
Diagnostic value and safety of endobronchial ultrasonography with a guide sheath transbronchial biopsy for diagnosing peripheral pulmonary lesions in patients with interstitial lung disease
Introduction
Low-dose computed tomography (LDCT) has been reported to facilitate the early detection of lung cancer and contribute to a reduction in the mortality rate (1). Therefore, it is gradually being adopted in clinical practice. Preclinical or asymptomatic interstitial lung disease (ILD) is sometimes detected by LDCT. ILD was reported to be highly associated with lung cancer (2-4).
All peripheral pulmonary lesions (PPLs) encountered in patients with ILD were not always malignant lesions (5). Therefore, these lesions were required for pathological diagnosis of whether the lesions were malignant or not. Furthermore, in determining the treatment policy in patients with lung cancer co-existing with idiopathic pulmonary fibrosis (IPF), anti-cancer therapies including radiotherapy, chemotherapy and surgical therapy have the possibility of causing acute exacerbation of ILD and/or fatal complications (6,7). Therefore, accurate diagnosis is more important to present treatment plan to patients with ILD. PPLs, including peripheral lung cancer, can be diagnosed based on bronchoscopy, transthoracic needle biopsy (TTNB) and surgical lung biopsy (SLB). According to previous reports on the diagnostic yield and complications, bronchoscopy can be an initial option for diagnosing PPLs (7,8). For the diagnosis of PPLs via bronchoscopy, radial endobronchial ultrasonography with a guide sheath transbronchial biopsy (EBUS-GS TBB) has improved the diagnostic yield of PPLs, including small PPLs (8-13).
Several studies have reported that the factors affecting the diagnostic yield based on EBUS-GS TBB were the probe position relative to the lesion, the bronchus sign, (represents a bronchus directly leading to the lesion) on computed tomography (CT), the lesion size, and its segment (14,15). Few reports have assessed the diagnostic yield of EBUS-GS TBB in lungs with a background pathology. In particular, regarding the presence of pulmonary emphysema influencing the diagnostic yield of EBUS-GS TBB, although the diagnostic yield varied depending on the severity of emphysema, the presence of emphysema itself has not been reported to reduce the yield. Furthermore, the presence of usual interstitial pneumonia (UIP) pattern on CT in patients with IPF considerably affected the EBUS-GS TBB results (16,17). Additionally, a previous study reported that the diagnostic yield of EBUS-GS TBB for PPLs in patients with ILD was reported to be lower, about 60%, as compared with the diagnostic yield of about 70% as described on the previous meta-analysis report, which might be related to the small sample size (15,18). Thus, there has been limited data assessing the association of patients with ILD regarding the diagnostic yield and complications of EBUS-GS TBB. In patients with IPF, honeycomb structures have been reported to show a patchy combination of hyper- and hypoechoic patterns on radial endobronchial ultrasonography in autopsied lungs. Therefore, these changes might prevent the recognition of lesions by radial endobronchial ultrasonography (19).
We hypothesised that the diagnostic yield of EBUS-GS TBB for PPLs in patients with ILD might be lower than that in patients without ILD. Hence, we evaluated the utility and safety of EBUS-GS TBB for PPLs in patients with ILD in the background lung, along with the factors affecting the diagnostic yield of EBUS-GS TBB. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-809/rc).
Methods
Patient enrolment
We performed a retrospective analysis of consecutive patients who underwent EBUS-GS TBB for PPLs at Nagoya University Hospital from April 1, 2011, to March 31, 2020. The PPLs were defined as lesions surrounded by normal lung parenchyma or interstitial lung area and were not visible on bronchoscopy (20). We excluded patients with endobronchial lesions and only pulmonary emphysema without ILD. A pneumonologist (T.I.) and an experienced radiologist (S.I.) identified ILD based on high-resolution CT (HRCT) images before performing bronchoscopy, as described below. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Nagoya University Hospital Institutional Review Board (No. 2021-0272). The requirement for patient consent was waived because of the retrospective nature of the study.
Bronchoscopy procedure
Spirometry was performed a day prior to the bronchoscopy in 64.0% cases. Before the procedure, all patients were locally anaesthetised with a 1% lidocaine spray, and an intravenous bolus of midazolam was administered. A thin bronchoscope (BF-P260F; Olympus, Tokyo, Japan) with a guide sheath (K-201; Olympus; external diameter, 1.95 mm) was used for the 1.4-mm probe. After the probe was inserted and the radial endobronchial ultrasound (R-EBUS) image was confirmed, it was withdrawn and transbronchial forceps biopsy (FB-233D; Olympus) was performed at least nine times under fluoroscopic guidance, according to the Kurimoto method (10). Samples for pathological evaluation were only collected by the guide sheath using forceps. We classified the EBUS probe positions into three as follows: (I) within, when the probe was located inside the PPL; (II) adjacent to, when the probe was located at the periphery of the PPL; and (III) outside, when the probe was located away from the PPL. Furthermore, if an EBUS image could not be visualized, as in the case of a solid lesion, the probe was manipulated under X-ray fluoroscopic guidance until a whitish acoustic shadow (e.g., a blizzard sign or mixed blizzard sign) could be visualized (21-23). The virtual bronchoscopic navigation (VBN) was created on the workstation (Ziostation2, Ziosoft Ltd., Tokyo, Japan, or SYNAPSE VINCENT version 4.0, Fuji Medical Systems, Tokyo, Japan) by an experienced chest radiologist (S.I.) in 81.3% of the total cases.
Variables
The following clinical information were collected from all patients who underwent the procedure: age, sex, pulmonary function test results, lesion size, lesion lobe, lesion location from the hilum, lesion structure, bronchus sign, visibility on chest X-ray, background lung, EBUS image, bronchoscopic diagnosis, and final diagnosis. The lesion location from the hilum was classified into two: “inner” for lesions within the inner and middle third ellipses and “outer” for lesions within the outer third ellipse (24). The lesion structure was classified into two groups as follows: solid and others (25). Based on the background lung, patients were classified into having ILD (ILD group) and not having ILD (without ILD). ILD was identified based on radiological findings according to the official ATS/ERS/JRS/ALAT Clinical Practice Guidelines for diagnosis of IPF and classified into the two groups of UIP and non-UIP patterns (probable UIP, indeterminate for UIP, and alternative diagnosis) (26). The final diagnosis was confirmed based on the pathological findings of biopsy specimens from bronchoscopy, TTNB, and SLB. When the collected specimens showed malignancy (i.e., specific findings on histology or cytology positive) and this was consistent with the final diagnosis, bronchoscopy was considered diagnostic. When the collected specimens showed specific benign findings (e.g., granuloma and organizing pneumonia) and the subsequent clinical course was assessed to have decreased radiologically and stabilised in size during follow-up of more than 2 years after the procedure, bronchoscopy was considered diagnostic. Moreover, when the samples were not adequate (e.g., peripheral lung tissue and peribronchial tissue), bronchoscopy was regarded as non-diagnostic. If the lesions of part-solid or pure ground-glass structures were undiagnosed by bronchoscopy, follow-up was performed using CT at the physician’s discretion, and a definite diagnosis and appropriate therapy were obtained by surgery.
Statistical analysis
The data are presented as median and range. Mann-Whitney U and Pearson chi-square tests were used in analysing continuous and categorical variables, respectively. Multivariable logistic regression analyses were performed to investigate the significant predictors of the positive results of EBUS-GS TBB for all patients and those with ILD. Statistical significance was set at P<0.05, and all reported P values were two-sided. All analyses were performed using SPSS Statistics version 28 (IBM, Armonk, NY, USA).
Results
Patient characteristics
During the study, 691 patients underwent diagnostic bronchoscopy with R-EBUS for one PPL. A total of 431 lesions in 431 patients were included in the analyses after excluding 87 endobronchial lesions, 27 lesions that had an uncertain final diagnosis, and 146 lesions presenting solely with pulmonary emphysema without ILD. Finally, we identified 69 lesions associated with ILD and 362 lesions without ILD (Figure 1). The characteristics of the patients in the two groups are shown in Table 1. In addition to lesion lobe, there were significant differences in proportion of males, outer lesions, and solid nodules between patients with ILD and those without ILD. Furthermore, the histological findings between the two groups are shown in Table 2. The most frequent histological finding was adenocarcinoma in both groups.
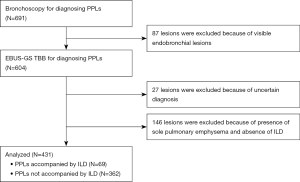
Table 1
| Variables | ILD (n=69) | Without ILD (n=362) | P value |
|---|---|---|---|
| Age, median [range], years | 73 [53–86] | 71 [43–88] | 0.097 |
| Sex, male, n (%) | 60 (87.0) | 178 (49.2) | <0.001 |
| FEV1/FVC, %, median [range] | 78 [42–131] | 76 [43–164] | 0.654 |
| FEV1, percent predicted, %, median [range] | 90 [57–117] | 92 [48–152] | 0.688 |
| FVC, percent predicted, %, median [range] | 99 [40–131] | 104 [58–157] | 0.069 |
| Size, median [range], mm | 26.5 [11–120] | 27 [10.8–150] | 0.983 |
| Lobe, n (%) | 0.036 | ||
| Right upper/left upper | 22 (31.9) | 171 (47.2) | |
| Right middle/lingula | 8 (11.6) | 45 (12.4) | |
| Right lower/left lower | 39 (56.5) | 146 (40.3) | |
| Location: outer, n (%) | 40 (58.0) | 161 (44.5) | 0.039 |
| Structure: solid nodule, n (%) | 62 (89.9) | 276 (76.2) | 0.012 |
| Bronchus sign: positive, n (%) | 66 (95.7) | 344 (95.0) | 0.559 |
| Chest X-ray: visible, n (%) | 61 (88.4) | 340 (93.9) | 0.087 |
ILD, interstitial lung disease; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity.
Table 2
| Diagnosis | ILD (n=69) | Without ILD (n=362) | P value |
|---|---|---|---|
| Malignant lesions | 66 | 339 | 0.042 |
| Diagnostic case | 41 | 252 | |
| Adenocarcinoma | 19 | 184 | |
| Squamous cell carcinoma | 11 | 32 | |
| Small cell carcinoma | 4 | 4 | |
| Non-small cell carcinoma | 6 | 19 | |
| Malignant lymphoma | 1 | 2 | |
| Metastatic lung cancer | 0 | 10 | |
| Spindle cell carcinoma | 0 | 1 | |
| Non-diagnostic case | 25 | 87 | |
| Benign lesions | 3 | 23 | 0.319 |
| Diagnostic case | 2 | 21 | |
| Inflammatory lesions | 2 | 9 | |
| Aspergillosis | 0 | 2 | |
| Cryptococcus | 0 | 1 | |
| NTM | 0 | 4 | |
| Organizing pneumonitis | 0 | 1 | |
| Sarcoidosis | 0 | 1 | |
| Actinomycosis | 0 | 1 | |
| Tuberculosis | 0 | 1 | |
| Abscess | 0 | 1 | |
| Non-diagnostic case | 1 | 2 |
ILD, interstitial lung disease; NTM, non-tuberculous mycobacteria.
The diagnostic yield according to lesion characteristics and EBUS images
The EBUS-GS TBB in patients with ILD had a significantly lower diagnostic yield than in patients without ILD (62.3% vs. 75.4%, P=0.024). The diagnostic yields of PPLs with larger lesions (>20 mm), upper lesions, solid lesions, positive bronchus sign, and malignant lesions were significantly lower in patients with ILD than in those without ILD. When the probe was located within, adjacent to, or outside the lesion, the diagnostic yields for the patients with ILD were 80%, 53.8%, and 0%, respectively, while the yields for the patients without ILD were 85.2%, 66.7%, and 10.7%, respectively. There was no significant difference in the diagnostic yields of the two groups based on the EBUS images (Table 3).
Table 3
| Variables | ILD (n=69) | Without ILD (n=362) | P value |
|---|---|---|---|
| Size | |||
| ≤20 mm | 7/14 (50.0) | 52/89 (58.4) | 0.554 |
| >20 mm | 36/55 (65.5) | 221/273 (81.0) | 0.011 |
| Location | |||
| Upper | 11/22 (50.0) | 126/171 (73.7) | 0.021 |
| Others | 32/47 (68.1) | 147/191 (77.0) | 0.207 |
| Location | |||
| Inner | 17/29 (58.6) | 152/201 (75.6) | 0.053 |
| Outer | 26/40 (65.0) | 121/161 (75.2) | 0.195 |
| Structure | |||
| Solid | 40/62 (64.5) | 221/276 (80.1) | 0.008 |
| Others | 3/7 (42.9) | 52/86 (60.5) | 0.300 |
| Bronchus sign | |||
| Positive | 42/66 (63.6) | 262/344 (76.2) | 0.033 |
| Negative | 1/3 (33.3) | 11/18 (61.1) | 0.388 |
| Visibility on chest X-ray | |||
| Visible | 40/61 (65.6) | 262/340 (77.1) | 0.055 |
| Invisible | 3/8 (37.5) | 11/22 (50.0) | 0.426 |
| EBUS image | |||
| Within | 36/45 (80.0) | 218/256 (85.2) | 0.380 |
| Adjacent to | 7/13 (53.8) | 52/78 (66.7) | 0.531 |
| Outside | 0/11 (0) | 3/28 (10.7) | 0.545 |
| Final diagnosis | |||
| Malignant | 41/66 (62.1) | 252/339 (74.3) | 0.042 |
| Benign | 2/3 (66.7) | 21/23 (91.3) | 0.319 |
| Total | 43/69 (62.3) | 273/362 (75.4) | 0.024 |
Data are shown as numbers of lesions/total lesions (%). EBUS, endobronchial ultrasound; ILD, interstitial lung disease.
Factors possibly affecting the successful diagnosis based on EBUS-GS TBB
Multivariate analysis showed that the presence of ILD as the background lung [odds ratio (OR) =0.517, 95% confidence interval (CI): 0.270–0.988, P=0.046], solid lesion (OR =1.946, 95% CI: 1.116–3.393, P=0.019), and probe position within the lesion (OR =4.654, 95% CI: 2.771–7.816, P<0.001) were significant factors that affected the diagnostic yield of EBUS-GS TBB (Table 4).
Table 4
| Variables | Reference | Multivariate | |
|---|---|---|---|
| OR (95% CI) | P value | ||
| Age ≥70 (n=262) | <70 (n=169) | 1.139 (0.700–1.854) | 0.599 |
| Sex, male (n=238) | Female (n=193) | 0.769 (0.461–1.283) | 0.315 |
| ILD (n=69) | Without ILD (n=362) | 0.517 (0.270–0.988) | 0.046 |
| Size >20 mm (n=328) | ≤20 mm (n=103) | 1.413 (0.798–2.503) | 0.235 |
| Lobe upper lobe (n=193) | Others (n=238) | 0.815 (0.502–1.324) | 0.410 |
| Location, outer (n=201) | Inner (n=230) | 0.928 (0.572–1.507) | 0.763 |
| Structure, solid nodule (n=338) | Others (n=93) | 1.946 (1.116–3.393) | 0.019 |
| Bronchus sign positive (n=410) | Negative (n=21) | 0.959 (0.318–2.897) | 0.941 |
| Chest X-ray visible (n=401) | Invisible (n=30) | 1.995 (0.827–4.812) | 0.124 |
| EBUS image within (n=301) | Others (n=130) | 4.654 (2.771–7.816) | <0.001 |
OR, odds ratio; CI, confidence interval; ILD, interstitial lung disease; EBUS, endobronchial ultrasound.
The diagnostic yield according to the pattern of ILD and factors associated with the diagnostic yield of EBUS-GS TBB in patients with ILD
The diagnostic yield of EBUS-GS TBB was not significantly different according to the pattern of ILD (P=0.447) (Table 5). In patients with ILD, the positional relationship of PPLs on EBUS images was the only significant predictor of the successful diagnosis based on EBUS-GS TBB (OR =12.074, 95% CI: 3.304–44.128, P<0.001) (Table 6).
Table 5
| UIP pattern (n=25) | Probable UIP pattern (n=21) | Indeterminate for UIP (n=15) | Alternative Diagnosis (n=8) | P value | |
|---|---|---|---|---|---|
| Diagnostic yield | 14/25 (56.0) | 12/21 (57.1) | 12/15 (80.0) | 5/8 (62.5) | 0.447 |
Data are shown as numbers of lesions/total lesions (%). EBUS-GS TBB, radial endobronchial ultrasonography with a guide sheath transbronchial biopsy; ILD, interstitial lung disease; UIP, usual interstitial pneumonia.
Table 6
| Variables | Reference | Multivariate | |
|---|---|---|---|
| OR (95% CI) | P value | ||
| Size, >20 mm (n=55) | ≤20 mm (n=14) | 1.348 (0.289–6.298) | 0.704 |
| UIP pattern (n=25) | Non-UIP pattern (n=44) | 0.453 (0.131–1.564) | 0.210 |
| Structure, solid nodule (n=62) | Others (n=7) | 3.359 (0.537–21.025) | 0.195 |
| The lesion location from the hilum, inner (n=29) | Outer (n=40) | 1.697 (0.499–5.775) | 0.397 |
| EBUS image, within (n=61) | Others (n=8) | 12.074 (3.304–44.128) | <0.001 |
ILD, interstitial lung disease; OR, odds ratio; CI, confidence interval; UIP, usual interstitial pneumonia; EBUS, endobronchial ultrasound.
Complications
The complications rate in patients with ILD were significantly higher than in those without ILD (8.7% vs. 1.1%, P=0.002). There was a significant difference in the prevalence of pneumothorax among the patients with ILD and those without ILD (4.3% vs. 0.6%, P=0.031) (Table 7). Pneumothorax occurred in three patients with ILD (4.3%). Of these, two required thoracic drainage. Conversely, two patients without ILD did not require thoracic drainage (0.6%).
Table 7
| Variables | ILD (n=69) | Without ILD (n=362) | P value |
|---|---|---|---|
| Complications, n (%) | 6 (8.7) | 4 (1.1) | 0.002 |
| Pneumothorax | 3 (4.3) | 2 (0.6) | 0.031 |
| Pneumonia | 2 (2.9) | 2 (0.6) | 0.122 |
| Mediastinal emphysema | 1 (1.4) | 0 (0) | 0.160 |
ILD, interstitial lung disease.
Discussion
Our results revealed that the diagnostic yield of EBUS-GS TBB in patients with ILD was significantly lower than that in those without ILD. Multivariate logistic analysis revealed that the presence of ILD as the background lung, lesion structure, and EBUS image significantly affected the diagnostic yield of EBUS-GS TBB. Furthermore, the probe position was considerably associated with the diagnostic yield of EBUS-GS TBB in patients with ILD. As for the complications, pneumothorax occurred more frequently in patients with ILD than in those without ILD.
Previous studies have reported that intra-procedural imaging facilitated accurate diagnoses based on EBUS-GS TBB (14,15). Similarly, the probe position relative to the lesion significantly affected the diagnostic yield. Furthermore, Yoshikawa et al. reported that solid lesions had a significantly higher diagnostic yield than ground-glass lesions (27). The reasons for the lower yields for ground-glass lesions include the difficulties in obtaining EBUS images for ground-glass lesions and ensuring an accurate position for ground-glass lesion fluoroscopy. Consistent with a previous report, lesion structure significantly affected the diagnostic yield of EBUS-GS TBB. Regarding the background lung in patients who underwent EBUS-GS TBB, the diagnostic yield and safety profile of EBUS-GS TBB in patients with IPF were reported. Lee et al. revealed that in patients with IPF, the presence of UIP pattern significantly influenced the lower diagnostic yield of PPLs with R-EBUS compared to the probable UIP/non ILD group (17). Our study indicated that the presence of ILD not limited to UIP pattern in patients with IPF had a significant influence on the lower diagnostic yield based on EBUS-GS TBB.
According to the lesion size (size ≤20 or >20 mm), lesion lobe (upper or others), lesion texture (solid or others), bronchus sign (positive or negative), underlying disease (malignant or benign), the diagnostic yield of EBUS-GS TBB with larger lesions (>20 mm), upper lesions, solid lesions, positive bronchus sign, and malignant lesions were significantly lower in patients with ILD than in those without ILD. In patients with ILD, there might be technical problems related to the difficulty of detecting the lesions because reticular shadows around PPLs preclude their detection and make it difficult to perform biopsies from the lesions appropriately during EBUS-GS TBB.
Moreover, in patients with ILD, the insertion of the device to target bronchus was reported to be difficult because of bronchial narrowing and torsion associated with traction bronchiectasis as anatomical changes (17). Conversely, Herth et al. reported that the diagnostic yield for lesions located within the right upper lobe was lower than that for other lesions because the right upper lesions were particularly difficult to reach due to affected manoeuvrability in the tortuous airways and sharp bends, resulting in a lower diagnostic yield (28). Furthermore, Kurimoto et al. reported that the lower diagnostic yield of PPLs located in the left upper lobe apical posterior segment is thought to be due to the difficulty in inserting the probe into B1+2 (10). In addition to anatomical changes associated with ILD, the difficulty of inserting the device into upper lesions might be linked to lower diagnostic yield in patients with ILD compared to those without ILD.
A previous report demonstrated that in patients with ILD, inflammatory cell infiltration and fibrotic changes increased in the PPL lung compared to the other one (29). Furthermore, in patients with ILD, the diagnostic yield of EBUS-GS TBB within or near fibrotic lesions was reported to be lower than that of PPLs distant from fibrotic lesions because small biopsy forceps may be associated with sampling only inflammatory cells or fibrotic changes around lung cancer co-existing with ILD (30).
In patients with ILD, EBUS image rather than the presence of UIP pattern was significantly associated with a successful bronchoscopic diagnosis with EBUS-GS TBB. We considered that the diagnostic yield of EBUS-GS TBB in patients with ILD was lower than in those without ILD, because of the difficulty in correctly reaching the lesions around reticular shadows and distinguishing lesions as background lung on EBUS image. However, when the probe was located within the lesions, the diagnostic yield of EBUS-GS TBB was not significantly different between the two groups. In patients with ILD, the detection of subsolid lesions on EBUS images might be difficult. However, when physicians performed EBUS-GS TBB for diagnosing solid lesions in these patients, they should perform biopsies in the position in which the probe was located within the lesion as much as possible (Figure 2). This result was similar to most previous studies which reported that the probe position relative to the lesion was a predictor of successful bronchoscopic diagnosis using R-EBUS (14,15,31).
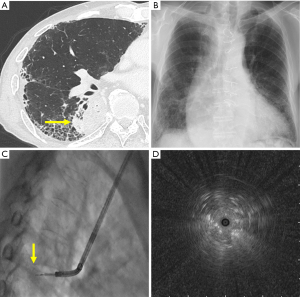
Previous reports showed that the rate of pneumothorax with EBUS-GS TBB was approximately 3% (32,33). In our patients with ILD, the rate of pneumothorax was 4.3%, which was significantly higher than that in patients without ILD. In our study, outer lesions were more prevalent in patients with ILD than in those without ILD. We considered that the damage to alveolar tissue accompanied by biopsy and the location of the lesion near the visceral pleura might be related to pneumothorax.
Our study has some limitations. First, it used a small cohort and was a retrospective study in a single centre. Second, the severity of ILD has not been fully investigated. Finally, before comparing the diagnostic outcomes and complications between ILD and without ILD patients, there was a bias in baseline characteristics between the two groups. A larger sample and a prospective randomized design will be needed to overcome these limitations.
Conclusions
In conclusion, the presence of ILD significantly affected the diagnostic yield of EBUS-GS TBB for PPLs. Moreover, in patients with ILD, the probe position relative to the lesion was a significant predictor of the diagnostic yield of EBUS-GS TBB. Regarding complications, the rate of pneumothorax during EBUS-GS TBB in patients with ILD was significantly higher than in those without ILD.
Acknowledgments
We would like to thank Editage (www.editagecom) for English language editing.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-809/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-809/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-809/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-809/coif). TFCY serves as an unpaid editorial board member of Journal of Thoracic Disease from April 2022 to March 2024. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was performed in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Nagoya University Hospital Institutional Review Board (No. 2021-0272). The requirement for informed consent was waived due to the retrospective design of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Aberle DR, DeMello S, Berg CD, et al. Results of the two incidence screenings in the National Lung Screening Trial. N Engl J Med 2013;369:920-31. [Crossref] [PubMed]
- Whittaker Brown SA, Padilla M, Mhango G, et al. Interstitial Lung Abnormalities and Lung Cancer Risk in the National Lung Screening Trial. Chest 2019;156:1195-203. [Crossref] [PubMed]
- Jin GY, Lynch D, Chawla A, et al. Interstitial lung abnormalities in a CT lung cancer screening population: prevalence and progression rate. Radiology 2013;268:563-71. [Crossref] [PubMed]
- Gibiot Q, Monnet I, Levy P, et al. Interstitial Lung Disease Associated with Lung Cancer: A Case-Control Study. J Clin Med 2020;9:700. [Crossref] [PubMed]
- Lee YH, Cha SI, Lim JK, et al. Clinical and radiological features of pulmonary tuberculosis in patients with idiopathic pulmonary fibrosis. Respir Investig 2019;57:544-51. [Crossref] [PubMed]
- Goto T. Measuring Surgery Outcomes of Lung Cancer Patients with Concomitant Pulmonary Fibrosis: A Review of the Literature. Cancers (Basel) 2018;10:223. [Crossref] [PubMed]
- Minami-Shimmyo Y, Ohe Y, Yamamoto S, et al. Risk factors for treatment-related death associated with chemotherapy and thoracic radiotherapy for lung cancer. J Thorac Oncol 2012;7:177-82. [Crossref] [PubMed]
- Wang Memoli JS, Nietert PJ, Silvestri GA. Meta-analysis of guided bronchoscopy for the evaluation of the pulmonary nodule. Chest 2012;142:385-93. [Crossref] [PubMed]
- Bai C, Choi CM, Chu CM, et al. Evaluation of Pulmonary Nodules: Clinical Practice Consensus Guidelines for Asia. Chest 2016;150:877-93. [Crossref] [PubMed]
- Kurimoto N, Miyazawa T, Okimasa S, et al. Endobronchial ultrasonography using a guide sheath increases the ability to diagnose peripheral pulmonary lesions endoscopically. Chest 2004;126:959-65. [Crossref] [PubMed]
- Rivera MP, Mehta AC, Wahidi MM. Establishing the diagnosis of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e142S-65S.
- Postmus PE, Kerr KM, Oudkerk M, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28:iv1-iv21. [Crossref] [PubMed]
- Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e93S-e120S.
- Guvenc C, Yserbyt J, Testelmans D, et al. Computed tomography characteristics predictive for radial EBUS-miniprobe-guided diagnosis of pulmonary lesions. J Thorac Oncol 2015;10:472-8. [Crossref] [PubMed]
- Ali MS, Trick W, Mba BI, et al. Radial endobronchial ultrasound for the diagnosis of peripheral pulmonary lesions: A systematic review and meta-analysis. Respirology 2017;22:443-53. [Crossref] [PubMed]
- Lee KM, Lee G, Kim A, et al. Clinical outcomes of radial probe endobronchial ultrasound using a guide sheath for diagnosis of peripheral lung lesions in patients with pulmonary emphysema. Respir Res 2019;20:177. [Crossref] [PubMed]
- Lee J, Kim C, Seol HY, et al. Safety and Diagnostic Yield of Radial Probe Endobronchial Ultrasound-Guided Biopsy for Peripheral Lung Lesions in Patients with Idiopathic Pulmonary Fibrosis: A Multicenter Cross-Sectional Study. Respiration 2022;101:401-7. [Crossref] [PubMed]
- Ito T, Kimura T, Kataoka K, et al. A Pilot Study of Transbronchial Biopsy Using Endobronchial Ultrasonography with a Guide Sheath in the Diagnosis of Peripheral Pulmonary Lesions in Patients with Interstitial Lung Disease. Diagnostics (Basel) 2021;11:2269. [Crossref] [PubMed]
- Omori S, Takiguchi Y, Hiroshima K, et al. Peripheral pulmonary diseases: evaluation with endobronchial US initial experience. Radiology 2002;224:603-8. [Crossref] [PubMed]
- Asano F, Shinagawa N, Ishida T, et al. Virtual bronchoscopic navigation combined with ultrathin bronchoscopy. A randomized clinical trial. Am J Respir Crit Care Med 2013;188:327-33. [Crossref] [PubMed]
- Sasada S, Izumo T, Chavez C, et al. Blizzard Sign as a specific endobronchial ultrasound image for ground glass opacity: A case report. Respir Med Case Rep 2014;12:19-21. [Crossref] [PubMed]
- Izumo T, Sasada S, Chavez C, et al. Radial endobronchial ultrasound images for ground-glass opacity pulmonary lesions. Eur Respir J 2015;45:1661-8. [Crossref] [PubMed]
- Nakai T, Izumo T, Matsumoto Y, et al. Virtual fluoroscopy during transbronchial biopsy for locating ground-glass nodules not visible on X-ray fluoroscopy. J Thorac Dis 2017;9:5493-502. [Crossref] [PubMed]
- Matsumoto Y, Izumo T, Sasada S, et al. Diagnostic utility of endobronchial ultrasound with a guide sheath under the computed tomography workstation (ziostation) for small peripheral pulmonary lesions. Clin Respir J 2017;11:185-92. [Crossref] [PubMed]
- Callister ME, Baldwin DR, Akram AR, et al. British Thoracic Society guidelines for the investigation and management of pulmonary nodules. Thorax 2015;70:ii1-ii54. [Crossref] [PubMed]
- Raghu G, Remy-Jardin M, Myers JL, et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med 2018;198:e44-68. [Crossref] [PubMed]
- Yoshikawa M, Sukoh N, Yamazaki K, et al. Diagnostic value of endobronchial ultrasonography with a guide sheath for peripheral pulmonary lesions without X-ray fluoroscopy. Chest 2007;131:1788-93. [Crossref] [PubMed]
- Herth FJ, Ernst A, Becker HD. Endobronchial ultrasound-guided transbronchial lung biopsy in solitary pulmonary nodules and peripheral lesions. Eur Respir J 2002;20:972-4. [Crossref] [PubMed]
- Fujita J, Yamadori I, Namihira H, et al. Increased intensity of lung infiltrates at the side of lung cancer in patients with lung cancer associated with pulmonary fibrosis. Lung Cancer 1999;26:169-74. [Crossref] [PubMed]
- Ito T, Okachi S, Kimura T, et al. Endobronchial Ultrasonography with a Guide Sheath Transbronchial Biopsy for Diagnosing Peripheral Pulmonary Lesions within or near Fibrotic Lesions in Patients with Interstitial Lung Disease. Cancers (Basel) 2021;13:5751. [Crossref] [PubMed]
- Okachi S, Imai N, Imaizumi K, et al. Factors Affecting the Diagnostic Yield of Transbronchial Biopsy Using Endobronchial Ultrasonography with a Guide Sheath in Peripheral Lung Cancer. Intern Med 2016;55:1705-12. [Crossref] [PubMed]
- Hayama M, Izumo T, Matsumoto Y, et al. Complications with Endobronchial Ultrasound with a Guide Sheath for the Diagnosis of Peripheral Pulmonary Lesions. Respiration 2015;90:129-35. [Crossref] [PubMed]
- Gotoh Y, Yamaguchi T, Yatsuya H, et al. Predictive risk factors for pneumothorax after transbronchial biopsy using endobronchial ultrasonography with a guide sheath. BMC Pulm Med 2021;21:181. [Crossref] [PubMed]


