Dacomitinib, an emerging HER-targeted therapy for non-small cell lung cancer
Lung cancer is the leading cause of cancer-related mortality worldwide. As much as 85% of primary lung cancers are non-small cell lung cancers (NSCLC) (1). A major characteristic of NSCLC is aberrant signaling pathways, including those mediated by the human epidermal growth factor receptor (HER) family of receptor tyrosine kinases, HER1 (epidermal growth factor receptor; EGFR), HER2, HER3 and HER4. At least one member of the HER family of receptors are expressed in 90% of solid tumors with 60% of these tumors containing abnormalities in a HER family member contributing to tumor development (2). EGFR overexpression is a common event in NSCLC. Estimates indicate that approximately 10-15% of Caucasian and 20-30% of Asian NSCLC patient tumors contain gain-of-function mutations in the EGFR gene. The most common EGFR mutations include deletions in exon 19 (45-50% of mutations) and the L858R point mutation in exon 21 (40-45% of mutations) (3-5). Presence of these mutations can make the NSCLC cells more dependent on EGFR for growth and more sensitive to EGFR tyrosine kinase inhibitors (TKIs), including gefitinib and erlotinib, the only two currently approved EGFR-targeted therapies for advanced NSCLC. Both agents are orally active, reversible, competitive inhibitors that mimic ATP binding to the kinase domain of EGFR. Despite the effectiveness of these drugs against mutant EGFR, most mutant EGFR-carrying NSCLC patients that initially respond to gefitinib/erlotinib acquire resistance to these inhibitors within 14 months leading to disease progression (6). The primary mechanism for the observed resistance to gefitinib/erlotinib was found to be the presence of a secondary mutation in EGFR (T790M) that is present in approximately 50% of patients with acquired resistance to gefitinib/erlotinib (7,8). This secondary point mutation alters the structure of the EGFR kinase domain with two consequences: (I) decreased binding of gefitinib/erlotinib to EGFR kinase domain, and (II) enhanced affinity of ATP for the EGFR kinase domain. These consequences ultimately mediate resistance to gefitinib/erlotinib in preclinical studies and patients (5,7,9-11).
These discoveries have made it necessary for developing second-generation EGFR TKIs, which led to irreversible TKIs such as dacomitinib, neratinib, and canertinib. Dacomitinib (PF00299804) is a pan-HER family, orally active inhibitor that has activity toward EGFR, HER2, and HER4. Whereas reversible EGFR TKIs compete with ATP in the kinase domain of EGFR, dacomitinib also competes for ATP binding but then covalently binds at the edge of the ATP binding cleft on Cys773 of EGFR via the Michael mechanism (addition of nucleophile to an α, β unsaturated carbonyl) (12). The result of this covalent binding is that the inhibitor irreversibly blocks binding of ATP to the kinase, rendering it inactive (12). Dacomitinib has been shown to be more potent than reversible EGFR inhibitors in cell-based assays (9). Due to its irreversible nature, dacomitinib has a low off-rate when compared to reversible inhibitors and its pharmacodynamics will be determined by its target (EGFR) half-life, meaning it can only be overcome by synthesis of new protein (9,13). Results of several preclinical studies demonstrated that dacomitinib displayed the ability to inhibit several different EGFR mutants, including the commonly found primary EGFR mutants (i.e. exon 19 deletion or L858R mutation) and the secondary EGFR T790M mutant (9,10). It has been shown that dacomitinib was effective at reducing the growth of gefitinib-resistant NSCLC xenografts (9).
Building upon the promising preclinical results, dacomitinib has undergone two Phase I clinical trials in patients with advanced solid tumor types (14,15). In these trials, patients with NSCLC comprised 47% (14) and 69% (15) of the study population with other tumor types comprising primarily breast and colorectal cancers. Maximum tolerated dose in these trials was established at 45 mg once daily and patients had manageable adverse events at this dosage. There was indication of a positive clinical response in both trials as one trial observed 4/110 patients had a partial response while 44/110 patients had stable disease (14). The other observed 1/13 patients had a partial response while 9/13 patients had stable disease ≥6 weeks while 4/8 NSCLC patients showed tumor shrinkage (15). NSCLC patients with EGFR mutations had either a partial response (2/29; 7%) or stable disease (12/29; 41%) in one trial (14) while the other found tumor shrinkage was 85% less in tumors with WT EGFR compared to mutant EGFR (15). These results suggested a potential clinical benefit to NSCLC patients leading to Phase II trials.
The potential clinical benefit of dacomitinib has been further tested in a Phase II clinical trial recently published by Ramalingam et al. in the Journal of Clinical Oncology (16). Patients treated in this trial had advanced NSCLC with disease progression following at least one chemotherapy regimen with no prior EGFR-targeted therapy. Investigators recruited 188 patients that were randomly assigned to either dacomitinib (45 mg once daily) or erlotinib (150 mg once daily) treatment. This is the first clinical trial published to directly compare dacomitinib (irreversible EGFR TKI) to erlotinib (reversible EGFR TKI) in any cancer type. There was a similar prevalence of EGFR mutations (16%) and KRAS mutations (16.4%) between groups. However, there was an imbalance in the amount of EGFR mutant patients receiving dacomitinib (20.2%) versus those receiving erlotinib (11.7%). Results showed a statistically significant difference in progression-free survival (PFS) in patients receiving dacomitinib (2.86 months) compared to erlotinib (1.91 months). The difference in PFS was even greater in patients with the molecular subset of KRAS-WT/EGFR any status (3.71 months for dacomitinib vs. 1.91 months for erlotinib). A significantly greater objective response rate for dacomitinib was also observed (17%) compared to erlotinib (5.3%). The clinical benefit response rate (complete response plus partial response plus stable disease ≥24 weeks) was significantly greater for dacomitinib (29.8%) compared to erlotinib (14.9%). Differences may be related to the observation that dacomitinib had a longer duration of response compared to erlotinib (16.56 versus 9.23 months, respectively).
Results of this Phase II trial are in agreement with dacomitinib performance in two earlier Phase I trials published to date (14,15). These trials observed dacomitinib administration resulted in stable disease for at least six weeks in up to 70% (15) of patients. One Phase I study suggested patients with EGFR mutations appear to respond more favorably to dacomitinib than other patients (15). However, Ramalingam et al. (16) did not find dacomitinib had a greater response in EGFR mutant patients compared to EGFR-WT patients. This is in contrast to erlotinib as previous trials clearly showed enhanced PFS and overall survival (OS) in EGFR-mutant NSCLC patients compared to EGFR-WT patients (Table 1) (16-26). Also, the higher PFS with dacomitinib observed by Ramalingam et al. (16) may have been due to an imbalance of patients with EGFR mutations (20% in dacomitinib group, 12% in erlotinib group). However, PFS was also improved in patients receiving dacomitinib with KRAS-WT/EGFR-WT, suggesting the inequality of EGFR mutants in this population did not entirely drive the differences in overall PFS. Dacomitinib also inhibits HER2 and HER4 in addition to EGFR. The improved PFS with dacomitinib may be due to simultaneous inhibition of all three HER family members.
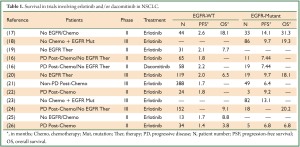
Full table
Adverse events (AEs) commonly produced by dacomitinib administration are manageable and clinical improvements are often observed. Phase I trials to determine safety of dacomitinib in patients primarily found AEs that were gastrointestinal or dermatological (14,15). These events primarily consisted of diarrhea, rash, dermatitis acneiform, and fatigue and were largely grade 1 or 2 in severity. Ramalingam et al. (16) found similar AEs in their study, although AEs were more common in patients receiving dacomitinib compared to erlotinib. Despite AEs being more common in the dacomitinib group, there were clinically meaningful improvements in disease symptoms such as cough, dyspnea, and chest pain among others. Together, these trials suggest dacomitinib does indeed cause mild to moderate AEs but also reduces disease symptoms.
There are several clinical trials ongoing to evaluate the efficacy of dacomitinib in the treatment of NSCLC. Several Phase I trials are ongoing to establish the safety of dacomitinib administration in combination with inhibitors that target mediators of gefitinib/erlotinib resistance (e.g., c-MET, IGF-1R). There are also multiple Phase II/III trials aimed at determining whether dacomitinib is better used as first-line therapy prior to gefitinib/erlotinib and whether it has efficacy in patients refractory to gefitinib/erlotinib. Phase III trials are also ongoing to extend the findings of Ramalingam et al. (16) comparing dacomitinib to erlotinib with the primary outcome of PFS. Results of these trials will have a large impact on whether dacomitinib has significant clinical use and will determine if it will be approved for broad clinical use in NSCLC patients.
These early trials using dacomitinib suggest potential benefits; however, its utility in patients is still unclear. This is suggested by the fact that dacomitinib did not yield significantly enhanced overall survival compared to erlotinib (16). Regardless, the prolonged PFS with dacomitinib over erlotinib in patients with WT EGFR may suggest usefulness in patients with amplified WT EGFR. In addition to genetic status of EGFR, other aspects of the complex EGFR signaling may play a role in NSCLC response to dacomitinib, such as, EGFR subcellular localization (27-29). Lastly, the enhancement in PFS by dacomitinib observed by Ramalingam et al. (16) could be due to inhibition of other HER family members, suggesting these tumors are dependent on not only EGFR but also HER2 and HER4, a topic preclinical studies have yet to fully address. Future preclinical and clinical studies should address these issues.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Ettinger DS, Akerley W, Bepler G, et al. Non-small cell lung cancer. J Natl Compr Canc Netw 2010;8:740-801.
- Woodburn JR. The epidermal growth factor receptor and its inhibition in cancer therapy. Pharmacol Ther 1999;82:241-50.
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129-39.
- Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004;304:1497-500.
- Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A 2004;101:13306-11.
- Nguyen KS, Kobayashi S, Costa DB. Acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancers dependent on the epidermal growth factor receptor pathway. Clin Lung Cancer 2009;10:281-9.
- Kobayashi S, Boggon TJ, Dayaram T, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med 2005;352:786-92.
- Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med 2005;2:e73.
- Engelman JA, Zejnullahu K, Gale CM, et al. PF00299804, an irreversible pan-ERBB inhibitor, is effective in lung cancer models with EGFR and ERBB2 mutations that are resistant to gefitinib. Cancer Res 2007;67:11924-32.
- Gonzales AJ, Hook KE, Althaus IW, et al. Antitumor activity and pharmacokinetic properties of PF-00299804, a second-generation irreversible pan-erbB receptor tyrosine kinase inhibitor. Mol Cancer Ther 2008;7:1880-9.
- Kwak EL, Sordella R, Bell DW, et al. Irreversible inhibitors of the EGF receptor may circumvent acquired resistance to gefitinib. Proc Natl Acad Sci U S A 2005;102:7665-70.
- Garuti L, Roberti M, Bottegoni G. Irreversible protein kinase inhibitors. Curr Med Chem 2011;18:2981-94.
- Singh J, Petter RC, Baillie TA, et al. The resurgence of covalent drugs. Nat Rev Drug Discov 2011;10:307-17.
- Jänne PA, Boss DS, Camidge DR, et al. Phase I dose-escalation study of the pan-HER inhibitor, PF299804, in patients with advanced malignant solid tumors. Clin Cancer Res 2011;17:1131-9.
- Takahashi T, Boku N, Murakami H, et al. Phase I and pharmacokinetic study of dacomitinib (PF-00299804), an oral irreversible, small molecule inhibitor of human epidermal growth factor receptor-1, -2, and -4 tyrosine kinases, in Japanese patients with advanced solid tumors. Invest New Drugs 2012. [Epub ahead of print].
- Ramalingam SS, Blackhall F, Krzakowski M, et al. Randomized Phase II Study of Dacomitinib (PF-00299804), an Irreversible Pan-Human Epidermal Growth Factor Receptor Inhibitor, Versus Erlotinib in Patients With Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2012;30:3337-44.
- Jänne PA, Wang X, Socinski MA, et al. Randomized phase II trial of erlotinib alone or with carboplatin and paclitaxel in patients who were never or light former smokers with advanced lung adenocarcinoma: CALGB 30406 trial. J Clin Oncol 2012;30:2063-9.
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46.
- Kobayashi T, Koizumi T, Agatsuma T, et al. A phase II trial of erlotinib in patients with EGFR wild-type advanced non-small-cell lung cancer. Cancer Chemother Pharmacol 2012;69:1241-6.
- Gridelli C, Ciardiello F, Gallo C, et al. First-Line Erlotinib Followed by Second-Line Cisplatin-Gemcitabine Chemotherapy in Advanced Non-Small-Cell Lung Cancer: The TORCH Randomized Trial. J Clin Oncol 2012;30:3002-11.
- Brugger W, Triller N, Blasinska-Morawiec M, et al. Prospective molecular marker analyses of EGFR and KRAS from a randomized, placebo-controlled study of erlotinib maintenance therapy in advanced non-small-cell lung cancer. J Clin Oncol 2011;29:4113-20.
- Spigel DR, Burris HA 3rd, Greco FA, et al. Randomized, double-blind, placebo-controlled, phase II trial of sorafenib and erlotinib or erlotinib alone in previously treated advanced non-small-cell lung cancer. J Clin Oncol 2011;29:2582-9.
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42.
- Herbst RS, Ansari R, Bustin F, et al. Efficacy of bevacizumab plus erlotinib versus erlotinib alone in advanced non-small-cell lung cancer after failure of standard first-line chemotherapy (BeTa): a double-blind, placebo-controlled, phase 3 trial. Lancet 2011;377:1846-54.
- Lilenbaum R, Axelrod R, Thomas S, et al. Randomized phase II trial of erlotinib or standard chemotherapy in patients with advanced non-small-cell lung cancer and a performance status of 2. J Clin Oncol 2008;26:863-9.
- Felip E, Rojo F, Reck M, et al. A phase II pharmacodynamic study of erlotinib in patients with advanced non-small cell lung cancer previously treated with platinum-based chemotherapy. Clin Cancer Res 2008;14:3867-74.
- Han W, Lo HW. Landscape of EGFR signaling network in human cancers: biology and therapeutic response in relation to receptor subcellular locations. Cancer Lett 2012;318:124-34.
- Wang YN, Hung MC. Nuclear functions and subcellular trafficking mechanisms of the epidermal growth factor receptor family. Cell Biosci 2012;2:13.
- Wheeler DL, Dunn EF, Harari PM. Understanding resistance to EGFR inhibitors-impact on future treatment strategies. Nat Rev Clin Oncol 2010;7:493-507.


