
SARC-F scores can predict health status and daily activity in patients with idiopathic pulmonary fibrosis
Introduction
Idiopathic pulmonary fibrosis (IPF) is a progressive pulmonary disease with a high mortality rate (1,2). With disease progression, patients with IPF experience exercise intolerance, physical inactivity, and impaired health-related quality of life. Patient-reported outcome evaluations, including questionnaires or surveys that assess patients’ perceptions of issues including symptoms or health-related quality of life, facilitate the multifaceted symptomatic management of patients with IPF (3).
The St. George’s Respiratory Questionnaire (SGRQ) is among the most widely used tools for assessing the health status of patients with respiratory diseases, including IPF (4-6). It is comprised of 50 items and can be completed within 10 min (578 s) (7); moreover, the complicated scoring systems impede its clinical use. Contrastingly, the chronic obstructive pulmonary disease assessment test (CAT) is easy to complete and is suitable for clinical use (6,7). It is comprised of only eight items, which are each scored on a scale from 1 to 5. The total score ranges from 0 to 40. Both the CAT and SGRQ scores were correlated with various clinical markers of IPF (7).
Sarcopenia is an age-related syndrome characterized by progressive and generalized loss of skeletal muscle mass and function. It is correlated with physical disability, poor quality of life, and death (8). Up to 32.1% of patients with interstitial lung diseases suffer from sarcopenia (9). The strength, assistance in walking, rising from a chair, climbing stairs, and falls questionnaire (SARC-F) is widely used for screening sarcopenia (8,10). It is comprised of only five items: strength, assistance in walking, rising from a chair, climbing stairs, and falls. The total score ranges from 0 to 10, with each item being scored from 0 to 2 points.
It remains unclear whether the SARC-F score can predict the quality of life and daily activity in patients with IPF. We aimed to confirm the association of SARC-F scores with the measurements of quality of life and activity. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-813/rc).
Methods
Patients
This single-center, cross-sectional pilot study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the ethics review board of Nagoya City University Hospital (No. 60-20-0190). Written informed consent was obtained from all participants.
IPF was diagnosed through multidisciplinary discussions based on the 2018 international guidelines (11). Between April 2021 and March 2022, outpatients with stable IPF were screened at our hospital. The inclusion criteria included having provided written informed consent and the ability to perform a 6-minute walk test (6MWT) for assessing the exercise capacity in patients with IPF. The exclusion criteria were as follows: long-term oxygen administration at rest, active cancer, and inability to comprehend the questionnaire. To clarify the clinical impact of SARC-F scores in patients with IPF, we excluded patients who required supplemental oxygen therapy at rest given their difficulty to perform 6MWT or pulmonary function tests.
Patients with acute exacerbations of IPF or pneumonia underwent pulmonary rehabilitation during hospitalization. Outpatients did not undergo any rehabilitation.
Pulmonary function tests and 6 MWT
All patients underwent pulmonary function tests using spirometry (CHESTAC-8900; Chest, Tokyo, Japan) based on the American Thoracic Society/European Respiratory Society criteria (12). Further, the diffusion capacity of carbon monoxide (DLCO) was measured (CHESTAC-8900). The percentage of predicted forced vital capacity (%FVC), percentage of predicted forced expiratory volume in 1.0 s (FEV1), and percentage of predicted DLCO (%DLCO) were calculated based on the patient’s height, age, and sex, according to standardized methods (13). The 6MWTs were performed without supplemental oxygen following the American Thoracic Society guidelines (14). The 6MWT recording was supported by pulse oximetry using dedicated software (Anypal walk, ATP-W03, Fukuda Denshi Co., Ltd, Tokyo, Japan).
Daily step count
The patients’ daily step count was assessed using a tri-axis accelerometer (FB-732-BK; Tanita, Japan). This device is water resistant, lightweight (30 g), and small (74×165×20 mm3), with a data storage capacity of 14 days. The participants were instructed to keep the device in the pocket of their shirts/pants or hang it around the neck using a strap for seven consecutive days, except while bathing and sleeping. The median value of the daily step counts for seven consecutive days was used for analysis.
Diagnosis of sarcopenia
Sarcopenia was defined based on the algorithm and criteria of the Asian Working Group for Sarcopenia 2019 (8). Accordingly, sarcopenia was diagnosed if the patient had low muscle mass, low muscle strength, and/or low physical performance, with the presence of all three indicating severe sarcopenia. The appendicular skeletal mass index (height squared-adjusted, kg/m2) was calculated using a multifrequency bioelectrical impedance analyzer (InBody 720; InBody Japan, Tokyo, Japan). The cut-off criterion for low muscle mass was <7.0 kg/m2 for men and <5.7 kg/m2 for women. Handgrip strength measurement is the recommended method for detecting low muscle strength. Handgrip strength was measured in the standing position with full elbow extension using an electronic dynamometer (HG-251; N-Force, Wakayama, Japan). Measurements were performed twice for each hand, with the largest grip strength value being used for analysis. The cut-off criterion for low muscle strength was defined as <28.0 kg for men and <18.0 kg for women. Physical performance was evaluated using the usual gait speed, which was calculated by measuring the time taken to walk down a 10-m corridor at the usual speed. The cut-off criterion for low physical performance was defined as <1.0 m/s for both sexes.
Clinical staging of IPF
The clinical staging of IPF was determined using the gender, age, and physiology (GAP) staging system (15).
Questionnaires
Symptoms and health-related quality of life were evaluated using the CAT (6,7) and SGRQ (4). The CAT comprises eight items related to respiratory disorder symptoms and their impact. Patients are asked to respond to all items using an identical 0–5 response scale. The total score ranges from 0 to 40, with a score of 0 indicating no impairment. The SGRQ is a specific questionnaire for respiratory diseases that contains three domains, which are all scored from 0 to 100. The score is positively correlated with the impairment of health-related quality of life. Psychological evaluation was performed using the Hospital Anxiety and Depression Scales (HADS) (16). The HADS is composed of 14 items; among them, seven comprise the anxiety subscale while the remaining seven comprise the depression subscale. Each item is rated on a 0–3 scale. Accordingly, the total subscale score ranges from 0 (no distress) to 21 (maximum distress), with higher scores indicating more severe distress. The SARC-F was used for analysis (8-10). The original SARC-F comprises five items, which are scored as follows: (I) Strength: How much difficulty do you have in lifting and carrying 10 pounds? None: 0, Some: 1, A lot or unable: 2. (II) Assistance in walking: How much difficulty do you have when walking across a room? None: 0, Some: 1, A lot, use aids, or unable: 2. (III) Rise from a chair: How much difficulty do you have transferring from a chair or bed? None: 0, Some: 1, A lot or unable without help 2. (IV) Climb stairs: How much difficulty do you have climbing a flight of 10 stairs? None: 0, Some: 1, A lot or unable: 2. (V) Falls: How many times have you fallen in the past year? None: 0, <3 falls: 1, ≥4 falls: 2. The SARC-F scores range from 0 (best) to 10 (worst). Patients with a total score ≥4 were considered to be at risk of sarcopenia (8).
Statistical analyses
Continuous variables were tested for normality using the Shapiro-Wilk test, with normally and non-normally distributed values being presented as mean ± standard deviation and median (interquartile range), respectively. The relationship of the clinical parameters with the SARC-F and CAT scores was evaluated using Spearman’s rank correlation coefficients. Differences between patients with and without sarcopenia were analyzed using the Mann-Whitney U test. Comparisons of the SARC-F score, CAT score, and daily step count among the robust group, non-severe sarcopenia group, and severe sarcopenia group were analyzed using an analysis of variance test. Receiver operating characteristic (ROC) curve analyses were performed to analyze the predictive utility of SARC-F and CAT scores for sarcopenia, respectively. Linear regression analyses were performed to investigate the predictive utility of the questionnaires and clinical parameters for daily step count. The correlations of distance walked during the 6MWT and daily step count with the SARC-F score were evaluated using Spearman’s rank correlation coefficients. Statistical significance was set at P<0.05. Statistical analyses were performed using SPSS version 28 (IBM Corp., Armonk, NY, USA).
Results
Patient characteristics
Among outpatients with stable IPF who were screened at our hospital, 69 patients were identified. Among them, we excluded four patients who required long-term oxygen therapy at rest; one and three patients unable to perform the 6MWT due to unstable angina and wheelchair use, respectively; five patients who declined to participate in this study, and two patients who did not submit records of their daily step counts. Finally, we enrolled 54 outpatients with IPF (age, 73.6±7.9 years; %FVC, 80.4%±15.6%). Table 1 presents the patient characteristics. Sarcopenia was diagnosed in 21 (38.9%) patients, with 7 (13.0%) patients being diagnosed with severe sarcopenia. Figure S1 shows the relationships among low muscle mass, low handgrip strength, and low gait speed.
Table 1
| Characteristics | Value |
|---|---|
| Age, years | 73.6±7.9 |
| Sex, female | 6 (11.1) |
| Body mass index, kg/m2 | 22.2±2.95 |
| Smoking history | |
| Never smoker | 9 (16.7) |
| Ex-smoker | 44(84.4) |
| Current smoker | 1 (1.9) |
| Pack-years | 30 [13–46] |
| Histological diagnosis | 18 (33.3) |
| GAP index | 3 [3–4] |
| GAP stage (I/II/III) | 32 (59.3)/21 (38.9)/1 (1.9) |
| FVC, L | 2.61±0.72 |
| FVC, % predicted | 80.4±15.6 |
| FEV1, L | 2.11±0.56 |
| FEV1, % predicted | 82.0±14.4 |
| FEV1/FVC, % | 81.9±8.98 |
| DLCO, % predicted | 66.7±18.6 |
| PaO2, mmHg | 87.3 [78.7–96.2] |
| Handgrip strength, kg | 31.5±9.9 |
| Low handgrip strength | 16 (29.6) |
| Usual gait speed, m/s | 1.08±0.26 |
| Low gait speed | 17 (31.5) |
| 6MWT, m | 410±88 |
| Lowest SpO2, % | 89 [85–92] |
| Skeletal muscle index, kg/m2 | 6.76±0.92 |
| Low muscle mass | 27 (50.0) |
| SGRQ score | |
| Symptom | 38.3 [19.4–67.6] |
| Activity | 39.3 [18.5–66.2] |
| Impact | 7.6 [7.9–37.7] |
| Total | 28.8 [14.4–46.9] |
| SARC-F score | 2 [1–3.25] |
| CAT score | 13 [7–22] |
| HADS score | |
| Anxiety | 4 [1–7] |
| Depression | 5.5 [3–8.25] |
| Comorbidity | |
| Hypertension | 27 (50.0) |
| Hyperlipidemia | 20 (37.0) |
| Diabetes | 11 (20.4) |
| Heart disease | 6 (11.1) |
| Stroke | 3 (5.5) |
| Sarcopenia | 21 (38.9) |
| Severe sarcopenia | 7 (13.0) |
| Daily step count, steps | 4,258 [2,190–6,917] |
Data are presented as mean ± standard deviation, median [interquartile range], or number (%). IPF, idiopathic pulmonary fibrosis; GAP, gender, age, and physiology; FVC, forced vital capacity; FEV1, forced expiratory volume in 1.0 s; DLCO, diffuse capacity of the lung for carbon monoxide; PaO2, partial pressure of oxygen; 6MWT, 6-minute walk test; SpO2, oxygen saturation by pulse oximetry; SGRQ, St. George’s Respiratory Questionnaire; SARC-F, strength, assistance in walking, rising from a chair, climbing stairs, and falls questionnaire; CAT, chronic obstructive pulmonary disease assessment test; HADS, Hospital Anxiety and Depression Scale.
Relationships of clinical parameters with the SARC-F and CAT scores
Table 2 shows the relationships of the clinical parameters with the SARC-F and CAT scores. The SARC-F score was moderately correlated with the pulmonary function tests, (P<0.001), partial pressure of oxygen at rest (P<0.001), and CAT scores (P<0.001); strongly correlated with the SGRQ score; and weakly correlated with the HADS score (P<0.05). Similar to the SARC-F score, the CAT scores were strongly and weakly correlated with the SGRQ (P<0.001) and HADS scores (P<0.05), respectively; however, they showed a weaker correlation with pulmonary function tests and partial pressure of oxygen at rest.
Table 2
| Parameters | SARC-F score | CAT score | |||||
|---|---|---|---|---|---|---|---|
| r | 95% CI | P | r | 95% CI | P | ||
| Age | 0.19 | −0.10 to 0.44 | 0.180 | 0.13 | −0.16 to 0.39 | 0.367 | |
| Pulmonary functions | |||||||
| FVC, % predicted | −0.51 | −0.68 to −0.26 | <0.001 | −0.29 | −0.53 to −0.02 | 0.032 | |
| FEV1, % predicted | −0.40 | −0.61 to −0.14 | <0.01 | −0.19 | −0.44 to 0.09 | 0.178 | |
| FEV1/FVC, % | 0.24 | −0.03 to 0.49 | 0.076 | 0.18 | −0.10 to 0.43 | 0.196 | |
| DLCO, % predicted | −0.49 | −0.68 to −0.25 | <0.001 | −0.26 | −0.50 to 0.02 | 0.059 | |
| PaO2 | −0.50 | −0.39 to −0.27 | <0.001 | −0.34 | −0.57 to −0.08 | 0.011 | |
| SGRQ score | |||||||
| Symptom | 0.52 | 0.29 to 0.69 | <0.001 | 0.83 | 0.73 to 0.90 | <0.001 | |
| Activity | 0.80 | 0.68 to 0.86 | <0.001 | 0.67 | 0.49 to 0.80 | <0.001 | |
| Impact | 0.67 | 0.48 to 0.79 | <0.001 | 0.75 | 0.59 to 0.85 | <0.001 | |
| Total | 0.77 | 0.63 to 0.86 | <0.001 | 0.83 | 0.72 to 0.90 | <0.001 | |
| HADS score | |||||||
| Anxiety | 0.31 | 0.03 to 0.54 | 0.025 | 0.32 | 0.05 to 0.55 | 0.020 | |
| Depression | 0.28 | 0.01 to 0.51 | 0.041 | 0.29 | 0.02 to 0.53 | 0.032 | |
| SARC-F score | 0.57 | 0.35 to 0.73 | <0.001 | ||||
| CAT score | 0.57 | 0.35 to 0.73 | <0.001 | ||||
SARC-F, strength, assistance in walking, rising from a chair, climbing stairs, and falls questionnaire; CAT, chronic obstructive pulmonary disease assessment test; CI, confidence interval; FVC, forced vital capacity; FEV1, forced expiratory volume in 1.0 s; DLCO, diffuse capacity of the lung for carbon monoxide; PaO2, partial pressure of oxygen; SGRQ, St. George’s Respiratory Questionnaire; HADS, Hospital Anxiety and Depression Scale.
Comparisons of the SARC-F and CAT scores between patients with and without sarcopenia
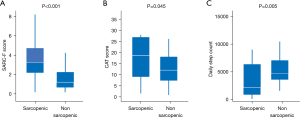
Figure 1 shows the comparisons of the SARC-F and CAT scores between patients with and without sarcopenia. The median SARC-F scores in the sarcopenia and non-sarcopenia groups were 3 [2–4] and 1 [1–2], respectively. Additionally, the median CAT scores in the sarcopenia and non-sarcopenia groups were 18 [9–26] and 11 [7–17], respectively. There were significant between-group differences in the SARC-F (P<0.001) (Figure 1A) and CAT scores (P=0.045) (Figure 1B).
We performed ROC curve analyses of sarcopenia according to the SARC-F and CAT scores. The areas under the curve (AUC) values of the SARC-F and CAT scores were 0.77 [95% confidence interval (CI), 0.63–0.92, P=0.001] and 0.66 (95% CI, 0.50–0.63, P=0.62), respectively.
Figure S2A,S2B shows the comparisons of the SARC-F and CAT scores among the robust, non-severe sarcopenia, and severe sarcopenia groups. There were significant among-group differences in the SARC-F scores (P<0.001) but not in the CAT scores (P=0.106).
Comparisons of the daily step count between patients with and without sarcopenia
There were significant between-group differences in the daily step counts [sarcopenia group, 2,138 (986–5,490); non-sarcopenic group, 4,652 (3,626–6,880); P=0.005; Figure 1C].
Figure S2C shows the comparisons of the daily step count among the robust, non-severe sarcopenia, and severe sarcopenia groups. There was no significant among-group difference in the daily step count (P=0.124).
Linear regression analyses of the daily step count
Table 3 shows the results of the linear regression analyses for the daily step counts. Distance walked in the 6MWT (standardized β=0.33, P=0.011) and the SARC-F score (standardized β=−0.39, P=0.005), but not the CAT or SQRQ scores, were significant predictors for daily step count.
Table 3
| Parameters | B | 95% CI | Standardized β | P |
|---|---|---|---|---|
| Age | −91.0 | −228 to 46.4 | −0.18 | 0.190 |
| Sex | 315.4 | −3,186 to 3,816 | 0.03 | 0.857 |
| FVC, % predicted | 35.3 | −35.1 to 106.0 | 0.14 | 0.319 |
| DLCO, % predicted | 37.6 | −21.3 to 96.4 | 0.18 | 0.206 |
| 6MWT, distance | 15.5 | 3.67 to 27.41 | 0.33 | 0.011 |
| 6MWT, lowest SpO2 | 167 | −3.1 to 337.4 | 0.27 | 0.054 |
| SARC-F score | −797 | −1,342 to −252 | −0.39 | 0.005 |
| CAT score | −103 | −225 to 20 | −0.23 | 0.099 |
| SGRQ score | −35.9 | −75.5 to −3.6 | −0.34 | 0.074 |
| HADS anxiety score | 29.5 | −256 to 316 | 0.03 | 0.837 |
| HADS depression score | −33.5 | −350 to 284 | −0.03 | 0.833 |
CI, confidence interval; FVC, forced vital capacity; DLCO, diffuse capacity of the lung for carbon monoxide; 6MWT, 6-minute walk test; SpO2, oxygen saturation by pulse oximetry; SARC-F, strength, assistance in walking, rising from a chair, climbing stairs, and falls questionnaire; CAT, chronic obstructive pulmonary disease assessment test; SGRQ, St. George’s Respiratory Questionnaire; HADS, Hospital Anxiety and Depression Scale.
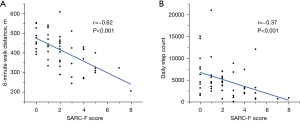
Correlations of the 6-minute walk distance and daily step count with the SARC-F score
Figure 2 shows the correlations of the distance walked during the 6MWT and the daily step count with the SARC-F score. The SARC-F score was significantly correlated with the distance walked during the 6MWT (r=−0.62, P<0.001) and daily step count (r=−0.37, P<0.001).
Discussion
To our knowledge, this is the first study to show that the SARC-F scores were correlated with measures of quality of life and daily activity in outpatients with IPF. The SARC-F score was correlated with pulmonary function tests, partial pressure of oxygen at rest, SGRQ scores, HADS scores, CAT score, and daily step count. Notably, the SARC-F score, but not the CAT or SQRQ score, was a predictor for daily step count in this cohort.
We observed good correlations of the SARC-F scores with the SGRQ activity score (r=0.80, P<0.001), and CAT score and SGRQ symptom score (r=0.83, P<0.001). Compared with the CAT score, the SARC-F score showed a stronger correlation with pulmonary function.
In patients with chronic obstructive pulmonary disease (COPD), the CAT score is associated with quality of life and mortality (17,18), which has been similarly reported in patients with IPF (19). The SARC-F is widely used to screen for sarcopenia (8-10). The SARC-F score has been associated with poor survival in patients with cancer receiving palliative care (20). The SARC-F score could be correlated with poor survival in patients with IPF; however, further research is warranted to confirm this hypothesis.
A meta-analysis showed that the daily step count was negatively associated with the risk of all-cause mortality, up to a level that varied with age (21). The daily step count is considered useful for assessing daily activity in COPD (22). Regarding IPF, daily activity has been associated with mortality (23). The cutoff value for predicting 1-year survival has been reported to be 3,473 steps per day (24).
Regarding sarcopenia, we observed a between-group difference in the SARC-F (P<0.001), CAT scores (P=0.045), and daily step count (P=0.005), which is consistent with previous reports that sarcopenia is associated with lower daily step count (25). A study on daily step counts continuously collected for 5 years reported a substantially lower risk of developing sarcopenia in older people taking at least 7,000–8,000 steps per day or exercising for at least 15–20 min per day at an intensity >3 metabolic equivalents (26). Both resistance and muscle strength training are effective interventions for preventing and treating sarcopenia (27).
As shown in Figure S2, there were significant differences in the SARC-F score (P<0.001), but not the CAT score (P=0.106) and daily step count (P=0.124), among the robust, non-severe sarcopenia, and severe sarcopenia groups. This could be attributed to our small sample size. The utility of distinguishing between non-severe and severe sarcopenia in patients with IPF remains unclear in clinical practice.
Similar to a previous report on patients with cancer (28), we observed a moderate AUC value (0.77) of the SARC-F score for detecting sarcopenia in patients with IPF. Given our small sample size, we could not calculate the cut-off SARC-F score for detecting sarcopenia or compare the AUC values of the SARC-F and CAT scores for sarcopenia.
This study has several limitations. First, this was a small-scale single-center study on patients of a single nationality. Only one patient with IPF had advanced disease (GAP stage III). Moreover, we could not examine a validation cohort. Therefore, large-scale multi-center studies on other nationalities or ethnic groups are warranted to examine the utility of the SARC-F. Second, we did not analyze seasonal changes in the daily step counts. Third, this was a cross-sectional study. A longitudinal survey is required to clarify the clinical significance of changes in the SARC-F score and its association with mortality. Therefore, this study can be considered a preliminary report. Fourth, we excluded patients on long-term oxygen treatment at rest. Therefore, our findings may not be representative of the overall population of patients with IPF.
In conclusion, SARC-F scores were associated with health status and daily activity in patients with IPF. Further studies are warranted to validate the utility of the SARC-F in patients with IPF.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Funding: This research was supported by grants from Fukuda Life Tech Chubu (Japan), and Nippon Boehringer Ingelheim Co., Ltd (Japan) to HO.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-813/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-813/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-813/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-813/coif). ANi serves as an unpaid editorial board member of Journal of Thoracic Disease. HO received research grants from Fukuda Life Tech Chubu, and Nippon Boehringer Ingelheim Co., Ltd, regarding the submitted work. The authors have the following competing interests outside the submitted work. HO received honoraria for lectures from Nippon Boehringer Ingelheim Co., Ltd. Kensuke Fukumitsu received research grants from Novartis Pharma and GSK. SF received honoraria for lectures from AstraZeneca and Eli Lilly. YK received research grants from Novartis Pharma, MSD, Sanofi, and Kyowa- Kirin Corporation and honoraria for lectures from GSK, Novartis Pharma, AstraZeneca, Sanofi, and Kyorin. TU received honoraria for lectures from AstraZeneca and Eli Lilly. KM received research support from Nippon Boehringer Ingelheim Co., Ltd., and honoraria for lectures from Pfizer and Chugai Pharmaceutical. TO reports honoraria for lectures from AstraZeneca, Eli Lilly Japan, Taiho Pharmaceutical, Pfizer, Chugai Pharmaceutical, MSD, Daiichi Sankyo, and Asahi Kasei Pharma, as well as research grants from Kyowa Hakko Kirin, Nippon Boehringer Ingelheim Co., Ltd., Ono Pharmaceutical, and Novartis. ANi reports honoraria for lectures from Astellas, AstraZeneca, Kyorin, GSK, MSD, Shionogi, Bayer, Sanofi, Taiho, and Nippon Boehringer Ingelheim Co., Ltd., and research grants from Astellas, Kyorin, Nippon Boehringer Ingelheim Co., Ltd., Novartis, MSD, Daiichi Sankyo, Taiho, Teijin, Ono, Takeda, and Sanofi Pharmaceutical. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the ethics review board of Nagoya City University Hospital (approval No. 60-20-0190). Written informed consent was obtained from all participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183:788-824. [Crossref] [PubMed]
- Lederer DJ, Martinez FJ. Idiopathic Pulmonary Fibrosis. N Engl J Med 2018;378:1811-23. [Crossref] [PubMed]
- Kalluri M, Luppi F, Ferrara G. What Patients With Idiopathic Pulmonary Fibrosis and Caregivers Want: Filling the Gaps With Patient Reported Outcomes and Experience Measures. Am J Med 2020;133:281-9. [Crossref] [PubMed]
- Jones PW, Quirk FH, Baveystock CM, et al. A self-complete measure of health status for chronic airflow limitation. The St. George's Respiratory Questionnaire. Am Rev Respir Dis 1992;145:1321-7. [Crossref] [PubMed]
- Swigris JJ, Esser D, Conoscenti CS, et al. The psychometric properties of the St George's Respiratory Questionnaire (SGRQ) in patients with idiopathic pulmonary fibrosis: a literature review. Health Qual Life Outcomes 2014;12:124. [Crossref] [PubMed]
- Ringbaek T, Martinez G, Lange P. A comparison of the assessment of quality of life with CAT, CCQ, and SGRQ in COPD patients participating in pulmonary rehabilitation. COPD 2012;9:12-5. [Crossref] [PubMed]
- Matsuda T, Taniguchi H, Ando M, et al. COPD Assessment Test for measurement of health status in patients with idiopathic pulmonary fibrosis: A cross-sectional study. Respirology 2017;22:721-7. [Crossref] [PubMed]
- Chen LK, Woo J, Assantachai P, et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J Am Med Dir Assoc 2020;21:300-307.e2. [Crossref] [PubMed]
- Hanada M, Sakamoto N, Ishimoto H, et al. A comparative study of the sarcopenia screening in older patients with interstitial lung disease. BMC Pulm Med 2022;22:45. [Crossref] [PubMed]
- Malmstrom TK, Morley JE. SARC-F: a simple questionnaire to rapidly diagnose sarcopenia. J Am Med Dir Assoc 2013;14:531-2. [Crossref] [PubMed]
- Raghu G, Remy-Jardin M, Myers JL, et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med 2018;198:e44-68. [Crossref] [PubMed]
- Laszlo G. Standardisation of lung function testing: helpful guidance from the ATS/ERS Task Force. Thorax 2006;61:744-6. [Crossref] [PubMed]
- Kubota M, Kobayashi H, Quanjer PH, et al. Reference values for spirometry, including vital capacity, in Japanese adults calculated with the LMS method and compared with previous values. Respir Investig 2014;52:242-50. [Crossref] [PubMed]
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002;166:111-7. [Crossref] [PubMed]
- Ley B, Ryerson CJ, Vittinghoff E, et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann Intern Med 2012;156:684-91. [Crossref] [PubMed]
- Matsudaira T, Igarashi H, Kikuchi H, et al. Factor structure of the Hospital Anxiety and Depression Scale in Japanese psychiatric outpatient and student populations. Health Qual Life Outcomes 2009;7:42. [Crossref] [PubMed]
- Karloh M, Fleig Mayer A, Maurici R, et al. The COPD Assessment Test: What Do We Know So Far?: A Systematic Review and Meta-Analysis About Clinical Outcomes Prediction and Classification of Patients Into GOLD Stages. Chest 2016;149:413-25. [Crossref] [PubMed]
- Casanova C, Marin JM, Martinez-Gonzalez C, et al. Differential Effect of Modified Medical Research Council Dyspnea, COPD Assessment Test, and Clinical COPD Questionnaire for Symptoms Evaluation Within the New GOLD Staging and Mortality in COPD. Chest 2015;148:159-68. [Crossref] [PubMed]
- Matsuda T, Kondoh Y, Furukawa T, et al. The prognostic value of the COPD Assessment Test in fibrotic interstitial lung disease. Respir Investig 2022;60:99-107. [Crossref] [PubMed]
- Mori N, Maeda K, Fukami Y, et al. High SARC-F score predicts poor survival of patients with cancer receiving palliative care. Support Care Cancer 2022;30:4065-72. [Crossref] [PubMed]
- Paluch AE, Bajpai S, Bassett DR, et al. Daily steps and all-cause mortality: a meta-analysis of 15 international cohorts. Lancet Public Health 2022;7:e219-28. [Crossref] [PubMed]
- Nakanishi M, Minakata Y, Tanaka R, et al. Simple standard equation for daily step count in Japanese patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2019;14:1967-77. [Crossref] [PubMed]
- Nishiyama O, Yamazaki R, Sano H, et al. Physical activity in daily life in patients with idiopathic pulmonary fibrosis. Respir Investig 2018;56:57-63. [Crossref] [PubMed]
- Shingai K, Matsuda T, Kondoh Y, et al. Cutoff Points for Step Count to Predict 1-year All-Cause Mortality in Patients with Idiopathic Pulmonary Fibrosis. Respiration 2021;100:1151-7. [Crossref] [PubMed]
- Park H, Park S, Shephard RJ, et al. Yearlong physical activity and sarcopenia in older adults: the Nakanojo Study. Eur J Appl Physiol 2010;109:953-61. [Crossref] [PubMed]
- Shephard RJ, Park H, Park S, et al. Objectively measured physical activity and progressive loss of lean tissue in older Japanese adults: longitudinal data from the Nakanojo study. J Am Geriatr Soc 2013;61:1887-93. [Crossref] [PubMed]
- Dhillon RJ, Hasni S. Pathogenesis and Management of Sarcopenia. Clin Geriatr Med 2017;33:17-26. [Crossref] [PubMed]
- Fu X, Tian Z, Thapa S, et al. Comparing SARC-F with SARC-CalF for screening sarcopenia in advanced cancer patients. Clin Nutr 2020;39:3337-45. [Crossref] [PubMed]


