275:32879-87. [PubMed]
- Santra M, Eichstetter I, Iozzo RV. An anti-oncogenic role for decorin. Down-regulation of ErbB2 leads to growth suppression and cytodifferentiation of mammary carcinoma cells. J Biol Chem 2000;275:35153-61. [PubMed]
- Zhang Z, Li XJ, Liu Y, et al. Recombinant human decorin inhibits cell proliferation and downregulates TGF-beta1 production in hypertrophic scar fibroblasts. Burns 2007;33:634-41. [PubMed]
- Huijun W, Long C, Zhigang Z, et al. Ex vivo transfer of the decorin gene into rat glomerulus via a mesangial cell vector suppressed extracellular matrix accumulation in experimental glomerulonephritis. Exp Mol Pathol 2005;78:17-24. [PubMed]
- Bi X, Tong C, Dockendorff A, et al. Genetic deficiency of decorin causes intestinal tumor formation through disruption of intestinal cell maturation. Carcinogenesis 2008;29:1435-40. [PubMed]
- Bi X, Pohl NM, Qian Z, et al. Decorin-mediated inhibition of colorectal cancer growth and migration is associated with E-cadherin in vitro and in mice. Carcinogenesis 2012;33:326-30. [PubMed]
- Oka H, Shiozaki H, Kobayashi K, et al. Expression of E-cadherin cell adhesion molecules in human breast cancer tissues and its relationship to metastasis. Cancer Res 1993;53:1696-701. [PubMed]
- Zasadkevich YM, Brilliant AA, Sazonov SV. Role of cadherins in health and in developing breast cancer. Arkh Patol 2015;77:57-64. [PubMed]
- Fiedler LR, Eble JA. Decorin regulates endothelial cell-matrix interactions during angiogenesis. Cell Adh Migr 2009;3:3-6. [PubMed]


