Five factors and three characteristics of coronary in-stent restenosis
In the field of interventional cardiology, the emerging drug-eluting stent (DES) has markedly reduced the number of patients requiring repeat revascularization. Nevertheless, several patient subsets still present with poor clinical and angiographic outcomes after DES implantation (1). It cannot be overlooked that the main reason for a poor clinical outcome after percutaneous coronary intervention (PCI) in comparison with after coronary artery bypass grafting for multivessel disease is still restenosis, even in the DES era (2). Although drug-coated balloon angioplasty is known as a safe and effective remedy for in-stent restenosis (ISR) (3), additional DES implantation provides superior long-term clinical and angiographic outcomes (4). However, additional DES implantation causes multiple layers of stent in the coronary artery (5). A better therapeutic strategy for ISR still needs to be investigated.
Intravascular ultrasound (IVUS) remains the gold standard to elucidate coronary artery disease (6). The utility of IVUS for exploring the mechanism of ISR has already been reported (7,8). Goto et al. used IVUS to identify the difference between the mechanism of ISR in various types of stents: bare metal stent (BMS) and first- and second-generation DES (9). Although reference lumen areas were similar in BMS and first- and second-generation DES, restenotic DES was significantly longer and stent areas were significantly smaller. Stent fracture was seen only in DES, whereas there was no difference between first- and second-generation DES. This paper concluded that restenotic first- and second-generation DES were characterized by less neointimal hyperplasia, smaller stent areas, longer stent lengths, and more stent fractures compared with restenotic BMS (9).
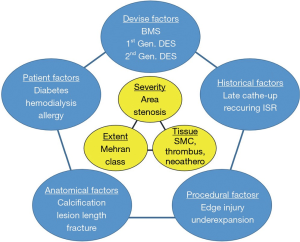
The author of this editorial comment supports the idea that ISR includes three characteristics that are caused by five factors (Figure 1). Goto’s study investigated four of five factors and two of three characteristics (9). This paper explored the impact of fracture on ISR, whereas baseline calcification (10), stent edge injury, and underexpansion, etc., which are visually and quantitatively identifiable on IVUS just after stent implantation, were not investigated. Serial findings should demonstrate the mechanism of ISR more clearly (11). This paper failed to show the difference in ISR tissue characterization. Smooth muscle migration is known as a major factor of ISR (12). However, a previous study showed that thrombus and inflammatory cell infiltration can be observed in DES-ISR tissue (8). Neoatherosclerosis is a known factor of ISR, which appears more than 1 year after DES implantation (13). Optical coherence tomography (OCT) has the potential to reveal more subtle features in restenotic tissue (14-16). Notably, previous investigations using OCT revealed that the neointima of diabetic patients frequently shows microvessels (16,17). The suppression of microvessel proliferation may be a key to reducing ISR in diabetic patients. Additionally, we reported that coexistence of eccentric tissue proliferation and strong signal attenuation detected in OCT images of ISR is related to TLR after PCI for DES-ISR especially in patients undergoing maintenance hemodialysis (18). To reduce further revascularization, a tailor-made strategy may be considered in accordance with the factors and characteristics of the individual ISR lesion.
Acknowledgements
None.
Footnote
Provenance: This is a Guest Editorial commissioned by the Section Editor Yue Liu (Department of Cardiology, the First Affiliated Hospital of Harbin Medical University, Harbin, China).
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Nomura T, Suzuki N, Takamura S, et al. Three-year clinical and angiographic outcomes after everolimus-eluting stent implantation in patients with a history of coronary artery bypass grafting. Int Heart J. [Epub ahead of print].
- Bangalore S, Guo Y, Samadashvili Z, et al. Everolimus-eluting stents or bypass surgery for multivessel coronary disease. N Engl J Med 2015;372:1213-22. [PubMed]
- Kawashima H, Suzuki N, Kyono H, et al. Incidence, predictors and outcomes of immediate decrease in thrombolysis in myocardial infarction flow immediately after paclitaxel-coated balloon angioplasty. Int J Cardiol 2015;191:223-4. [PubMed]
- Alfonso F, Pérez-Vizcayno MJ, Cárdenas A, et al. A Prospective Randomized Trial of Drug-Eluting Balloons Versus Everolimus-Eluting Stents in Patients With In-Stent Restenosis of Drug-Eluting Stents: The RIBS IV Randomized Clinical Trial. J Am Coll Cardiol 2015;66:23-33. [PubMed]
- Khouzam RN, Dahiya R, Schwartz R. A heart with 67 stents. J Am Coll Cardiol 2010;56:1605. [PubMed]
- Suzuki N, Kozuma K. Coronary Imaging Modalities for Forecasting the “Eruption of the Volcano”. Circ J 2015;79:2112-3. [PubMed]
- Suzuki N, Nanda H, Angiolillo DJ, et al. Assessment of potential relationship between wall shear stress and arterial wall response after bare metal stent and sirolimus-eluting stent implantation in patients with diabetes mellitus. Int J Cardiovasc Imaging 2008;24:357-64. [PubMed]
- Suzuki N, Angiolillo DJ, Monteiro C, et al. Variable histological and ultrasonic characteristics of restenosis after drug-eluting stents. Int J Cardiol 2008;130:444-8. [PubMed]
- Goto K, Zhao Z, Matsumura M, et al. Mechanisms and Patterns of Intravascular Ultrasound In-Stent Restenosis Among Bare Metal Stents and First- and Second-Generation Drug-Eluting Stents. Am J Cardiol 2015;116:1351-7. [PubMed]
- Honda Y, Toyama T, Miyaishi Y, et al. Coronary artery calcification as a new predictor of non-target lesion revascularization during the chronic phase after successful percutaneous coronary intervention. Cardiovasc Interv Ther 2014;29:315-23. [PubMed]
- Maeda T, Okamura T, Yamada J, et al. Serial three-dimensional optical coherence tomography assessment of strut coverage and intraluminal structures after drug-eluting stent implantation. Cardiovasc Interv Ther 2014;29:31-9. [PubMed]
- Costa MA, Simon DI. Molecular basis of restenosis and drug-eluting stents. Circulation 2005;111:2257-73. [PubMed]
- Nakazawa G, Otsuka F, Nakano M, et al. The pathology of neoatherosclerosis in human coronary implants bare-metal and drug-eluting stents. J Am Coll Cardiol 2011;57:1314-22. [PubMed]
- Fu Q, Suzuki N, Kozuma K, et al. Quantitative optical coherence tomography analysis for late in-stent restenotic lesions. Int Heart J 2015;56:13-7. [PubMed]
- Kawashima H, Suzuki N, Kyono H, et al. Incidence and distribution of thin-high signals detected by coronary optical coherence tomography in patients treated with paclitaxel-coated balloon angioplasty for in-stent restenosis. Int J Cardiol 2016;202:892-3. [PubMed]
- Suzuki N, Kozuma K, Kyono H, et al. Predominant microvessel proliferation in coronary stent restenotic tissue in patients with diabetes: insights from optical coherence tomography image analysis. Int J Cardiol 2013;168:843-7. [PubMed]
- Gao L, Park SJ, Jang Y, et al. Comparison of Neoatherosclerosis and Neovascularization Between Patients With and Without Diabetes: An Optical Coherence Tomography Study. JACC Cardiovasc Interv 2015;8:1044-52. [PubMed]
- Suzuki N, Kozuma K, Kyono H, et al. The clinical characteristics and prognosis of lesions with in-stent eccentric tissue proliferation and strong signal attenuation detected by optical coherence tomography. Cardiovasc Interv Ther 2015. [Epub ahead of print]. [PubMed]


