Comparison of the lung function change in patients with COPD and bronchial asthma before and after treatment with budesonide/formoterol
Abstract
Objective: This study investigated the rapid onset of bronchodilation effect and compared lung function changes following budesonide/formoterol (Symbicort Turbuhaler®) inhalation in Chinese patients with moderate-severe chronic obstructive pulmonary disease (COPD) and bronchial asthma.
Methods: In this open-label, parallel-group clinical study, patients eligible for study were divided into COPD group (n=62, mean age 68.16±8.75 years) and asthma group (n=30, mean age 45.80±12.35 years). Lung function tests (include FEV1, FVC, FEV1/FVC, and IC) were performed at baseline (t=0 min time point, value before inhalation of budesonide/ formoterol), and then eligible patients received two inhalations of budesonide/formoterol (160/4.5 μg). Lung function tests were reassessed at t=3, 10 and 30 min time point. The primary end-point was lung function change 3 min after drug inhalation, and the secondary end-points were comparison of the gas flow rate (ΔFEV1) and volume responses (ΔFVC, ΔIC) between COPD and asthma patients after inhalation of budesonide/formoterol.
Results: Compared with the baseline, all patients significantly improved their lung function (included FEV1, FVC, FEV1/ FVC, and IC) at 3 min (P<0.05). Greater bronchodilation efficacy was found in the asthma group compared with the COPD group (P<0.05). In the asthmatic patients, the curves of FEV1, FVC, FEV1/FVC, IC, showed improvement with an ascending trend at all time points from 3 to 30 min. Whereas in the COPD patients, only the curves of FEV1, FVC, IC showed similar pattern. We found that ΔFVC was significantly higher than ΔFEV1 in both groups (P<0.05), but no significant difference between ΔIC and ΔFEV1 (P>0.05). Compared with COPD group, asthma group had higher level of ΔFEV1 and ΔIC (P<0.05), but no significant difference for ΔFVC can be found.
Conclusions: Budesonide/formoterol has a fast onset of bronchodilation effect in patients with moderate-severe COPD and asthma. Greater efficacy was found in the asthma group compared with the COPD group. The gas flow rate and volume responses in patients with COPD differ from those with asthma after inhalation of Budesonide/formoterol.
Key words: Budesonide/formoterol; chronic obstructive pulmonary disease; bronchial asthma; lung function test
Chronic obstructive pulmonary disease (COPD) and bronchial asthma (asthma) have important similarities and differences. Both are chronic inflammatory diseases that cause airflow limitation (1,2). However, there are great difference between the two in terms of genetic basis, idiosyncratic reaction, airway hyper-responsiveness, inflammatory mediators and response to the treatment (3). There is neither study about the rapid onset of effect of ICS/LABA combination therapy (budesonide/formoterol) in China nor report about the differences of lung function change before and after treatment between patients with COPD and asthma all over the world.
It is traditionally thought that the responses in bronchodilatation test of asthma and COPD are “gas flow rate responses” and “volume responses” respectively (1). But yet no report about the “gas flow rate responses” and “volume responses” of ICS/LABA such as budesonide/formoterol in patients with COPD and asthma is available.
In this trial, we will determine the rapid onset of effect of budesonide/formoterol in patients with COPD and asthma by testing the lung function in several time points in a short time. In addition, we will discuss the differences of the gas flow rate and volume responses in patients with asthma and COPD after budesonide/formoterol inhalation.
Materials and methods
Patients
Subjects enrolled in this study were outpatients referred to Department of Pulmonary & Critical Care Medicine, the First Affiliated Hospital of Sun Yat-sen University, Guangzhou, P. R. China, from May 2008 to April 2010, diagnosed as bronchial asthma and chronic obstructive pulmonary disease (4,5).
Materials and drugs
Materials: lung functions were measured by computerized automated Hyp’Air equipment (Medisoft, Belgium) in the Room, of Lung Function Test the First Affiliated Hospital of Sun Yat-sen University.
Name of the Medicinal Product: budesonide/formoterol combination therapy, brand name: Symbicort Turbuhaler® (AstraZeneca, Sweden) 160/4.5 μg ×60.
Method for the determination of lung function
The technique of the lung function equipment meets the criteria of American Thoracic Society/European Respiratory Society (6), and the scaler (3.0 L as recommendation) was performed every day after startup to keep the machine working normally (error should ≤3%) and then technicians would adjust the room temperature, room pressure, humidity, atmospheric pressure, body temperature and pressure, saturated (BTPS). SABA, LABA, phylline, hormone, anti-histamine, and anti-leukotriene therapy were all suspended prior to the trial. Lung function tests were performed at baseline (t=0 min time point, value before inhalation of budesonide/formoterol), and then eligible patients received two inhalations of budesonide/formoterol (160/4.5 μg). Lung function was reassessed at t=3, 10 and 30 min time point.
Primary end-points
(I) FEV1, predicted FEV1%, FVC, FEV1/FVC, and IC.
(II) Change of lung function before and after treatment (T30min–Tbaseline): ΔFEV1, ΔFVC, and ΔIC.
Statistical analysis
SPSS 13.0 package was used for statistical analyses. Parameters of lung function were expressed in mean ± standard deviation (x±s) using repeated measures analysis of variance. When the spherical test condition (α=0.1) was unsatisfied, Greenhouse-Geisser adjustment was applied. Paired t test or paired rank sum test method were used for the self-comparison in each time point. Trends in lung function over time were described. T test or rank sum test method was used for the comparison of the changes of lung function with the significance level of P<0.05. Sigmaplot 10.0 software was used for plotting.
Results
Baseline characteristics
A total of 92 patients were divided into asthma group (n=30, mean age 45.80±12.35 years) and COPD group (n=62, mean age 68.16±8.75 years). The patients of asthma group was significantly younger than those in COPD group (P<0.001), and there was no statistically significant difference in height and weight between the two groups (P>0.05) (see Table 1 for detail).

Full table
Change and trend of lung function before and after budesonide/formoterol inhalation
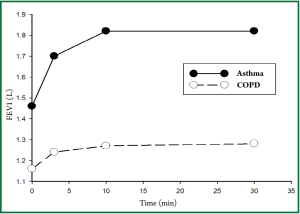
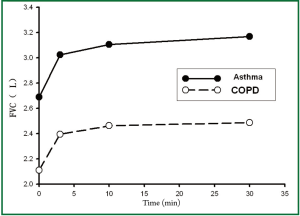
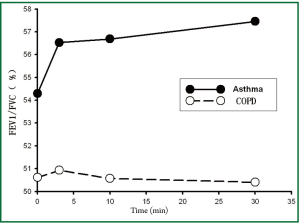
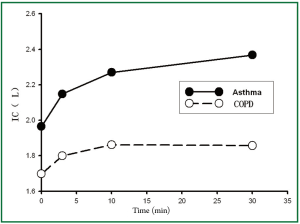
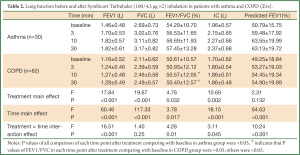
Table 2 showed the differences of lung functions before and after treatments between the two groups. We could see that there were significant differences among the trends in FEV1, predicted % FEV1, FEV1/FVC, and IC after treatment in both groups (all P values of treatment × time interaction effects were <0.05), and there was no significant difference of FVC in both groups (P=0.132). There were significant differences of FEV1, FVC, FEV1/FVC, and IC after treatment overall and in each time point in both group (all P values of main effects of treatment and time were <0.05). Thus, it could be inferred that patients in asthma group could gain greater improvement in lung function in terms of FEV1, FEV1/FVC, and IC after Symbicort Turbuhaler inhalation than those in COPD group.
Full table
According to Table 2 and the Figures 1-4, we could find significant improvement of lung function comparing with baseline in each time point after treatment (P<0.05 for all comparisons) and upward trends in all measurements over time in asthma group. Patients in both groups showed significant improvement 3 min after inhalation (P<0.05 for all comparisons).
Comparison of the change of lung function before and after treatment
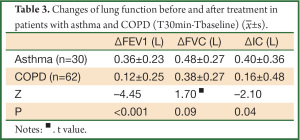
We found greater improvement of ΔFEV1, ΔIC 30 min after treatment in asthma group than COPD group (P<0.05 for all comparisons), however, the difference of ΔFVC was not significant (P=0.09) (Table 3).
Full table
Self-comparison of ΔFEV1 and ΔFVC
Paired test in COPD group showed that ΔFEV1 was less than ΔFVC (Z=–5.503, P<0.001), and the similar result was found in asthma group (t=2.2, P=0.036).
Self-comparison of ΔFEV1 and ΔIC
(I) 0.08L was used as the interval to make a percentage distribution histogram of ΔFEV1, and ΔIC in COPD group. We could see that the proportion of patients with ΔFEV1>0.24L in COPD group was about 22.5%, and those with ΔIC>0.24L was 48.3%, indicating that there were more patients with IC improvement than with FEV1 improvement. However, the paired test show that there was no statistical difference between ΔFEV1 and ΔIC in COPD group (Z=–1.848, P=0.065).
(II) A similar percentage distribution histogram of ΔFEV1, and ΔIC in asthma group was drawn. We could see that the proportion of patients with ΔFEV1>0.3 L in asthma group was about 76.6%, and those with ΔIC>0.3 L was 66% suggesting that there were more patients with FEV1 improvement than with IC improvement, while the paired test show that there was no statistical difference between ΔFEV1 and ΔIC in asthma group (t=0.807, P=0.426).
Discussion
This open-label, parallel-group clinical study confirmed that there was rapid onset of effect significantly improving lung function just 3 min after budesonide/formoterol (Symbicort Turbuhaler®160/4.5 μg ×2) inhalation in patients with moderate to severe COPD and asthma in stable phase, and the treatment effect was more obvious in asthma group than in COPD group.
Lindberg et al. (7) randomly divided 90 patients with moderate to severe COPD in stable phase into 4 groups, giving budesonide/formoterol (160/4.5 μg), salmeterol/fluticasone (25/250 μg), salbutamol 100 μg and placebo, ×2 inhalations to each group as treatment. Results showed that FEV1 improved significantly 5 min after budesonide/formoterol inhalation and this effect was greater than salmeterol/fluticasone and placebo, while comparable with salbutamol. Balanag et al. (8) used a double-blind and double-mimic trial to compare the effects of Symbicort Turbuhaler (overall dose 1,280/36 μg) and salbutamol (overall dose 1,600 μg) and find a comparable and rapid onset of effect.
Our study found that 3 min after budesonide/formoterol (overall dose 320/9 μg) inhalation in patients with moderate to severe COPD and asthma in stable phase, there were obvious improvements of FEV1, FVC, FEV1/FVC and IC compared with baseline. The treatment effect was more obvious in asthma group than COPD group. The advantage in asthma group persisted to the time point of 30 min after treatment which was also the end of the study. The result confirmed that Symbicort Turbuhaler inhalation could render rapid onset of effect in Chinese patient similar with foreign studies. The possible reasons that the effect was greater in asthma group than COPD group may include: (I) Airway stenosis of asthma is mainly caused by spasm of airway smooth muscle, which has good response to bronchodilators and inhaled corticosteroids. (II) Airflow limitation of COPD is mainly due to the structural changes in small airways, mucus hypersecretion, and decreased elastic recoil force caused by pulmonary alveolar destruction. Spasm and contraction of airway smooth muscle cover only a small part of the factors (9) which limit the effect of inhaled bronchodilators. (III) Patients in asthma group were younger with greater baseline lung function. Those in COPD group were older with relatively weaker physical conditions and respiratory muscle strength. It may more difficult to achieve improvement in the latter patients due to the “ceiling effect”.
Our study also found that ΔFVC was more distinct than ΔFEV1 30 min after Symbicort Turbuhaler inhalation, and ΔFEV1 in asthma group was greater than in COPD group. The non-significant difference of ΔFVC between the two groups indicated that the “gas flow rate response” in patients with asthma was greater than patients with COPD, however, the “volume response” using ΔFVC as index was comparable in these two groups.
Most scholars insist that the reaction of COPD to bronchodilator belongs to “volume response” and asthma as “gas flow rate response” (1). In our study, the response of COPD was similar to traditional view. However, the response of asthma was different from the previous studies. The possible reason may be due to the limited sample in our study. And the therapy used in this study consists of both LABA and ICS. Besides, asthma and COPD have many similarities in the response to bronchodilator. In conclusion, there was no wonder to find the result that “volume response” was more obvious than “gas flow rate response” in patients with asthma. Certain study (10) has reported that the “volume response” was the prominent response in patients with severe asthma after bronchodilator inhalation. Patients needed to exhale rapidly and forcibly when FVC was measured and in the forced expiratory process of patients with COPD, gas trapping may exist, resulting in increased residual volume, so FVC may not fully reflect the volume change after treatment. However, the other indices such as inspiratory capacity, IC, show no such shortcomings. Thus, we compared ΔIC and ΔFEV1 and found there were more patients with COPD having greater improvement in IC than FEV1, suggesting more patients with COPD showed “volume response” after Symbicort Turbuhaler inhalation, despite the comparable improvement in mean IC and FEV1 change. In contrast, there were more patients with asthma having greater improvement in FEV1 than IC, indicating that the “volume response” and “gas flow rate response” should be different among patients with asthma and COPD. Furthermore, the improvement of IC in asthma group was greater than in COPD group meaning that the effect of Symbicort Turbuhaler comes off more rapid in asthma group than in COPD group. Chinese researcher Jiang Han (11) stratified patients with COPD and asthma by the baseline lung function to compare the difference of ΔFEV1 and ΔFVC after salbutamol 400 μg inhalation, finding that the “volume response” and “gas flow rate response” of patients with COPD was different obviously than patients with asthma, and the bronchodilation effect was different depends on the baseline lung function. The sample in our study was small, and only moderate to severe patients were selected, thus, stratification analysis was not conducted.
In sum, our study showed that Symbicort Turbuhaler inhalation could rapidly improve the lung function of patients with moderate to severe COPD and asthma in a short time and the improvement was greater in asthma group than in COPD group. There were a bit differences in “gas flow rate response” and “volume response” of patients with COPD and asthma after budesonide/formoterol inhalation.
Acknowledgements
Funding: This work was supported in part by a grant from the Science and Technology Plan Project of Guangdong (No. 2009B03081171); in part by a grant from the 2009 Outstanding Young Teachers Cultivation & Training Program of Sun Yat-sen University.
Disclosure: The authors declare no conflict of interest.
References
- Cui DJ. Strengthening understanding of the differences and similarities between asthma and chronic obstructive pulmonary disease to improve treatment outcomes. Zhonghua Jie He He Hu Xi Za Zhi 2006;29:361-2.
- Buist AS. Similarities and differences between asthma and chronic obstructive pulmonary disease: treatment and early outcomes. Eur Respir J Suppl 2003;39:30s-35s.
- Yao WZ, Sun DJ. eds. Hot topics of chronic obstructive pulmonary disease. Beijing: People’s Medical Publishing House, 2009:47-53,150-66.
- Chinese Respiratory Disease Association. Diagnosis and treatment guideline of chronic obstructive pulmonary disease (revised edition in 2007). Zhonghua Jie He He Hu Xi Za Zhi 2007;30:8-17.
- Chinese Respiratory Disease Association. Guideline of prevention and treatment of bronchial asthma. Chin J Tuberc Respir Dis 2008;31:177-85.
- Miller MR, Crapo R, Hankinson J, et al. General considerations for lung function testing. Eur Respir J 2005;26:153-61.
- Lindberg A, Szalai Z, Pullerits T, et al. Fast onset of effect of budesonide/formoterol versus salmeterol/fluticasone and salbutamol in patients with chronic obstructive pulmonary disease and reversible airway obstruction. Respirology 2007;12:732-9.
- Balanag VM, Yunus F, Yang PC, et al. Efficacy and safety of budesonide/formoterol compared with salbutamol in the treatment of acute asthma. Pulm Pharmacol Ther 2006;19:139-47.
- Yao WZ. Clinical significance of bronchial reversibility test in the diagnosis and treatment of chronic obstructive pulmonary disease. Zhonghua Jie He He Hu Xi Za Zhi 2009;32:243-4.
- Paré PD, Lawson LM, Brooks LA. Patterns of response to inhaled bronchodilators in asthmatics. Am Rev Respir Dis 1983;127:680-5.
- Jiang H, Zhang Q, Lin KX, et al. Differences in gas flow rate and volume responses after inhalation of bronchodilator between patients with COPD and asthma. Med J Chin PLA 2009;8:940-4.