Basic pathophysiology and options of treatment for surgical management of functional tricuspid regurgitation: a systematic review
Introduction
There has been an increasing interest in the tricuspid valve, which was still called the ‘forgotten’ or ‘neglected’ valve in the 1990s within the last two decades. Every aspect of tricuspid valve disease, and primarily of tricuspid regurgitation (TR), is being investigated today (1-3).
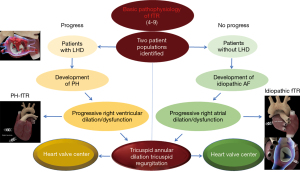
While organic (or primary) TR is a rare disease having many causes and various pathophysiological mechanisms, functional TR affects up to 1% of the people of developed countries and is almost always caused by the right ventricular (RV) dilation/dysfunction and pulmonary hypertension secondary to left-sided heart valve diseases, with atrial fibrillation (AF) being a very important contributing factor. In the natural history of left-sided heart diseases, the onset of TR coincides with the significant worsening of signs and symptoms of heart failure and is associated with poor patient survival. Approximately 30% of individuals with severe mitral valve disease have significant TR, and up to 50% of patients undergoing mitral valve surgery suffer from associated TR. As functional TR can increase if left untreated (this fact occurs quite unpredictably), even after complete correction of the left-sided heart disease, its surgical treatment should still be recommended during primary left-sided cardiac operations; especially since cardiac reoperations due to residual/recurrent TR may be very risky (sometimes prohibitive) procedures (1-3).
However, despite significant improvements in knowledge of its pathophysiology [causes and basic pathophysiology of functional TR are summarized in Table 1 and Figure 1 (4-9)], several issues of functional TR, which concern primarily indications for treatment and options of repair remain to be solved.
Table 1
| A. Left-sided heart diseases |
| Left ventricular dysfunction (diastolic, systolic) |
| Ischemic |
| Non-ischemic |
| Valvular |
| Mitral |
| Aortic |
| B. Pulmonary hypertension |
| Primary |
| Post-embolism |
| Chronic obstructive pulmonary disease |
| C. RV diseases |
| Cardiomyopathy |
| Myocardial infarction |
| Volume overload |
| D. Supraventricular tachyarrhythmias (supraventricular paroxysmal tachycardia, atrial flutter, AF) |
TR, tricuspid regurgitation; RV, right ventricular; AF, atrial fibrillation.
The purpose of the present systematic review is to foster a broader understanding of the basic pathophysiology of functional TR and of providing guidance for clinicians. The current evidence base for the surgical treatment of functional TR is discussed here. An evidence-based algorithm for the choice among the different surgical options has been finally suggested. We present the following article in accordance with the PRISMA reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-661/rc).
Methods
Search method
Databases (Embase, n=753; Ovid Medline, n=447; Cochrane, Web of Science, and Google, n=379) have been scrutinized from inception, and only articles in English language were examined. The first inclusion criterion was significant TR (by transthoracic echocardiography) due to tricuspid annular dilatation and leaflet tethering, and secondary to right or left cardiomyopathy (regardless of etiology). The studies pertained to adult (18+ years) human beings eligible for surgical repair or replacement of tricuspid valve (the choice of surgical treatment was decided by the surgeon). Consequently, pediatric age and organic TR due to structural valve disease for which tricuspid valve repair was not recommended were the exclusion criteria. Randomized controlled trials, propensity-matched studies, and observational studies have been included in this review. Recent meta-analyses and reviews on this topic for potential additional references were searched. Three investigators (GG, AF, and FN) searched papers published between January 1, 2000, and December 31, 2021 using the following keywords: ‘tricuspid regurgitation’, ‘organic tricuspid regurgitation’, and ‘functional tricuspid regurgitation’. These keywords were coupled with ‘tricuspid valvuloplasty’, ‘tricuspid valve repair’, ‘tricuspid annuloplasty’, and ‘tricuspid valve replacement’. Investigators (GG, AF, and FN) independently reviewed all citations and any disagreement has been resolved by consensus.
Data extraction and quality assessment
Studies were selected for inclusion from prospective randomized trials or propensity-matched retrospective studies. Preoperative and postoperative data were extracted from the main publication, and searches were performed by two independent researchers (GG and AF) using blind method. A third independent investigator (FN) has assessed adequacy. Data to be extracted included: study demographics (sample size, number of institutions involved, publication year, study period, design, country, length of follow-up), patient demographics (age, sex), RV and left ventricular (LV) function, and procedural details (use of double-level repair combined to single-level repair). Postoperative data (in-hospital mortality, postoperative complications, residual TR, reoperation), long-term results (survival, freedom from reoperation, freedom from rehospitalization) were also included.
Results
A total of 1,579 studies were reviewed. Thirty-two (2%) of these met inclusion criteria and were enclosed in the final systematic review (Figure 2): 13 studies were primarily focused on pathophysiology and preoperative assessment of functional TR; 19 studies on surgical treatment of functional TR. There were 8 prospective and 24 retrospective studies. A total of 15,509 patients were included. The number of patients in the individual study ranged from 11 to 5,223. The mean age ranged from 52 to 70.4 years. The grade of TR ranged from 0 (null, trace, and trivial) to 3+ (moderate-to-severe and severe). The tricuspid valve evaluation was always performed using two-dimensional color Doppler echocardiography; in one study was added the real time three-dimensional echocardiography. In 28 studies the patients underwent surgery, which was most frequently mitral valve repair or replacement combined with tricuspid valve suture (De Vega) or device annuloplasty. Two studies explored tricuspid valve replacement for treatment of functional TR. The mean follow-up period ranged from 5 days to 17 years (Tables 2,3).
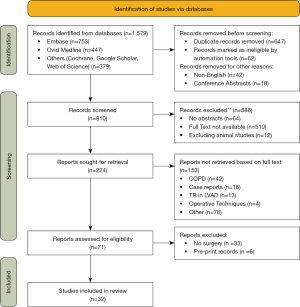
Table 2
| First author (ref. no.) | No. of patients (mean age, years) | Type of study | Follow-up | Assessment/procedure | Main findings |
|---|---|---|---|---|---|
| Nath (1) | 5,223 (66.5±12.8) | Retrospective | Mean, 498±402 days | 2D color Doppler echocardiography/no surgery | Increasing TR severity is associated with worse survival in men regardless of LVEF or pulmonary artery pressure. Severe TR is associated with a poor prognosis, independent of age, biventricular systolic function, RV size, and dilation of the inferior vena cava |
| Koelling (2) | 1,421 (66.5±12.8) | Retrospective | Mean, 365±364 days | 2D color Doppler echocardiography/no surgery | Patients with severe MR or TR represent high-risk subsets of patients with LVEF ≤35% |
| Kim (6) | 64 (N/A) | Prospective | – | 2D color Doppler echocardiography/no surgery | The findings underscore the importance of eccentric RV dilation for determining functional TR severity |
| Calafiore (10) | 110 (N/A) | Retrospective | 5 years | 2D color Doppler echocardiography/MV surgery ± TV annuloplasty | Tricuspid annuloplasty is an easy and safe procedure, mandatory in case of at least moderate functional TR to achieve better mid-term outcome in patients with functional MR undergoing MV surgery |
| Song (11) | 638 (52±14) | Retrospective | 101 months | 2D color Doppler echocardiography/left-sided valve surgery | Age, female gender, rheumatic etiology, AF, and peak pressure gradient of TR at follow-up were independent risk factors for development of late significant TR. Early surgical intervention for TR in selected patients with these risk factors may be justified, even though they have only mild TR |
| Dreyfus (12) | 311 (N/A) | Retrospective | 3, 5, and 10 years | 2D color Doppler echocardiography/MV surgery ± TV annuloplasty | Remodeling TV annuloplasty based on tricuspid dilation improves functional status irrespective of the grade of regurgitation. Considerable tricuspid dilation can be present even in the absence of substantial TR. Tricuspid dilation is an ongoing disease process that will lead with time to severe TR |
| Matsunaga (13) | 124 (N/A) | Retrospective | <1, 1, 3, and >3 years | 2D color Doppler echocardiography/MV repair + CABG ± TV annuloplasty | Functional TR is frequently associated with functional ischemic MR. After MV repair, close to 50% of patients have TR. The incidence of postoperative TR increases with time. Preoperative tricuspid annulus dilation might be a predictor of late TR |
| Di Mauro (14) | 165 (N/A) | Retrospective | 5 years | 2D color Doppler echocardiography/MV surgery | Patients with untreated moderate-or-more functional TR had survival and survival in New York Heart Association class I–II lower than patients with untreated less-than-moderate functional TR |
| Yilmaz (15) | 699 (60.4) | Retrospective | <1, 1, 3, 5, and >5 years | 2D color Doppler echocardiography/MV repair | Clinical silent non-severe TR in patients with degenerative MV disease is unlikely to progress after MV repair. TV surgery is rarely necessary for most patients undergoing repair of isolated MV prolapse |
| Van de Veire (16) | 182 (N/A) | Retrospective | 2 years | 2D color Doppler echocardiography/MV repair ± TV annuloplasty | Concomitant TV annuloplasty during MV repair should be considered in patients with tricuspid annular dilation despite the absence of important TR at baseline because this improves echocardiographic outcome |
| Kwak (17) | 615 (N/A) | Retrospective | Mean, 11.6±2.1 years | 2D color Doppler echocardiography/left-sided valve surgery | The development of significant TR long after left-sided valve surgery is not uncommon with an estimated incidence of 27% and is closely associated with a poor prognosis. The presence of preoperative AF was identified as the only independent predictor of the development of late TR |
| Colombo (18) | 50 (N/A) | Prospective | Mean, 25±15.9 months | 2D color Doppler echocardiography/MV replacement ± TV suture annuloplasty | The choice to treat TR according to indexed tricuspid annulus dimension (>21 mm/m2) has been effective in terms of clinical efficacy and of late functional result. Fractional shortening of the tricuspid annulus affects the postoperative evolution of TR |
| Mutlak (19) | 2,139 (N/A) | Retrospective | – | 2D color Doppler echocardiography/no surgery | Although pulmonary hypertension is a strong determinant of TR and pulmonary artery pressure systolic values correlate with TR severity, many patients with pulmonary hypertension do not exhibit significant TR. Demographic characteristics, mechanical factors, right heart remodeling, AF, and occult organic TV disease are predictive of TR |
TR, tricuspid regurgitation; 2D, two-dimensional; LVEF, left ventricular ejection fraction; RV, right ventricular; MR, mitral regurgitation; N/A, not available; MV, mitral valve; TV, tricuspid valve; AF, atrial fibrillation; CABG, coronary artery bypass grafting.
Table 3
| First author (ref. no.) | No. of patients (mean age, years) | Type of study | Follow-up | Assessment/procedure | Main findings |
|---|---|---|---|---|---|
| Gatti (20) | 22 (N/A) | Retrospective | Mean, 19.9±9.7 months | 2D color Doppler echocardiography/TV annuloplasty using flexible prosthetic ring (Cosgrove-Edwards Annuloplasty System*) | TV annuloplasty using flexible prosthetic ring is very effective in the treatment of functional TR, even in the presence of pulmonary hypertension |
| Gatti (21) | 53 (66.2±8.5) | Retrospective | Mean, 19.2±14 months | 2D color Doppler echocardiography/TV annuloplasty using flexible prosthetic band (Koehler Mitral Repair System†) | TV annuloplasty using flexible prosthetic band is very effective in the treatment of functional TR, even in the presence of pulmonary hypertension |
| Fukuda (22) | 136 (N/A) | Prospective | Mean, 5±5 days (>1 year for 28 patients) | 2D color Doppler echocardiography/TV annuloplasty using 3D prosthetic ring (Edwards MC3 Tricuspid Annuloplasty Ring*) | TV annuloplasty using 3D ring is effective for the management of TR and may be superior to conventional techniques. Patients with extensive leaflet tethering (>1.0 cm) require additional maneuvers to ensure valve competence |
| De Bonis (23) | 140 (63.8±11.6) | Retrospective | Mean, 22±9.5 months | 2D color Doppler echocardiography/TV annuloplasty using 3D prosthetic ring (Edwards MC3 Tricuspid Annuloplasty Ring*) | TV annuloplasty using 3D ring provides satisfactory early results which remain stable at mid-term follow-up. The presence of other mechanisms besides annular dilation leads to residual valve insufficiency after ring annuloplasty alone |
| Ratschiller (24) | 200 (70.4±9.1) | Retrospective | Mean, 1±0.7 years | 2D color Doppler echocardiography/TV annuloplasty using 3D prosthetic ring (Contour 3D Tricuspid Annuloplasty Ring‡) | TV annuloplasty using 3D ring can be performed with a low rate of residual TR at hospital discharge, a low reoperation rate, and with an excellent early functional outcome |
| Min (25) | 59 (N/A) | Prospective | Mean, 4.7±2.1 days | Real time 3D and 2D color Doppler echocardiography/TV suture or device annuloplasty | Tenting angles of all three leaflets increase, whereas annulus diameters decrease, after TV annuloplasty. Pre-TV annuloplasty tenting volume and antero-posterior annulus diameter are independent predictors of residual TR severity, and measurement of these parameters may help to identify patients at high risk for severe residual TR, for whom TV replacement should possibly be initially considered |
| Rabago (26) | 150 (N/A) | Retrospective | 6 to 30 months | 2D color Doppler echocardiography/TV suture (De Vega) annuloplasty | The addition of tricuspid circular annuloplasty to mitral and aortic valve replacements has resulted in a gratifying fail in operative mortality and better long term functional result |
| Dreyfus (27) | 15 (N/A) | Prospective | – | 2D color Doppler echocardiography/tricuspid leaflet augmentation (pericardial patch) + TV annuloplasty using flexible prosthetic ring (Carpentier-Edwards Tricuspid Annuloplasty Ring*) | The anterior tricuspid leaflet is augmented by use of an autologous pericardial patch, which increases its size, surface, and surface coaptation; a TV flexible annuloplasty ring is then implanted. This technique is effective to treat severe TR due to tethering of the TV leaflets |
| Ghanta (28) | 237 (N/A); TV bicuspidization, 157, annuloplasty, 80 | Retrospective, comparative | Mean, 3 years | 2D color Doppler echocardiography/TV suture bicuspidization vs. TV ring annuloplasty | Bicuspidalization annuloplasty and ring annuloplasty were equally effective at eliminating TR and should be used for all patients with moderate-to-severe TR |
| De Bonis (29) | 14 (57±17) | Prospective | Mean, 12±6.3 months | 2D color Doppler echocardiography/TV clover technique + TV prosthetic ring annuloplasty | This approach (for severe TR due to complex lesions) consists of stitching together the central part of the free edges of the leaflets producing a “clover” shape valve. It is effective even in the presence of huge RV dilation and pulmonary hypertension |
| Couetil (30) | 11 (66.8±12.8) | Prospective | Mean, 12.8±6.3 months | 2D color Doppler echocardiography/papillary muscle septalization + TV prosthetic ring annuloplasty | Papillary muscle septalization combined with TV annuloplasty allows to correct the leaflet tenting, favors RV remodeling, and decreases recurrent TR |
| Do (31) | 29 (N/A); biological, 23, mechanical prostheses, 6 | Retrospective | Mean, 67.7 months | 2D color Doppler echocardiography/isolated TV replacement | Isolated TV replacement remains a high-risk procedure |
| Filsoufi (32) | 81 (61, range 19–83); biological, 34, mechanical prostheses, 47 | Retrospective | 2.5, 5, and 10 years | 2D color Doppler echocardiography/TV replacement, biological vs. mechanical prostheses | Patients requiring TV replacement are high-risk with a high percentage of reoperations, concomitant cardiac procedures, and end-stage functional class. Operative and overall mortality remains high |
| Tang (33) | 702 (N/A); TV suture, 493, or ring annuloplasty, 209 | Retrospective, comparative | Mean, 5.9±4.9 years | 2D color Doppler echocardiography/TV suture (primarily De Vega) vs. ring annuloplasty | TV ring annuloplasty is associated with improved survival and event-free survival |
| Fukuda (34) | 39 (N/A) | Prospective | Mean, 5±2 days, 20±6 months | 2D color Doppler echocardiography/TV annuloplasty | TV tethering, LV and RV function and pressures impact repair durability |
| McCarthy (35) | 790 (65±12) | Retrospective | 1 week, 8 years | 2D color Doppler echocardiography/TV suture (De Vega) and device annuloplasty | TV annuloplasty using De Vega and customized semicircular annuloplasty should be abandoned. Trans-tricuspid pacing leads should be replaced with epicardial leads |
| Gatti (36) | 527 (69.6±9.5) | Retrospective | Mean, 5.2±3.5 years | 2D color Doppler echocardiography/TV suture (De Vega) or device annuloplasty | After device tricuspid annuloplasty and in the absence of preoperative LV dysfunction and severe tricuspid annular dilation, functional TR is generally controlled within grade 1+. Recurrent TR is associated with new left-sided valvular lesions, primarily MR |
| Gatti (37) | 462 (69.2±9.5) (flexible band, 345, rigid ring, 117); 98 propensity-matched pairs | Retrospective, comparative, propensity-matched | Flexible band, mean, 5.7±3.1 years; rigid ring, mean, 3.2±4.2 years | 2D color Doppler echocardiography/TV flexible band vs. rigid ring annuloplasty | TV annuloplasty using flexible band or rigid ring are equally effective in the treatment of functional TR. However, TV rigid ring annuloplasty causes over time a more complete right heart reverse remodelling |
| Ghoreishi (38) | 101 (N/A) | Retrospective | Mean, 17±9 years | 2D color Doppler echocardiography/undersized TV annuloplasty using 3D prosthetic ring | Undersized TV annuloplasty using 3D prosthetic ring (size 26 or 28) is the method of choice for reliable and durable treatment of functional TR |
*, Edwards Lifesciences Corp., Irvine, CA, USA; †, Koehler Medical Ltd., Swillington, Leeds, UK; ‡, Medtronic Comp., Brampton, ON, Canada. TR, tricuspid regurgitation; N/A, not available; 2D, two-dimensional; TV, tricuspid valve; 3D, three-dimensional; RV, right ventricular; LV, left ventricular; MR, mitral regurgitation.
Discussion
The tricuspid anatomy for surgical handling
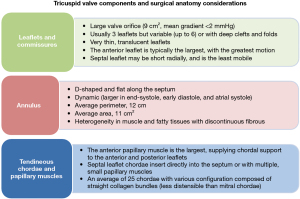
For surgeons engaged in handling the tricuspid valve, accurate knowledge of the anatomy of the tricuspid apparatus and the RV is required. The tricuspid valve and its subvalvular apparatus are formed by leaflets, annulus, papillary muscles, and chordae tendineae (Figure 3) (39-49).
Tricuspid valve leaflets
The valve is made of three leaflets of different sizes; however, in several cases the identification of two (bicuspid), four or more leaflets may be revealed. As stated by their anatomic disposition, the three leaflets are called septal, anterior-superior, and posterior-inferior leaflets. The most common features of the anterior leaflet are the development in length and width with radial direction, wider area, and maximum movement. The posterior leaflet is sometimes distinguished by multiple scallops. The septal leaflet is the less developed, with least mobility and it is placed into the tricuspid annulus straight over the interventricular septum. The commissure between the septal and posterior leaflets is generally found close to the ostium of the coronary sinus in the right atrium (RA). The anteroseptal commissure between the septal and anterior leaflets is the greatest when compared to the posterior one, and is located near the non-coronary sinus of Valsalva of the aortic root (Figure 4) (39-43).
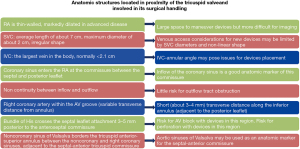
Tricuspid valve annulus
The valvular annulus is usually individualized by a well-defined fibrous structure. However, anatomical studies on healthy cadavers suggested that this designation is inappropriate for the tricuspid valve annulus. Rather, the scarcity of fibrous tissue, which is primarily localized in the septal segment of the annulus is disclosed. Anatomical studies reported that the normal tricuspid annulus has a D-shape configuration with two distinct segments. Given this peculiarity, therefore, the tricuspid annulus is represented by a larger C-shaped portion that corresponds to the free wall of the RA and RV, and by a shorter segment that appears linear and correlates with the septal leaflet and the ventricular septum. The structure of the tricuspid valve annulus contributes to its brittleness and lower tensile strength during surgical manipulation. This architecture is characterized by the presence of multiple muscle filaments of about 2–4 mm in diameter, which extend from the free wall of the RV and the septal wall. The described morphostructure is organized to form muscle bands that can offer support to the non-fibrous RA-RV junction, which is the weak part of the right heart. Its histology consists of very little fibrous tissue together with the endocardium (Figure 4) (42-45).
Subvalvular apparatus
The anatomy of the tensor apparatus supporting the stress load of tricuspid valve includes the papillary muscles and chordae tendineae (42-45), both of which play a decisive role in tricuspid valve repair, especially when a cryopreserved homograft is used (46-48). The subvalvular apparatus of the tricuspid valve encompasses the anterior papillary muscle and posterior papillary muscle. A third papillary muscle is not always obvious. The chordae tendinae of the tricuspid valve are made up of fibrous cords of various lengths that join the papillary muscles to the leaflets of the tricuspid valve. The anterior papillary muscle is usually the most evolved, giving rise the chordae tendineae that are inserted on the anterior and posterior leaflet. The moderating band (trabecula septomarginalis) can be inserted into this papillary muscle. On the other hand, the morphostructure of posterior papillary muscle includes two or three chordae tendinae heads that are attached to the posterior and septal leaflets. The septal papillary muscle (Lancisi muscle) forms part of the posterior papillary muscle and is often fluctuating, small in appearance, multiple or even absent in up to 20% of normal subjects. In a subvalvular apparatus lacking the septal papillary muscle, the septal chordae tendinae can start directly from the ventricular septum heading towards the anterior and septal leaflets. From a pathoanatomical point of view, the anterior and posterior leaflets of the tricuspid valve are related to the septum and may rely on a large anterior papillary muscle situated along the anterolateral wall of the RV. Commonly, the number of chordae fluctuate from 17 to 36 with an average of 25. They are distinguished in five types according to their morphology and place of fixing to the leaflet: fan-shaped, rough zone, basal, free edge, and deep chordae. Histology of the chordae tendinae revealed the presence of collagen for approximately 80%, whereas the remaining 20% is made of elastin and endothelial cells (42-45).
In the course of a tricuspid valve repair, it is crucial to know that the true chordae are connected to the leaflet and in most cases spring out from the apical third of the papillary muscle, although they can also arise from the ventricular wall. The presence of accessory chordae tendinae can be misleading for the surgeon because they can be generated from the free wall of the RV and the trabecula septomarginalis. In addition, the presence of false chordae in the subvalvular apparatus is an eventuality that is suggested by their insertion in various parts of the RV and the possibility of connecting the two papillary muscles, a papillary muscle with the ventricular wall, or two points of the ventricular wall Figure 4 (42,49).
Basic pathophysiology
Natural history of functional TR
Functional TR will progress if left untreated (10). Six hundred thirty-eight patients who underwent left-sided heart valve surgery with no tricuspid valve intervention were included in a study by Song et al. (11). It was noted that within 5 years of surgery, moderate or severe TR was found in 7.3% of those who preoperatively had no or trivial TR, and in 20% of those who presented mild TR. The survival rate decreased in patients who developed late TR. Similarly, Dreyfus et al. (12) described that significant late TR occurred in 34% of patients who sustained mitral valve repair with no tricuspid valve procedure and this was linked to the worse functional class. Moderate-to-severe TR was recorded by Matsunaga and Duran (13) in 75% of the patients in just three years following mitral valve repair for ischemic mitral regurgitation. Another study by Calafiore et al. (10) disclosed that 40% of the patients presented with worsening severity of TR directly after mitral valve surgery where the tricuspid valve had not been operated on. A similar situation was observed by Yilmaz et al. (15) when the TR grade went up a significant amount from 1.84 to 2.11 (P=0.03) in patients during the 5 years that followed mitral valve repair; moderate or severe TR was seen in 29.4% of patients in comparison with 16.5% preoperatively.
Signs and symptoms of functional TR
The onset of an also mild functional TR, perhaps following AF significantly worsens symptoms of the underlying left-sided heart disease. In addition, the signs and symptoms of the right-sided heart failure gradually appear. Actually, TR may not produce any symptoms if the patient does not have increased values of pulmonary artery pressure. If moderate or severe TR exists along with pulmonary hypertension, signs and symptoms may include jugular venous distension, visible jugular pulse, palpable hepatomegaly, pulsatile liver, hepatojugular reflux, as well as swelling of the abdomen, feet, and/or ankles. Ascites, important peripheral edema till to anasarcatic state, decreased urine output, and irreversible liver insufficiency usually appear in more advanced stages of disease. Dyspnea, reduced exercise tolerance, and generalized weakness are quite always present but to varying degrees (10-15).
Stages of functional TR
The natural history of functional TR can be schematically divided into stages. The increase in the stage of TR may be influenced by factors such as the presence of RV dilation/dysfunction (tricuspid annular dilation, leaflet tethering), pulmonary hypertension, LV dysfunction, and AF. It is crucial to deal with significant tricuspid annular dilation and leaflet tethering at the time of surgery on the left-sided heart, because if not the functional TR may advance. On the contrary, it is unlikely that tricuspid valve disease will get worse over time in individuals having null or trivial dilation of the tricuspid annulus and leaflet tethering (16,17).
Depending on the degree of tricuspid annular dilation and leaflet tethering, functional TR can be divided into four stages (Table 4) (50,51):
- Stage A: tricuspid annular dilation is absent or mild. Tricuspid leaflet tethering is absent. TR is absent or trivial;
- Stage B: tricuspid annular dilation is moderate. Tricuspid leaflet tethering is mild or moderate. TR is frequently mild or even not present, but may become more severe over time due to changes in RV preload, afterload, and contractility. The patients are asymptomatic.
- Stage C: in this stage both the leaflet tethering and the tricuspid annular dilation are significant. A progressive increase of the following factors such as the dilation of the tricuspid annulus, enlargement of the RV, and inadequate leaflet coaptation, can aid the development of TR. It can develop under all physiological conditions, even if the degree of deterioration of the condition may be affected by the subsequent contributors: RV preload, afterload, and contractility. However, the patients remain asymptomatic, or only mildly symptomatic, due to activation of the cardiovascular compensation mechanisms;
- Stage D: both tricuspid annular dilation and leaflet tethering are severe. Given the attachment of the papillary muscles to the free wall of the RV, severe tethering of the tricuspid leaflets occur in addition to annular dilation and following progressive enlargement of the RV, with regards to its abnormal shape or geometry. TR can also be caused when the anterior and posterior leaflets are pushed further apart and this does not allow coaptation to happen with the septal leaflet and the other leaflet(s). The patients are symptomatic with signs of right-sided heart failure.
Table 4
| Stage | Definition | Valve anatomy | Valve hemodynamics† | Hemodynamic consequences | Symptoms and presentation |
|---|---|---|---|---|---|
| A. | At risk of TR | Normal | No or trace TR | None | None |
| Early annular dilation | |||||
| B. | Progressive TR | Early annular dilation | Central jet area <50% of RA area | None | None |
| Moderate leaflet tethering | Vena contracta width <0.7 cm EROA <0.40 cm2 Regurgitant volume <45 mL |
||||
| C. | Asymptomatic severe TR | Severe annular dilation (≥40 mm or 21 mm/m2) | Central jet area >50% of RA area | RV and RA dilation | Increased venous pressure |
| Marked leaflet tethering | Vena contracta width ≥0.7 cm EROA ≥0.40 cm2 Regurgitant volume ≥45 mL Dense continuous wave signal with triangular shape |
Increased RA pressure with “c-V” wave | No symptoms | ||
| D. | Symptomatic severe TR | Severe annular dilation (≥40 mm or >21 mm/m2) | Central jet area >50% of RA area | RV and RA dilation | Increased venous pressure |
| Marked leaflet tethering | Vena contracta width ≥0.7 cm EROA ≥0.40 cm2 Regurgitant volume ≥45 mL Dense continuous wave signal with triangular shape Hepatic vein systolic flow reversal |
Increased RA pressure with “c-V” wave | Dyspnea on exertion, fatigue, ascites, edema |
†, several valve hemodynamic criteria are provided for assessment of severity of TR, but not all criteria for each category will necessarily be present in every patient. Categorization of severity of TR as mild, moderate, or severe also depends on image quality and integration of these parameters with clinical findings. “c-V” wave, systolic positive wave. TR, tricuspid regurgitation; RA, right atrial; EROA, effective regurgitant orifice area; RV, right ventricular.
Preoperative assessment
Transthoracic echocardiography is the diagnostic imaging modality of first choice to assess severity of TR (grading), which should be based on qualitative, semiquantitative, and quantitative methods. In patients with significant TR, transesophageal approach can confirm etiology of disease and add additional aspects regarding mechanism of regurgitation and probability of treatment strategy success. As TR is sensitive to preload and afterload, respirophasic variability and changes in loading conditions will introduce significant variability in TR severity. To assess TR severity prior to consideration for intervention, it is recommended that the patient be in a euvolemic state, with measurements performed during quiet respirations, and 5–10 beats averaged when the rhythm is irregular. Blood pressure and heart rate should be recorded. The tricuspid annular dilation and leaflet tethering, as well as the pulmonary artery pressure values and the RV and LV function have to be evaluated before surgery. Cardiac magnetic resonance imaging (CMRI), three-dimensional echocardiography, and exercise testing may be useful diagnostic tools to improve evaluation of tricuspid valve as well as of the RV and LV morphology and function (Table 5) (41,51). A 5-grade TR classification based on objective quantitative parameters has been recently introduced by Hahn et al. (41).
Table 5
| Management | 2021 ESC/EACTS Guidelines for the management of VHD (52) | 2020 ACC/AHA Guideline for the management of patients with VHD (51) | 2020 JCS/JSCS/JATS/JSVS Guidelines on the management of VHD (53) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Recommendation | COR | LOE | Recommendation | COR | LOE | Recommendation | COR | LOE | |||
| Diagnosis and follow-up | – | – | – | In patients with TR, TTE is indicated to evaluate the presence and severity of TR, determine the etiology, measure the sizes of the right-sided chambers and inferior vena cava, assess RV systolic function, estimate pulmonary artery systolic pressure, and characterize any associated left-sided heart disease | I | C-LD | TTE is indicated to evaluate the severity of TR, determine etiology, assess RV and RA, estimate pulmonary artery systolic pressure, and assess concomitant left-sided heart disease | I | C | ||
| In patients with TR, invasive measurement of the cardiac index, right-sided diastolic pressures, pulmonary artery pressures, and pulmonary vascular resistance, as well as right ventriculography, can be useful when clinical and noninvasive data are discordant or inadequate | IIa | C-LD | Cardiac catheterization is reasonable for patients with TR to assess pulmonary artery pressures, pulmonary vascular resistance and RA pressure if there is a discrepancy between clinical findings and noninvasive tests | IIa | C | ||||||
| TEE may be considered when TTE images are inadequate | IIb | C | |||||||||
| CMRI or real-time 3D echocardiography may be considered to assess RV volume and systolic function | IIb | C | |||||||||
| Exercise echocardiography or cardiopulmonary exercise test may be considered for patients with severe TR with no or minimal symptoms to assess exercise capacity | IIb | C | |||||||||
| Medical therapy | – | – | – | In patients with signs and symptoms of right-sided HF attributable to severe TR (stages C and D), diuretics can be useful | IIa | C-EO | – | – | – | ||
| In patients with signs and symptoms of right-sided HF attributable to severe secondary TR (stages C and D), therapies to treat the primary cause of HF (e.g., pulmonary vasodilators to reduce elevated pulmonary artery pressures, GDMT for HF with reduced LVEF, or rhythm control of AF) can be useful | IIa | C-EO | |||||||||
| Intervention | Surgery is recommended in patients with severe TR undergoing left-sided valve surgery | I | B | In patients with severe TR (stages C and D) undergoing left-sided valve surgery, TV surgery is recommended | I | B-NR | Concomitant TV repair (or replacement) is recommended for patients with severe TR | I | C | ||
| Surgery should be considered in patients with mild or moderate TR with a dilated annulus (≥40 mm or >21 mm/m2 by 2D echocardiography) undergoing left-sided valve surgery | IIa | B | In patients with progressive TR (stage B) undergoing left-sided valve surgery, TV surgery can be beneficial in the context of either (I) tricuspid annular dilation (tricuspid annulus end-diastolic diameter >4.0 cm) or (II) prior signs and symptoms of right-sided HF | IIa | B-NR | Concomitant TV repair is reasonable for patients with moderate TR | IIa | C | |||
| Surgery should be considered in patients with severe TR (with or without previous left-sided surgery) who are symptomatic or have RV dilatation, in the absence of severe RV or LV dysfunction and severe pulmonary vascular disease/hypertension | IIa | B | In patients with signs and symptoms of right-sided HF and severe isolated secondary TR attributable to annular dilation (in the absence of pulmonary hypertension or left-sided disease) who are poorly responsive to medical therapy (stage D), isolated TV surgery can be beneficial to reduce symptoms and recurrent hospitalizations | IIa | B-NR | Concomitant TV repair may be considered for patients with mild TR and tricuspid annular dilation (≥40 mm or 21 mm/m2) | IIb | C | |||
| Transcatheter treatment of symptomatic severe TR may be considered in inoperable patients at a Heart Valve Centre with expertise in the treatment of tricuspid valve disease | IIb | C | In patients with signs and symptoms of right-sided HF and severe TR (stage D) who have undergone previous left-sided valve surgery, reoperation with isolated TV surgery may be considered in the absence of severe pulmonary hypertension or severe RV systolic dysfunction | IIb | B-NR | Concomitant TV repair may be considered for patients with mild TR and persistent AF | IIb | C | |||
| TV repair (or replacement) is reasonable for patients with severe TR who suffer from recurrent HF despite medical therapy and without either of severe RV dysfunction, irreversible pulmonary hypertension, or irreversible liver dysfunction | IIa | C | |||||||||
| TV repair (or replacement) is reasonable for patients with severe TR who suffer from recurrent HF despite medical therapy | IIa | C | |||||||||
| TV repair may be considered for patients with severe TR and progressive RV dysfunction and/or dilation despite medical therapy regardless of the presence of symptoms | IIb | C | |||||||||
TR, tricuspid regurgitation; ESC/EACTS, European Society of Cardiology/European Association for Cardiothoracic Surgery; VHD, valvular heart disease; ACC/AHA, American College of Cardiology/American Heart Association; JCS/JSCS/JATS/JSVS, Japanese Circulation Society/Japanese Society for Cardiovascular Surgery/Japanese Association for Thoracic Surgery/Japanese Society for Vascular Surgery; COR, class of recommendation; LOE, level of evidence; TTE, transthoracic echocardiography; RV, right ventricular; LD, limited data; RA, right atrial; CMRI, cardiac magnetic resonance imaging; 3D, three-dimensional; HF, heart failure; GDMT, guideline-directed medical therapy; LVEF, left ventricular ejection fraction; AF, atrial fibrillation; EO, expert opinion; 2D, two-dimensional; NR, nonrandomized; TV, tricuspid valve.
Tricuspid annular dilation
The tricuspid annular diameter can be measured by transthoracic echocardiography in a four-chamber view. The starting point to take this measurement is the middle of the septal annulus and it goes up until the middle of the anterior annulus. If the diameter is greater than or equal to 40 mm (or 21 mm/m2) in diastole, it should be considered dilated (18,19,54,55). The tricuspid annular diameter, similarly with TR severity can also be affected by the RV preload, afterload, and contractility.
The diameter of the tricuspid annulus obtained using transthoracic echocardiography differs from the intraoperative surgical measurement. If we proceed in an intraoperative manner, the measurement of the tricuspid annular diameter within a heart at rest is taken. We measure the annular diameter from the anteroseptal commissure to the anteroposterior commissure. Unlike in the case of echocardiographic measurements, this is easily reproducible because it has a fixed dimension and remains unchanged regardless of different physiological conditions. If the diameter is assessed to be greater than 70 mm, the tricuspid annulus should be considered significantly dilated; this fact occurred in 48% of patients undergoing mitral valve surgery in a study by Dreyfus et al. (12). Surgical measurement of the tricuspid annular diameter corresponds to the one obtained by transesophageal echocardiography (transgastric view).
Tricuspid leaflet tethering
Transthoracic echocardiography in a four-chamber view is the optimal method for determining the severity of the tricuspid leaflet tethering. However, transthoracic approach can provide extremely good views also from the parasternal long axis (RV inflow), the modified apical two-chamber view of the right heart, not to mention transesophageal echocardiography. It is crucial to assess the tethering height which is obtained by measuring how far apart the coaptation point of the anterior and septal leaflets (or theoretical coaptation point if no leaflet coaptation is found) is from the plane of the tricuspid annulus. This distance can also be called coaptation depth, or tenting height. Coaptation depth of greater than 8 mm at end-systole is considered significant (6,19).
Indications for surgery
There is a substantial agreement concerning the indications for surgery of functional TR between the 2021 European Society of Cardiology (ESC)/European Association for Cardio-Thoracic Surgery (EACTS), the 2020 American Heart Association (AHA)/American College of Cardiology (ACC), and the 2020 Japanese Circulation Society (JCS)/Japanese Society for Cardiovascular Surgery (JSCS)/Japanese Association for Thoracic Surgery (JATS)/Japanese Society for Vascular Surgery (JSVS) guidelines for the management of valvular heart disease (51-53).
Patients having severe TR and undergoing left-sided valve surgery should have the tricuspid valve operated upon as well. Furthermore, the surgical treatment may also be indicated for lower degrees of TR, and this approach should be well taken into consideration. The level of evidence for this recommendation (to date, IIa for moderate TR, and IIa/IIb for mild TR) is bound to increase. A recently published, randomized controlled trial that explored outcomes at 2 years of patients having degenerative mitral regurgitation combined with moderate TR or less-than-moderate TR with annular dilation, and undergoing mitral valve surgery with or without tricuspid valve annuloplasty, has shown less frequent progression to severe TR in patients who had concomitant tricuspid annuloplasty (56). It is customary to measure the tricuspid annular dilation during left-sided heart surgery using transthoracic echocardiography. If ≥40 mm dilation is noted preoperatively, or >70 mm measured intraoperatively, it is best practice to intervene in these cases as well. In the absence of annular dilation but the presence of documented signs and symptoms of right-sided heart failure, tricuspid valve surgery should be considered also for patients with mild or moderate TR (2020 AHA/ACC guidelines). According to the 2020 JCS/JSCS/JATS/JSVS guidelines, surgery may also be considered in presence of mild TR combined with persistent AF. Reoperations on the tricuspid valve entail very high risk (3), hence why concomitant surgery at the time of the left-sided heart surgery is recommended. Reoperation for isolated, severe TR should be considered in patients with signs and symptoms of progressive RV dilation/dysfunction, provided that there are no severe RV/LV dysfunction, pulmonary hypertension, or irreversible liver dysfunction (2020 JCS/JSCS/JATS/JSVS guidelines). Finally, transcatheter treatment of symptomatic severe TR may be considered in inoperable patients at a Heart Valve Centre with expertise in the treatment of tricuspid valve disease (2021 ESC/EACTS guidelines) (Table 5).
As stated before, it is suggested that a surgical operation on the tricuspid valve should take place if the tricuspid annular diameter detected is higher or equal to 40 mm using transthoracic echocardiography and higher than 70 mm when using an intraoperative approach. Nonetheless, the ideal limit beyond which surgical intervention would be advantageous has yet to be identified and evidence has shown that a lower value should be utilized (7,10,52).
Surgical therapy
The optimal anatomical acquaintance of the entire structure of the tricuspid valve and its morphofunctional relationship with the RV is crucial in approaching tricuspid valve surgery. In the course of tricuspid valve repair operation, the coaptation of the tricuspid valve is usually at, or just below, the annulus plane and the coaptation length is measured at 5 to 10 mm (42). Excessive coaptation length should be taken into account for homograft usage to replace the tricuspid valve (46-48). Likewise, it is important to focus on the fact that the length of the chordae tendinae is fixed. Consequently, the displacement of the ventricular septum or of the RV free wall may affect the coaptation of the tricuspid leaflets. This means that wider coaptation length may function as a ‘reserve’ when performing restrictive annuloplasty. Finally, the network of chordae can meddle with catheters and devices used during the interventional cardiological approach, making a transcatheter tricuspid valve procedure more challenging (Figures 3,4).
When possible, surgical repair of the tricuspid valve should be favored over replacement. A wide area of leaflet coaptation should be the goal of any repair. Another objective is trying to preserve adequate mobility of the leaflet, as well as aiming to bring back the annulus to its original size and geometry.
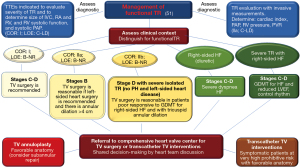
A clinical algorithm for the management of secondary TR based on transthoracic echocardiography is depicted in Figure 5. In brief, assessment of functional TR begins with evaluation of the diseased tricuspid valve and right-sided pressure measurements with transthoracic echocardiography. Following this, severity of disease and the presence of signs and symptoms of heart failure are used to suggest intervention. Lastly, referral to a specialist heart team is recommended for guiding any procedure.
Tricuspid valve annuloplasty
Tricuspid ring annuloplasty is the most common technique of tricuspid valve repair today (20). A 32-mm ring is frequently used for males and a 30-mm ring for females, even though direct sizing would always be preferable (12). The anterior leaflet and a minute part of the posterior share the characteristic that their chordae stem from the same papillary muscle. A preferred manner through which they can be unfolded is by exerting traction on the chordae which support them. It is possible to measure the unfolded leaflets by using a ring sizer.
The most common option is to select a ring of the same measurement. However, if undersizing is preferred, a smaller one is selected. Typically, every tricuspid annuloplasty ring has to be interrupted (it should be called more properly incomplete ring or band) due to the strict topographic interrelation of the tricuspid valve to the atrioventricular node. Since the depth of the tricuspid annulus is greater in the septal annulus than it is in the anterior one, the ideal tricuspid annuloplasty ring (or band) should respect this peculiar morphology and be either flexible [e.g., the Duran AnCore Annuloplasty System (Medtronic Comp.; Brampton, ON, Canada), the Cosgrove-Edwards Annuloplasty System (Edwards Lifesciences Corp., Irvine, CA, USA), and the Koehler Mitral Repair System (Koehler Medical Ltd., Swillington, Leeds, UK)] or semi-rigid, to be manually shaped into this geometry [e.g., the Simulus Semi-Rigid Annuloplasty Band (Medtronic Comp.) and the classic Carpentier-Edwards Tricuspid Annuloplasty Ring (Edwards Lifesciences Corp.)] (9,20,21). A more recent version of the rings has been thought for tricuspid annuloplasty, i.e., the Carpentier-Edwards Physio Tricuspid Annuloplasty Ring (Edwards Lifesciences Corp.), the Edwards MC3 Tricuspid Annuloplasty Ring (Edwards Lifesciences Corp.), and the Contour 3D Tricuspid Annuloplasty Ring (Medtronic Comp.), that have the characteristic of being preemptively shaped into the desired geometry (22-24).
To achieve a reduction annuloplasty, horizontal mattress sutures are the preferred choice. They are positioned commencing from the anteroseptal commissure and in a circular motion that goes up until the anterior and posterior annulus. The middle of the septal annulus is where they finish, right above the coronary sinus, taking great care to avoid the atrioventricular node. The next maneuver is to separate the leaflet from the annulus, by pulling them away from one another; the goal is to provide better clarity on the type of hinge that should be used for the next suture placement. The next step is then to pass the sutures through the annuloplasty ring sewing band while keeping an equal spacing at the anterior and posterior annulus.
The annuloplasty ring has the great task of restoring the dilated tricuspid annulus to its normal size and shape, uniting the leaflets and increasing coaptation, therefore obtaining valve competency. As the annuloplasty ring does not correct leaflet tethering (in fact it increases it), it is unlikely that it may be effective on a long-term basis if it is the only strategy used in dealing with significant leaflet tethering. Therefore, it is necessary to include other repair techniques in the process for having a better chance of increasing the surface of coaptation. Tricuspid valve replacement might be considered in cases of extreme leaflet tethering (25-27,57). Nowadays, suture annuloplasty is less and less frequently adopted. The De Vega repair utilizes a double-pledgeted suture that starts at the anteroseptal commissure, curving right around until the anterior and posterior annulus is reached, and terminating just beyond the posteroseptal commissure. An important part of the procedure is to tie the suture over a sizer, also called an obturator, to reduce the annulus to the desired size (26). This strategy has the advantage of reducing the tricuspid annular size and improving leaflet coaptation. However, the drawback is that its original geometry is never fully recovered.
Tricuspid leaflet augmentation
Recent studies have demonstrated that: (I) annuloplasty on its own is not an effective method in addressing leaflet tethering; (II) when dealing with significant leaflet tethering, it is pivotal to restore the leaflet surface area to provide the right conditions for coaptation yielding the highest possibility of the leaflets overlapping one another; this ensures a correct valve competency (7,25).
According to these two very important concepts, tricuspid leaflet augmentation should be adopted specifically for patients who present with significant leaflet tethering caused by RV dilation and the displacement of the papillary muscle (27,57). This method rectifies the tethering effect of the dilated RV by widening the anterior tricuspid leaflet (and its surface of coaptation) and therefore the coaptation zone is reduced down into the RV, into the level of the restricted posterior and septal leaflets. A preferred choice is the anterior leaflet due to its characteristic of being most often the restricted leaflet. This is caused by the fact that the papillary muscle belonging to it is attached to the anterior wall of the RV. It is also gifted with the property of being the most easily enlargeable.
The anterior leaflet is separated from its annular bond along the entirety of its length. A patch of autologous pericardium is pre-treated with 0.6% glutaraldehyde; it is then cut into an oval shape (this is done for it to avoid shrinkage). The native leaflet is transformed into the new coaptation surface and the pericardial patch develops as the primary body of the leaflet. An important measurement is the height of the patch to be the greatest length that separates the annulus from the detached leaflet. Instead, its diameter is considered by assessing the interval that sets the anteroseptal and the anteroposterior commissure apart. Each commissure, the middle of the anterior annulus, to the detached anterior leaflet, and lastly to the annulus, are all components onto which the patch has to be secured. The chosen suture is a running 5/0 interlocked one. An annuloplasty ring is implanted to strengthen the repair. The autologous pericardial patch has the great advantage of increasing the surface of coaptation by a factor of three, and favors the attainment of leaflet coaptation, free of tension, within the RV, at the septal and posterior leaflet levels (27,57).
Other repair techniques
A repair technique obtains tricuspid valve bicuspidisation through the operation of suturing the anteroposterior and posteroseptal commissure. To do this, a double pledgeted 2/0 ethibond mattress suture is adopted, therefore joining them along the posterior annulus. Once the suture is tied, the effect is to obliterate the posterior leaflet and annulus and reduce the annular size (28).
The clover technique achieves valve continence because it uses a 5/0 polypropylene suture to tie the boundaries of the three leaflets together with one another at their midpoints. The repair is usually aided by an annuloplasty. This technique has been used in cases of severe (organic) TR due to trauma, myxomatous degeneration, or extreme leaflet tethering (29). In this last case, the drawback is that it restricts leaflet motion even more and worsens leaflet tension, and does not alleviate the tethering effect of the dilated RV.
Another repair technique involves the transposition of the papillary muscle from the anterior free wall of the RV to the septum (papillary muscle septalization). It has the same indications as the tricuspid leaflet augmentation and the clover technique (30).
Tricuspid valve replacement
Tricuspid valve replacement is exceptionally used to treat functional TR, usually following previous failed repairs (this fact explains the limited number of studies dealing with tricuspid valve replacement for functional TR, that have been included in the present review). In these very rare cases, biologic valves are universally preferred owing to the increased risk of thrombosis in the right heart due to slower blood flow. However, a mechanical substitute might be considered for young patients in the presence of conditions requiring long-term oral anticoagulation such as permanent AF, a history of pulmonary embolism, or a left-sided mechanical valve. The need, current or future, for an intraventricular pacemaker would contraindicate the choice of a mechanical valve, though epicardial pacemakers can be an alternative option. No significant difference in survival or reoperation rates between the use of mechanical and biological valves in the tricuspid position (31,32,58).
Choice of surgical option based on the stage of functional TR
The choice among all the available surgical techniques for treating functional TR depends on the advancement of the disease. In the so-called stages B and C, where tricuspid annular dilation is present but severe leaflet tethering is absent, the adoption of ring annuloplasty alone can provide good results, although, if necessary, in stage C a more substantial ring undersizing may have to be adopted. In stage D of the disease, where there is a combination of severe tricuspid annular dilation and severe leaflet tethering (coaptation depth >8 mm), the isolated presence of ring annuloplasty is linked to significant recurrent TR; additional techniques such as the anterior leaflet augmentation, the clover technique, or the papillary muscle septalization are necessary to achieve late durability of repair; exceptionally, tricuspid valve replacement also may be carried out. Concomitant ablation of AF may be helpful. Good long-term outcome of concomitant surgery of left-sided heart diseases is a required condition.
Surgical results
In the case of functional TR, a satisfying outcome has been recorded using tricuspid valve annuloplasty in individuals presenting with dilation of the tricuspid annulus and mild-to-moderate TR at the time of mitral valve surgery. In a particular study, it was shown that 97% of individuals with very limited or even completely absent traces of TR had improved symptoms of heart failure six years after their procedure in comparison with patients who had not undergone tricuspid valve repair (12). RV reverse remodeling was provoked by tricuspid valve annuloplasty (16). However, tricuspid annuloplasty is a less successful procedure in patients with severe TR; due to the reappearance of this disease within the range from 15% up to 39% of individuals within the case study (24,32). Risk factors correlated with the lack of success of the operation include preoperative severe TR, tricuspid annular dilation and leaflet tethering, impaired RV and LV function, pulmonary hypertension, and persistent AF, as well as permanent intraventricular pacemaker and suture annuloplasty use (15,25,34-36,51,52). Tang et al. reported a 15-year freedom from recurrent TR of 39%±11%, for suture annuloplasty (De Vega procedure), and 82%±5% for ring annuloplasty (P=0.003) (33). A strong link (P<0.0001) between significant recurrent TR and new left-sided valvular lesions, primarily mitral regurgitation, was also shown (36). Flexible and rigid (or semi-rigid) ring annuloplasty seem to be equally effective in the long-term treatment of functional TR (37,59). Flexible rings could offer specific benefits (over rigid and semi-rigid rings) due to the inherent flexibility and the simpler design and technique of implantation, at least on a speculative basis. First, there is a lower risk of device dehiscence or fracture and tricuspid stenosis, even after undersized annuloplasty. Second, there is virtually no risk of injuring the conduction tissue and the right coronary artery, or the aortic box during implantation within a beating heart. Third, flexible rings could best preserve RV function and help RV functional recovery after surgery. Nevertheless, despite all these benefits, there is no evidence of the superiority of flexible over rigid (or semi-rigid) tricuspid annuloplasty rings. If anything, the opposite may be true. The present authors have shown indeed that there could be two different patterns of RV reverse remodeling, which would be more complete when a rigid ring had been used (37). Even though a more significant undersizing of the annuloplasty ring may affect the durability of the process (38), supplementary repair techniques would be necessary when severe tricuspid leaflet tethering is present. The survival rate is not as good in people developing recurrent TR and the estimated risk of needing an additional operation is very high, up to about 40% (34). While encouraging early results have been achieved using either the tricuspid leaflet augmentation or the clover technique (27,29,57), at least moderate TR has been reported in 25% of patients at 3 years with the suture bicuspidisation technique (28). Only one preliminary clinical experience has been reported to date on papillary muscle septalization (30).
The mortality rate for tricuspid valve replacement is said to be up to 22%. These poor results are primarily connected to the severity of the disease in the patients, in whom multiorgan failure is frequently present. Complete heart block needing a permanent pacemaker is a recognized complication in up to 30% of patients (31,32,58).
Yet, no significant differences in postoperative outcomes have been shown when the surgical technique had been adopted according to a specific preoperative evaluation and decision-making process. This fact, albeit desirable, is very difficult to prove.
Special topics
Predicting mortality in patients with TR
Classic surgical risk stratification tools such as the Society of Thoracic Surgeons (STS) and the European System for Cardiac Operative Risk Evaluation (EuroSCORE) scores fail to predict perioperative mortality if there was evidence of severe liver dysfunction (60). As TR may cause damage to liver and kidney function, the Model for End-Stage Liver Disease excluding international normalized ratio (MELD-XI) and the model with albumin replacing international normalized ratio [Model for End-Stage Liver Disease including albumin (MELD-Albumin)] scores, which include both liver and kidney function indexes, may predict mortality in patients with TR, at least from a theoretical point of view. This concept has been recently confirmed by Lv et al. (61) who have shown that MELD-XI [net reclassification index (95% confidence interval), 0.237 (0.138–0.323)] and MELD-Albumin scores [net reclassification index (95% confidence interval), 0.220 (0.122–0.302)] provided incremental prognostic information, and could play important roles in risk assessment in patients with significant TR.
Recently, two prediction models have been independently devised to stratify preoperatively the risk of mortality and major morbidity for symptomatic patients with severe TR undergoing isolated tricuspid valve surgery. The first model, Clinical Risk Score (62), was developed, using multiple-level mixed effects regression, from the STS database records (2,050 isolated tricuspid valve operations for any etiology performed at 50 hospitals between 2002 and 2014); according to this model (area under the receiver-operating characteristics curve, 0.74), age, sex, stroke, hemodialysis, ejection fraction (EF), lung disease, New York Heart Association class, reoperation, and urgent or emergency status were independent predictors of mortality. The second model, TRI-SCORE (63), was developed, using multivariable logistic regression, from records of 12 French centres (466 isolated tricuspid valve operations for non-congenital etiology performed between 2007 and 2017); age ≥70 years, New York Heart Association class III–IV, right-sided heart failure signs, daily dose of furosemide ≥125 mg, glomerular filtration rate <30 mL/min, elevated bilirubin, LVEF <60%, and moderate/severe RV dysfunction (apparent and bias-corrected areas under the receiver-operating characteristics curves, 0.81 and 0.75, respectively; much higher than the logistic EuroSCORE, 0.67, or EuroSCORE II, 0.63). Both scores were proposed to inform patients and physicians regarding the risk of isolated tricuspid valve surgery and guide the clinical decision-making process of patients with severe TR, especially as transcatheter therapies are emerging.
Residual TR in patients with left ventricular assist device (LVAD)
Preoperative functional TR is frequently present in patients with left cardiomyopathy in need of LV mechanical support. Implantation of an LVAD generally improves TR on a short-term basis. However, the clinical significance of residual TR in LVAD-patients with longer follow-up is unknown. In a study performed by Nakanishi et al. (64) on a cohort of 127 patients who underwent LVAD implantation without tricuspid valve procedure, LVAD implantation significantly improved the ratio of TR color jet area/right atrial area as well as LV and RV systolic function and tethering distance (all P<0.05), whereas it enlarged the tricuspid valve annulus (P=0.002), with significant residual TR observed in 30 (23.6%) patients; age, preoperative tricuspid valve annulus diameter, and residual mitral regurgitation were significantly associated with significant residual TR (all P<0.05), whereas TV tethering was not; during a mean follow-up of 21±17 months, patients with residual TR had significantly higher mortality than those without residual TR (log-rank, P<0.001).
The effect of concomitant tricuspid valve repair/replacement with LVAD implantation has been explored by Fujino et al. (65) in a retrospective observational study on 194 patients (in 108 patients, tricuspid valve surgery was performed in addition to LVAD implantation). Based on the results of this study, concomitant tricuspid valve surgery combined with LVAD implantation improved TR in most patients but did not have an impact on clinical outcomes. Significant postoperative TR after LVAD implantation, in patients with and without tricuspid valve surgery, was associated with worse heart failure-free outcomes.
Uncorrected TR pre-LVAD and post-LVAD are associated with increased early and late mortality. Nevertheless, on average, TR diminishes progressively without intervention after an LVAD implant. Therefore, the patient selection for concomitant tricuspid valve surgery should not be based solely on TR grade (66).
Assessment of functional TR: the role of CMRI
The search for suitable tricuspid transcatheter devices has fueled renewed enthusiasm for the accurate characterization of tricuspid valve disease. CMRI, traditionally used as the gold standard in the assessment of RV size and function, has recently seen its use expanded to assess both the structure and function of the tricuspid apparatus. CMRI has the advantage of high spatial resolution and excellent endocardial border delineation. These aspects are specifically valuable for the assessment of complex RV structure and function. TR can be qualitatively assessed by the signal drop (spin, dephasing) that occurs within areas of non-laminar flow/areas of flow acceleration. Due to only modest correlation with quantitative assessment, grading TR severity by qualitative assessment is limited. From a technical perspective, CMRI is an optimal method for quantitating TR and there is satisfactory accuracy between echocardiographic and CMRI for TR quantification but often a deviation by 1 grade of severity. Intra- and inter-rater reproducibility was demonstrated to be adequate for CMRI assessed regurgitant volume and fraction. One advantage is that the reference stroke volume can be calculated reliably from three different methods (phase contrast imaging from the pulmonic valve or aortic valve, and volumetric LV stroke volume) (41). A recent study has demonstrated the prognostic value of the regurgitant volume and fraction by CMRI regarding subsequent mortality in an all-comer cohort (67). Another important feature of CMRI is tissue characterization. Late gadolinium enhancement and more recently T1 mapping as well as extracellular volume quantitation can provide information about myocardial impairment and fibrotic remodelling. Limitations of CMRI include the presence of arrhythmias and transvalvular pacemaker leads often present in patients with TR. More validation and differences in specific patient cohorts will be mandatory to strengthen the role of CMRI TR quantification in clinical practice (41,53,67,68).
Conclusions
A strict evaluation of the tricuspid valve should be always performed in patients undergoing left-sided valve surgery. In the case of concomitant functional TR, the decision regarding surgical correction and option of treatment depend on TR severity, tricuspid annular dilation, and tricuspid leaflet tethering, as well as on the presence of signs and symptoms of right heart failure and pulmonary hypertension. CMRI, real-time three-dimensional echocardiography, exercise echocardiography, and cardiopulmonary exercise tests may be useful companion tools for standard diagnostics. Lastly, following the relentless progress of technology, the percutaneous techniques for treating TR are gaining increasing success and could be even preferred to surgery for a few specific cases in the near future.
Acknowledgments
The present manuscript is part of the subgroup analyses of the following project: ‘Overall and Cause-Specific Mortality in Randomized/Non-Randomized Clinical Studies Comparing Percutaneous Transcatheter Mitral-Valve Repair versus Surgical Intervention for Secondary Mitral Regurgitation’ (PROSPERO CRD42021112920).
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-661/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-661/coif). GG serves as an unpaid editorial board member of Journal of Thoracic Disease from August 2021 to July 2023. FN serves as an unpaid editorial board member of Journal of Thoracic Disease from August 2021 to July 2023. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nath J, Foster E, Heidenreich PA. Impact of tricuspid regurgitation on long-term survival. J Am Coll Cardiol 2004;43:405-9. [Crossref] [PubMed]
- Koelling TM, Aaronson KD, Cody RJ, et al. Prognostic significance of mitral regurgitation and tricuspid regurgitation in patients with left ventricular systolic dysfunction. Am Heart J 2002;144:524-9. [Crossref] [PubMed]
- Russo M, Di Mauro M, Saitto G, et al. Beating Versus Arrested Heart Isolated Tricuspid Valve Surgery: Long-term Outcomes. Ann Thorac Surg 2022;113:585-92. [Crossref] [PubMed]
- Joudinaud TM, Flecher EM, Duran CM. Functional terminology for the tricuspid valve. J Heart Valve Dis 2006;15:382-8. [PubMed]
- Seccombe JF, Cahill DR, Edwards WD. Quantitative morphology of the normal human tricuspid valve: Autopsy study of 24 cases. Clin Anat 1993;6:203-12. [Crossref]
- Kim HK, Kim YJ, Park JS, et al. Determinants of the severity of functional tricuspid regurgitation. Am J Cardiol 2006;98:236-42. [Crossref] [PubMed]
- Spinner EM, Shannon P, Buice D, et al. In vitro characterization of the mechanisms responsible for functional tricuspid regurgitation. Circulation 2011;124:920-9. [Crossref] [PubMed]
- Martinez RM, O’Leary PW, Anderson RH. Anatomy and echocardiography of the normal and abnormal tricuspid valve. Cardiol Young 2006;16:4-11. [Crossref] [PubMed]
- Fukuda S, Saracino G, Matsumura Y, et al. Three-dimensional geometry of the tricuspid annulus in healthy subjects and in patients with functional tricuspid regurgitation: a real-time, 3-dimensional echocardiographic study. Circulation 2006;114:I492-8. [Crossref] [PubMed]
- Calafiore AM, Gallina S, Iacò AL, et al. Mitral valve surgery for functional mitral regurgitation: should moderate-or-more tricuspid regurgitation be treated? a propensity score analysis. Ann Thorac Surg 2009;87:698-703. [Crossref] [PubMed]
- Song H, Kim MJ, Chung CH, et al. Factors associated with development of late significant tricuspid regurgitation after successful left-sided valve surgery. Heart 2009;95:931-6. [Crossref] [PubMed]
- Dreyfus GD, Corbi PJ, Chan KM, et al. Secondary tricuspid regurgitation or dilatation: which should be the criteria for surgical repair? Ann Thorac Surg 2005;79:127-32. [Crossref] [PubMed]
- Matsunaga A, Duran CM. Progression of tricuspid regurgitation after repaired functional ischemic mitral regurgitation. Circulation 2005;112:I453-7. [Crossref] [PubMed]
- Di Mauro M, Bivona A, Iacò AL, et al. Mitral valve surgery for functional mitral regurgitation: prognostic role of tricuspid regurgitation. Eur J Cardiothorac Surg 2009;35:635-9; discussion 639-40. [Crossref] [PubMed]
- Yilmaz O, Suri RM, Dearani JA, et al. Functional tricuspid regurgitation at the time of mitral valve repair for degenerative leaflet prolapse: the case for a selective approach. J Thorac Cardiovasc Surg 2011;142:608-13. [Crossref] [PubMed]
- Van de Veire NR, Braun J, Delgado V, et al. Tricuspid annuloplasty prevents right ventricular dilatation and progression of tricuspid regurgitation in patients with tricuspid annular dilatation undergoing mitral valve repair. J Thorac Cardiovasc Surg 2011;141:1431-9. [Crossref] [PubMed]
- Kwak JJ, Kim YJ, Kim MK, et al. Development of tricuspid regurgitation late after left-sided valve surgery: a single-center experience with long-term echocardiographic examinations. Am Heart J 2008;155:732-7. [Crossref] [PubMed]
- Colombo T, Russo C, Ciliberto GR, et al. Tricuspid regurgitation secondary to mitral valve disease: tricuspid annulus function as guide to tricuspid valve repair. Cardiovasc Surg 2001;9:369-77. [Crossref] [PubMed]
- Mutlak D, Aronson D, Lessick J, et al. Functional tricuspid regurgitation in patients with pulmonary hypertension: is pulmonary artery pressure the only determinant of regurgitation severity? Chest 2009;135:115-21. [Crossref] [PubMed]
- Gatti G, Maffei G, Lusa AM, et al. Tricuspid valve repair with the Cosgrove-Edwards annuloplasty system: early clinical and echocardiographic results. Ann Thorac Surg 2001;72:764-7. [Crossref] [PubMed]
- Gatti G, Marcianò F, Antonini-Canterin F, et al. Tricuspid valve annuloplasty with a flexible prosthetic band. Interact Cardiovasc Thorac Surg 2007;6:731-5. [Crossref] [PubMed]
- Fukuda S, Gillinov AM, McCarthy PM, et al. Echocardiographic follow-up of tricuspid annuloplasty with a new three-dimensional ring in patients with functional tricuspid regurgitation. J Am Soc Echocardiogr 2007;20:1236-42. [Crossref] [PubMed]
- De Bonis M, Lapenna E, Taramasso M, et al. Mid-term results of tricuspid annuloplasty with a three-dimensional remodelling ring. J Card Surg 2012;27:288-94. [Crossref] [PubMed]
- Ratschiller T, Guenther T, Guenzinger R, et al. Early experiences with a new three-dimensional annuloplasty ring for the treatment of functional tricuspid regurgitation. Ann Thorac Surg 2014;98:2039-44. [Crossref] [PubMed]
- Min SY, Song JM, Kim JH, et al. Geometric changes after tricuspid annuloplasty and predictors of residual tricuspid regurgitation: a real-time three-dimensional echocardiography study. Eur Heart J 2010;31:2871-80. [Crossref] [PubMed]
- Rabago G, De Vega NG, Castillon L, et al. The new De Vega technique in tricuspid annuloplasty (results in 150 patients). J Cardiovasc Surg (Torino) 1980;21:231-8. [PubMed]
- Dreyfus GD, Raja SG, John Chan KM. Tricuspid leaflet augmentation to address severe tethering in functional tricuspid regurgitation. Eur J Cardiothorac Surg 2008;34:908-10. [Crossref] [PubMed]
- Ghanta RK, Chen R, Narayanasamy N, et al. Suture bicuspidization of the tricuspid valve versus ring annuloplasty for repair of functional tricuspid regurgitation: midterm results of 237 consecutive patients. J Thorac Cardiovasc Surg 2007;133:117-26. [Crossref] [PubMed]
- De Bonis M, Lapenna E, La Canna G, et al. A novel technique for correction of severe tricuspid valve regurgitation due to complex lesions. Eur J Cardiothorac Surg 2004;25:760-5. [Crossref] [PubMed]
- Couetil JP, Nappi F, Spadaccio C, et al. Papillary muscle septalization for functional tricuspid regurgitation: Proof of concept and preliminary clinical experience. JTCVS Tech 2021;10:282-8. [Crossref] [PubMed]
- Do QB, Pellerin M, Carrier M, et al. Clinical outcome after isolated tricuspid valve replacement: 20-year experience. Can J Cardiol 2000;16:489-93. [PubMed]
- Filsoufi F, Anyanwu AC, Salzberg SP, et al. Long-term outcomes of tricuspid valve replacement in the current era. Ann Thorac Surg 2005;80:845-50. [Crossref] [PubMed]
- Tang GH, David TE, Singh SK, et al. Tricuspid valve repair with an annuloplasty ring results in improved long-term outcomes. Circulation 2006;114:I577-81. [Crossref] [PubMed]
- Fukuda S, Gillinov AM, McCarthy PM, et al. Determinants of recurrent or residual functional tricuspid regurgitation after tricuspid annuloplasty. Circulation 2006;114:I582-7. [Crossref] [PubMed]
- McCarthy PM, Bhudia SK, Rajeswaran J, et al. Tricuspid valve repair: durability and risk factors for failure. J Thorac Cardiovasc Surg 2004;127:674-85. [Crossref] [PubMed]
- Gatti G, Dell’Angela L, Morosin M, et al. Tricuspid Annuloplasty for Tricuspid Regurgitation Secondary to Left-Sided Heart Valve Disease: Immediate Outcomes and Risk Factors for Late Failure. Can J Cardiol 2016;32:760-6. [Crossref] [PubMed]
- Gatti G, Dell’Angela L, Morosin M, et al. Flexible band versus rigid ring annuloplasty for functional tricuspid regurgitation: two different patterns of right heart reverse remodelling. Interact Cardiovasc Thorac Surg 2016;23:79-89. [Crossref] [PubMed]
- Ghoreishi M, Brown JM, Stauffer CE, et al. Undersized tricuspid annuloplasty rings optimally treat functional tricuspid regurgitation. Ann Thorac Surg 2011;92:89-95; discussion 96. [Crossref] [PubMed]
- Xanthos T, Dalivigkas I, Ekmektzoglou KA. Anatomic variations of the cardiac valves and papillary muscles of the right heart. Ital J Anat Embryol 2011;116:111-26. [PubMed]
- Hahn RT. State-of-the-Art Review of Echocardiographic Imaging in the Evaluation and Treatment of Functional Tricuspid Regurgitation. Circ Cardiovasc Imaging 2016;9:e005332. [Crossref] [PubMed]
- Hahn RT, Badano LP, Bartko PE, et al. Tricuspid regurgitation: recent advances in understanding pathophysiology, severity grading and outcome. Eur Heart J Cardiovasc Imaging 2022;23:913-29. [Crossref] [PubMed]
- Silver MD, Lam JH, Ranganathan N, et al. Morphology of the human tricuspid valve. Circulation 1971;43:333-48. [Crossref] [PubMed]
- Rogers JH, Bolling SF. The tricuspid valve: current perspective and evolving management of tricuspid regurgitation. Circulation 2009;119:2718-25. [Crossref] [PubMed]
- Messer S, Moseley E, Marinescu M, et al. Histologic analysis of the right atrioventricular junction in the adult human heart. J Heart Valve Dis 2012;21:368-73. [PubMed]
- Tretter JT, Sarwark AE, Anderson RH, et al. Assessment of the anatomical variation to be found in the normal tricuspid valve. Clin Anat 2016;29:399-407. [Crossref] [PubMed]
- Nappi F, Nenna A, Petitti T, et al. Long-term outcome of cryopreserved allograft for aortic valve replacement. J Thorac Cardiovasc Surg 2018;156:1357-65.e6. [Crossref] [PubMed]
- Nappi F, Spadaccio C, Acar C. Use of allogeneic tissue to treat infective valvular disease: Has everything been said? J Thorac Cardiovasc Surg 2017;153:824-8. [Crossref] [PubMed]
- Olivito S, Lalande S, Nappi F, et al. Structural deterioration of the cryopreserved mitral homograft valve. J Thorac Cardiovasc Surg 2012;144:313-20, 320.e1.
- Karas S Jr, Elkins RC. Mechanism of function of the mitral valve leaflets, chordae tendineae and left ventricular papillary muscles in dogs. Circ Res 1970;26:689-96. [Crossref] [PubMed]
- Dreyfus GD, Chan KM. Functional tricuspid regurgitation: a more complex entity than it appears. Heart 2009;95:868-9. [Crossref] [PubMed]
- Writing Committee Members. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Thorac Cardiovasc Surg 2021;162:e183-353. [Crossref] [PubMed]
- Vahanian A, Beyersdorf F, Praz F, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur J Cardiothorac Surg 2021;60:727-800. [Crossref] [PubMed]
- Izumi C, Eishi K, Ashihara K, et al. JCS/JSCS/JATS/JSVS 2020 Guidelines on the Management of Valvular Heart Disease. Circ J 2020;84:2037-119. [Crossref] [PubMed]
- Sugimoto T, Okada M, Ozaki N, et al. Long-term evaluation of treatment for functional tricuspid regurgitation with regurgitant volume: characteristic differences based on primary cardiac lesion. J Thorac Cardiovasc Surg 1999;117:463-71. [Crossref] [PubMed]
- Ubago JL, Figueroa A, Ochoteco A, et al. Analysis of the amount of tricuspid valve anular dilatation required to produce functional tricuspid regurgitation. Am J Cardiol 1983;52:155-8. [Crossref] [PubMed]
- Gammie JS, Chu MWA, Falk V, et al. Concomitant Tricuspid Repair in Patients with Degenerative Mitral Regurgitation. N Engl J Med 2022;386:327-39. [Crossref] [PubMed]
- Chan KMJ, Dreyfus GD. Tricuspid valve surgery. In: Moorjani N, Ohri SK, Wechsler AS. editors. Cardiac Surgery: Recent Advances and Techniques. 1st edition. Boca Raton, FL, USA: CRC Press Taylor & Francis Group, LLC, 2014:67-75.
- Rizzoli G, Vendramin I, Nesseris G, et al. Biological or mechanical prostheses in tricuspid position? A meta-analysis of intra-institutional results. Ann Thorac Surg 2004;77:1607-14. [Crossref] [PubMed]
- Zhu TY, Wang JG, Meng X. Is a rigid tricuspid annuloplasty ring superior to a flexible band when correcting secondary tricuspid regurgitation? Interact Cardiovasc Thorac Surg 2013;17:1009-14. [Crossref] [PubMed]
- Färber G, Marx J, Scherag A, et al. Risk stratification for isolated tricuspid valve surgery assisted using the Model for End-Stage Liver Disease score. J Thorac Cardiovasc Surg 2022; Epub ahead of print. [Crossref] [PubMed]
- Lv J, Ye Y, Li Z, et al. Prognostic Value of Modified Model for End-Stage Liver Disease Scores in Patients With Significant Tricuspid Regurgitation. Eur Heart J Qual Care Clin Outcomes 2022; Epub ahead of print. [Crossref] [PubMed]
- LaPar DJ, Likosky DS, Zhang M, et al. Development of a Risk Prediction Model and Clinical Risk Score for Isolated Tricuspid Valve Surgery. Ann Thorac Surg 2018;106:129-36. [Crossref] [PubMed]
- Dreyfus J, Audureau E, Bohbot Y, et al. TRI-SCORE: a new risk score for in-hospital mortality prediction after isolated tricuspid valve surgery. Eur Heart J 2022;43:654-62. [Crossref] [PubMed]
- Nakanishi K, Homma S, Han J, et al. Prevalence, Predictors, and Prognostic Value of Residual Tricuspid Regurgitation in Patients With Left Ventricular Assist Device. J Am Heart Assoc 2018;7:008813. [Crossref] [PubMed]
- Fujino T, Imamura T, Nitta D, et al. Effect of Concomitant Tricuspid Valve Surgery With Left Ventricular Assist Device Implantation. Ann Thorac Surg 2020;110:918-24. [Crossref] [PubMed]
- Veen KM, Mokhles MM, Soliman O, et al. Clinical impact and ‘natural’ course of uncorrected tricuspid regurgitation after implantation of a left ventricular assist device: an analysis of the European Registry for Patients with Mechanical Circulatory Support (EUROMACS). Eur J Cardiothorac Surg 2021;59:207-16. [Crossref] [PubMed]
- Wang TKM, Akyuz K, Reyaldeen R, et al. Prognostic Value of Complementary Echocardiography and Magnetic Resonance Imaging Quantitative Evaluation for Isolated Tricuspid Regurgitation. Circ Cardiovasc Imaging 2021;14:e012211. [Crossref] [PubMed]
- Hinojar R, Gómez AG, García-Martin A, et al. Impact of right ventricular systolic function in patients with significant tricuspid regurgitation. A cardiac magnetic resonance study. Int J Cardiol 2021;339:120-7. [Crossref] [PubMed]