
Extended use of rh-endostatin improves prognosis in patients with advanced non-small cell lung cancer: an analysis of retrospective study
Introduction
Lung cancer ranks first in incidence and mortality rates among malignant tumors worldwide, of which non-small cell lung cancer (NSCLC) is the most common type, accounting for 75–80% of all the lung cancer patients (1). Most patients are at an advanced stage (stage IIIB/IV) when they are diagnosed, and according to current guidelines, first-line therapy is platinum-base doublet chemotherapy, especially for those not harboring any driver-gene mutations (2-4).
It has been well established that angiogenesis plays a critical role in the process of tumorigenesis (5-7). Several agents, including bevacizumab, nintedanib, and ramucirumab, targeting VEGFR-1/2 have been demonstrated in the therapy of NSCLC (8-10). In some landmark clinical trials, the addition of angiogenic inhibitors to chemotherapy significantly improved survival. For example, in the E4599 trial, bevacizumab in combination with paclitaxel and carboplatin as first-line therapy in non-squamous NSCLC patients prolonged overall survival (OS) and progression-free survival (PFS) by 2 and 1.7 months, respectively (8). Unfortunately, the severe treatment-related hemorrhage rate in this trial was 4.4%, including 5 cases of fatal lung hemoptysis. In addition, squamous cell NSCLC is more prone to risk of bleeding compared to adenocarcinoma because it is more frequently centrally located and has a greater tendency to cavitate. In the trial conducted by Johnson et al., which included 20 squamous cell carcinoma (SCC) patients receiving paclitaxel, carboplatin and bevacizumab, 4 patients suffered fatal massive hemoptysis (11).
Hence, there is an urgent need for the development of a new therapeutic combination with improved efficacy and acceptable toxicity. Recombinant human endostatin (rh-endostatin) is a type of targeted agent capable of inhibiting neovascularization. It has a C-terminal fragment type of XVIII collagen which could bind to neo-capillary endothelial cells in a tumor directly (12). The randomized double-blind phase 3 clinical trial confirmed that time to progression was significantly increased in NSCLC patients receiving rh-endostatin plus vinorelbine/cisplatin (6.3 vs. 3.6 months, P<0.001) (13). A growing amount of data from real-world studies has shown an efficacy benefit with quality of life improving in these patients (14,15). Of note, several studies have reported a more favorable safety profile for patients with SCC (14-16). Therefore, it is feasible that long-term use of endostatin will be more practicable. However, although many clinical studies of rh-endostatin have reported a significant survival increase, most patients of which were treated with fewer than 4 cycles in first-line therapy (17,18). Up to now, we are not sure whether long term use of re-endostatin with or without chemotherapy could improve prognosis in maintenance therapy and second- or later-line until disease progression or intolerable toxicity as those reported in E4599 trial.
In this retrospective study, we evaluated the efficacy and safety of long-term and extended use of rh-endostatin with platinum-based chemotherapy for advanced squamous and non-squamous NSCLC patients after a long follow-up. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1292/rc).
Methods
Patients
We conducted a retrospective analysis of data screened between January 2008 and June 2018 from a lung cancer database in Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (Wuhan, China). The eligibility inclusion criteria were as follows: pathologically or cytologically confirmed NSCLC; Locally advanced or metastastic disease (IIIB or IV) defined by the AJCC v7.0; newly diagnosed without systematic anticancer treatment previously; ECOG PS (Eastern Cooperative Oncology Group performance status) score 0–1; having ≥1 measurable disease lesion according to RECIST (Response Evaluation Criteria in Solid Tumors) v1.1; and having adequate hepatic, renal and bone marrow functions. The exclusion criteria were as follows: patients with rapid progression of disease (PD) or intolerable toxicities during treatment, breast-feeding or pregnant women, and incomplete clinical data for analysis. The primary endpoints were PFS and OS, while secondary endpoints were ORR (objective response rate), DCR (disease control rate) and safety.
This study was conducted in compliance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics board of Tongji Medical College, Huazhong University of Science and Technology (No. 2019S121) and individual consent for this retrospective analysis was waived.
Treatment
All eligible patients were treated with at least 2-cycle platinum-based doublet chemotherapy as well as rh-endostatin for at least 1 cycle every 21 or 28 days. The chemotherapy regimens for patients included pemetrexed and cisplatin (DDP), gemcitabine and DDP, paclitaxel and carboplatin, docetaxel and DDP, irinotecan (CPT-11) and DDP, pemetrexed and nedaplatin (NDP), and gemcitabine and NDP. Pemetrexed was administered intravenously at 500 mg/m2 on day 1; gemcitabine was administered intravenously at 1,250 mg/m2 on days 1 and 8; paclitaxel was administered intravenously at 150–175 mg/m2 on day 1; docetaxel was administered intravenously at 75 mg/m2 on day 1; and CPT-11 was administered intravenously at 65 mg/m2 on days 1, 8, and 15. DDP and NDP were administered intravenously at 25 mg/m2 on days 1–3 or 75 mg/m2 on day 1, and carboplatin was administered intravenously at the dosage reaching an area under the curve (AUC) of 5 or 6 (AUC =5 or 6 mg/mL/min) on day 1.
Endostatin (Simcere Pharmaceutical Group, Nanjing, China) was infused intravenously at 7.5 mg/m2 over 3 hours, once daily for 14 days, or 15 mg/m2 over 3 hours, once daily for 7 days, along with the chemotherapy. Continued use of endostatin with or without chemotherapy as second- or later-line therapy was acceptable after PD following the first-line regimens above.
Trial design and treatment evaluation
Patients were assigned to 1 of 2 groups for analysis in this study. Patients who received ≥4 cycles of rh-endostatin were designated as the extended treatment group, while those who received <4 cycles were designated as the non-extended treatment group.
Imaging evaluation by computed tomography (CT) scan was conducted at the start of chemotherapy and then every 2 cycles of chemotherapy to evaluate tumor response by RECIST v1.1. The evaluable patients were defined as those treating with ≥2 cycles chemotherapy and having ≥1 measurable tumor lesion.
The basic clinical characteristics of the 2 groups were compared, including age, gender, ECOG PS score, disease stage, pathological subtype, and smoking status. Efficacy outcomes were recorded in terms of ORR, DCR, PFS, and OS. ORR was defined as the percentage of patients who had a tumor complete response (CR) or partial response (PR). DCR was defined as the percentage of patients who had CR/PR or stable disease (SD). The best overall response was defined as the best response recorded from the start of treatment until PD or withdrawal from treatment. PFS was measured as the interval between the date of first treatment dose and the date of PD or intolerable toxicity. OS was measured as the interval between the date of first treatment dose and the date of death or last follow-up. Patients were excluded from the study if they were treated or followed up for fewer than 6 months.
Safety assessment included adverse event documents, physical examinations, and laboratory abnormalities, which were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.0.
Statistical analysis
Survival analysis was estimated using the Kaplan-Meier method, and differences based on treatment were compared with the log-rank test. A Cox proportional hazards regression model was used in multivariate analyses of PFS and OS to test the effect of independent variables, including age, gender, ECOG PS, smoking status, stage, histology, and rh-endostatin cycles. Statistical analyses were performed using SPSS version 20 (IBM Corp., Armonk, NY, USA). All tests were 2-sided, and a P value <0.05 was considered statistically significant.
Detecting bias can influence prognosis in patients using extended endostatin who have better treatment compliance. However, we could not tell whether these patients were benefiting from endostatin or other factors from before this study.
Assuming a median OS for metastatic NSCLC of 6–9 months and a hazard ratio (HR) <0.66, we needed about 100 patients in total to achieve 80% statistical power and 5% significance.
Results
Patient characteristics
A total of 115 patients were enrolled in this study, in which 105 patients were eligible for analysis, and 10 patients didn’t have the follow-up report needed. The median (range) number of cycles of chemotherapy with rh-endostatin was 4 [2–19]. Thirty-three patients received rh-endostatin for first-line therapy as well as second- or later-line therapy. Baseline characteristics and demographics were similar between the extended group (n=56) and nonextended group (n=49) (Table 1). The mean age was 57.9 and 56.8 years in the extended and nonextended groups, respectively. There were more male patients in the extended group than in the nonextended group (75.0% vs. 67.3%), but the difference was not statistically significant. The proportion of patients with squamous cell carcinoma in the 2 groups was 48.2% and 42.9%, respectively, and the majority of patients in both groups had stage IV disease and good performance (PS score 0–1). A total of 44.6% patients in the extended group and 46.9% in the nonextended group were smokers.
Table 1
| Characteristic | Extended group (n=56) | Nonextended group (n=49) | P |
|---|---|---|---|
| Age, years, n (%) | 0.688 | ||
| ≤60 | 36 (64.3) | 29 (59.2) | |
| >60 | 20 (35.7) | 20 (40.8) | |
| Gender, n (%) | 0.397 | ||
| Male | 42 (75.0) | 33 (67.3) | |
| Female | 14 (25.0) | 16 (32.7) | |
| Smoking status, n (%) | 0.846 | ||
| Current/former smoker | 25 (44.6) | 23 (46.9) | |
| Nonsmoker | 31 (55.4) | 26 (53.1) | |
| ECOG PS score, n (%) | 1.000 | ||
| 0–1 | 39 (69.6) | 34 (69.4) | |
| 2 | 17 (30.4) | 15 (30.6) | |
| Pathology, n (%) | 0.695 | ||
| Squamous cell carcinoma | 27 (48.2) | 21 (42.9) | |
| Adenocarcinoma and others | 29 (51.8) | 28 (57.1) | |
| Disease stage, n (%) | 0.528 | ||
| IIIb | 15 (26.8) | 16 (32.7) | |
| IV | 41 (73.2) | 33 (67.3) | |
| Previous thoracic surgery, n (%) | 0.215 | ||
| Yes | 15 (26.8) | 19 (38.8) | |
| No | 41 (73.2) | 30 (61.2) |
ECOG PS, Eastern Cooperative Oncology Group performance status.
Efficacy
Survival outcome data were cut off on January 1, 2020. After a median follow-up time of 12.7 months (range, 9.4–32.1 months), 82 (78.1%) patients experienced disease progression (PD) or died. Kaplan-Meier curves for PFS and OS were shown in Figure 1. The median [95% confidence interval (CI)] PFS in the extended and nonextended groups was 8.2 (4.2–12.3) vs. 3.2 (1.3–5.0) months (HR =0.50, 95% CI: 0.33–0.78, P=0.002) and the median (95% CI) OS in the 2 groups was 25.1 (20.5–29.7) vs. 14.0 (9.2–18.8) months (HR =0.50, 95% CI: 0.32–0.79, P=0.003), respectively. The median (range) OS for patients receiving rh-endostatin both in first- and later-line therapy was 25.3 (19.4–30.5) months.
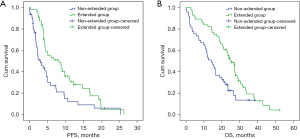
Among all the patients and therapy characteristics, univariate multivariate analysis found that only extended use of rh-endostatin (≥4 cycles) was significantly correlated with better PFS and OS (Table 2).
Table 2
| Characteristic | OS | PFS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Multivariate analysis | Univariate analysis | Univariate analysis | Multivariate analysis | ||||||||
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | ||||
| Age, years | |||||||||||
| ≤60 | 1 | 1 | 1 | 1 | |||||||
| >60 | 0.769 (0.482–1.227) | 0.271 | 0.798 (0.501–1.269) | 0.340 | 0.804 (0.511–1.265) | 0.345 | 0.825 (0.515–1.321) | 0.422 | |||
| Gender | |||||||||||
| Male | 1 | 1 | 1 | 1 | |||||||
| Female | 0.881 (0.458–1.694) | 0.703 | 1.061 (0.661–1.703) | 0.806 | 0.960 (0.594–1.550) | 0.886 | 0.928 (0.485–1.773) | 0.821 | |||
| Smoking status | |||||||||||
| Former/nonsmoker | 1 | 1 | 1 | 1 | |||||||
| Current smoker | 0.991 (0.557–1.762) | 0.975 | 1.097 (0.709–1.697) | 0.678 | 1.241 (0.800–1.925) | 0.335 | 1.196 (0.653–2.189) | 0.563 | |||
| ECOG PS score | |||||||||||
| 0–1 | 1 | 1 | 1 | 1 | |||||||
| 1 | 1.358 (0.812–2.271) | 0.243 | 1.223 (0.763–1.961) | 0.403 | 0.945 (0.581–1.538) | 0.820 | 1.095 (0.629–1.904) | 0.749 | |||
| Pathology | |||||||||||
| Squamous cell | 1 | 1 | 1 | 1 | |||||||
| Adenocarcinoma and others | 0.759 (0.469–1.228) | 0.261 | 0.923 (0.595–1.433) | 0.721 | 0.912 (0.586–1.419) | 0.682 | 0.758 (0.464–1.239) | 0.269 | |||
| Disease stage | |||||||||||
| IIIb | 1 | 1 | 1 | 1 | |||||||
| IV | 1.097 (0.650–1.852) | 0.729 | 0.954 (0.577–1.577) | 0.853 | 1.018 (0.615–1.683) | 0.946 | 1.109 (0.639–1.927) | 0.712 | |||
| Previous thoracic surgery | |||||||||||
| No | 1 | 1 | 1 | 1 | |||||||
| Yes | 0.854 (0.512–1.425) | 0.545 | 1.007 (0.631–1.607) | 0.976 | 0.755 (0.462–1.233) | 0.261 | 0.623 (0.366–1.063) | 0.082 | |||
| Extended treatment of rh-endostatin | |||||||||||
| No | 1 | 1 | 1 | 1 | |||||||
| Yes | 0.438 (0.268–0.717) | 0.001 | 0.501 (0.320–0.785) | 0.003 | 0.504 (0.325–0.784) | 0.002 | 0.463 (0.290–0.740) | 0.001 | |||
OS, overall survival; PFS, progression-free-survival; HR, hazard ratio; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status.
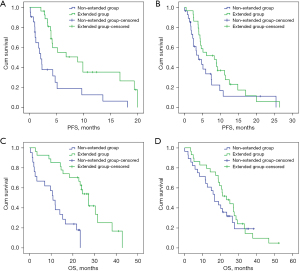
Patients with squamous cell cancers (n=48) significantly benefited from the extended use of rh-endostatin (≥4 cycles); the median (95% CI) PFS and OS were 8.9 (4.0–13.8) vs. 2.0 (1.3–2.9) months (HR =0.33, 95% CI: 0.16–0.64, P=0.001) and 27.2 (23.1–31.3) vs. 10.8 (7.6–11.1) months in the extended and nonextended groups, respectively (HR =0.20, 95% CI: 0.09–0.46, P=0.001). However, there was no significant survival increase in patients with adenocarcinoma (n=57); the median (95% CI) PFS and OS were 8.2 (2.0–14.5) vs. 3.8 (1.6–6.1) months (HR =0.612, 95% CI: 0.338–1.109, P=0.105) and 23.3 (16.2–30.4) vs. 16.6 (11.4–21.7) months, respectively (HR =0.767, 95% CI: 0.424–1.384, P=0.378) (Figure 2).
The ORR and DCR were 23.2% and 92.9% in the extended group, respectively, and 14.3% and 81.6% in the non-extended group, respectively, and no statistical difference was found (P=0.25 and P=0.08 for ORR and DCR, respectively). Similar response outcomes in women, non-smokers, stage IIIB or IV disease, and ECOG PS 0–1 were found between the 2 groups (Table 3).
Table 3
| Efficacy parameter | Extended group (n=56) | Nonextended group (n=49) | P |
|---|---|---|---|
| CR | 0 | 0 | |
| PR | 13 | 7 | |
| SD | 39 | 33 | |
| PD | 4 | 9 | |
| ORR | 13 (23.2%) | 7 (14.3%) | 0.25 |
| DCR | 52 (92.9%) | 40 (81.6%) | 0.08 |
NSCLC, non-small cell lung cancer; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease. ORR, objective response rate; DCR, disease control rate.
Safety
The incidence of therapy-related adverse events (AE) for the whole patients in this study is listed in Table 4. No difference was found between the extended and non-extended groups with respect to the frequency of all AEs (100% vs. 95.9%; P=0.17) or grade 3–4 AEs (12.5% vs. 12.2%; P=0.97). Transaminase increase, hematological and gastrointestinal toxicity (nausea and vomiting) were the most common drug-related AEs, in which hematologic and gastrointestinal toxicities occurred more frequently in the extended group but was not significantly different from those in the nonextended group. A relatively higher rate of hypertension was observed in the extended group (12.5% vs. 6.1% for all grades), with only 1 case suffering severe hypertension in the whole population.
Table 4
| AEs | All | Grade 3–4 | |||||
|---|---|---|---|---|---|---|---|
| Extended group (n=56) | Nonextended group (n=49) | P | Extended group (n=56) | Nonextended group (n=49) | P | ||
| Any, n (%) | 56 (100.0) | 47 (95.9) | 0.17 | 7 (12.5) | 6 (12.2) | 0.97 | |
| Hematological toxicity, n (%) | |||||||
| Myelosuppression | 55 (98.2) | 44 (89.8) | 0.06 | 6 (10.7) | 4 (8.2) | 0.66 | |
| Thrombocytopenia | 10 (17.9) | 9 (18.4) | 0.95 | 2 (3.6) | 1 (2.0) | 0.64 | |
| Hemoglobin reduction | 12 (21.4) | 7 (14.3) | 0.34 | 1 (1.8) | 1 (2.0) | 0.92 | |
| Nonhematological toxicity, n (%) | |||||||
| Increased transaminase | 11 (19.6) | 14 (28.6) | 0.28 | 0 | 1 (2.0) | 0.28 | |
| Hypertension | 7 (12.5) | 3 (6.1) | 0.27 | 1 (1.8) | 0 | 0.35 | |
| Cardiotoxicity | 4 (7.1) | 2 (4.1) | 0.50 | 0 | 0 | ||
| Rash | 5 (8.9) | 4 (8.2) | 0.89 | 0 | 0 | ||
| Nausea | 55 (98.2) | 48 (98.0) | 0.92 | 2 (3.6) | 1 (2.0) | 0.64 | |
| Vomiting | 48 (85.7) | 37 (75.5) | 0.18 | 1 (1.8) | 1 (2.0) | 0.92 | |
| Fatigue | 12 (21.4) | 10 (20.4) | 0.90 | 0 | 0 | ||
| Neurotoxicity | 8 (14.3) | 5 (10.2) | 0.53 | 0 | 0 | ||
| Renal dysfunction | 3 (5.4) | 1 (2.0) | 0.38 | 0 | 0 | ||
AEs, adverse events.
Discussion
The antiangiogenic inhibitor rh-endostatin combined with chemotherapy drugs can increase efficacy in advanced NSCLC and may improve the quality of life of patients without triggering more adverse events. In this study, we found that the extended use of rh-endostatin (≥4 cycles) was significantly correlated with PFS and OS in adenocarcinoma as well as squamous cell NSCLC patients, and no severe toxicities, including hemoptysis, were reported after a long follow-up.
As a novel antiangiogenic agent approved in China for the treatment of NSCLC, rh-endostatin possesses the function of impeding angiogenesis by inhibiting the expression of VEGF and the activity of proteolytic enzymes. Rh-endostatin has been reported to have several mechanisms, including organizing neovascularization, conducive to chemotherapy drugs entering into tumor cells, inducing apoptosis of endothelial cells, and affecting nutrient supply to tumors and inhibiting the growth of tumors and micrometastases (12,19,20). Meta-analysis studies have revealed that in advanced NSCLC patients, the ORR and DCR of rh-endostatin plus chemotherapy were significantly higher than those of chemotherapy alone, with 14.7% and 13.5% improvement, respectively (P<0.001) (16,21). Moreover, in a phase 3 randomized trial, the addition of 4 cycles of rh-endostatin was correlated with higher rate of TTP and OS compared to chemotherapy alone (median 6.61 vs. 3.65 months (P<0.001) and median 13.75 vs. 9.77 months (P<0.001), respectively), regardless of histological subtype (13,22).
We first reported the findings of our study that long-term (≥4 cycles) use of angiogenesis inhibitors rh-endostatin could play a significant role for NSCLC patients at the 2019 American Society of Clinical Oncology (ASCO) meeting. It was notable that PFS and OS were significantly increased for the extended rh-endostatin group versus the nonextended group (8.2 vs. 3.2 for median PFS, P=0.002; and 25.1 vs. 14.0 for median OS, P=0.003) after a long follow-up. These survival data are comparable with those from well-known randomized trials using bevacizumab in non-squamous NSCLC patients (23,24). In the E4599 study (8), the addition of bevacizumab to paclitaxel plus carboplatin significantly increased OS (12.3 vs. 10.3 months, HR =0.79, P=0.003) and PFS (6.2 vs. 4.5, HR =0.66, P<0.001). The BEYOND study (25) showed that OS could be prolonged to even 24.3 months in patients treated with carboplatin + paclitaxel and continuous bevacizumab as first-line therapy.
Our study showed a statistically significant increase in survival for squamous cell lung cancer patients in the extended group (8.9 vs. 2.0 for median PFS, P=0.001; and 27.2 vs. 10.8 for median OS, P=0.001). Other angiogenesis inhibitors, such as bevacizumab, sunitinib, sorafenib, vandetanib, and nitedanib, have not been demonstrated to be helpful or improve OS for SCC patients (11,26-29) due to the increased incidence rates of hemorrhage toxicity in these patients. It is estimated that such an adverse event is detrimental to survival, which may counteract the benefit of angiogenesis inhibitors such as bevacizumab. As a result, the National Comprehensive Cancer Network (NCCN) and European Society for Medical Oncology (ESMO) guidelines recommend that bevacizumab should only be used in non-squamous cell lung cancer (2,3). However, researchers were pleasantly surprised to find that there were no SCC patients with level 3–5 hemorrhage events reported after treatment with rh-endostatin and chemotherapy in the phase 3 study led by Sun et al. (13,30). In another phase 3 randomized trial including 126 advanced stage NSCLC patients treated with rh-endostatin with carboplatin and paclitaxel, no cases of hemoptysis were found in squamous cell lung cancer patients either (31). A real-world study (14) also found that only 1/54 (3.7%) patients receiving rh-endostatin suffered level 3–4 hemoptysis. Based on these findings, we have good reason to believe that the extended use of rh-endostatin is suitable for NSCLC patients, especially SCC patients. Following previous phase 3 trials using endostatin plus chemotherapy as first-line regimens, this study supplied further evidence showing that maintenance or later-line continuous use of endostatin may be effective for SCC patients.
In our study, 33 of 105 patients using endostatin continuously after first-line therapy obtained long OS, suggesting the important contribution from angiogenic inhibitors through persistent VEGF suppression. However, bevacizumab treatment after progression did not provide significant clinical benefit in NSCLC patients, although it has been fully accepted in breast and metastatic colorectal cancers (32-34). In the phase IIIb trial AvaALL (MO22097) with 485 NSCLC patients, the median OS was 11.9 versus 10.2 months in patients receiving continuous bevacizumab treatment compared with those with standard care beyond first progression (HR =0.84; P=0.104) (35). To our knowledge, this study is the first reporting a significant increase in OS through the extended use of angiogenic inhibitors, although this was not the primary endpoint.
Currently, programmed cell death 1 (PD-1)/programmed death-ligand 1 (PD-L1) inhibitors are recommended as the preferred option for continuous maintenance therapy in metastatic NSCLC patients with PD-L1 ≥1% (2,3). However, the benefit of PD-1/PD-L1 inhibitors for maintenance in those with PD-L1 <1% or an unknown level is not clear, although several phase 3 randomized trials, such as Keynote-189, 407, and IMpower 150 have reported data (36-38). In addition, the options of chemotherapy + bevacizumab and chemotherapy + PD-1/PD-L1 inhibitors in first-line therapy may be both acceptable in driver-gene-negative NSCLC patients. So far, there are no data from randomized trials comparing these 2 regimens, and the IMpower 150 study of non-squamous cell lung cancer patients indicated that OS of arm A (atezolizumab + chemotherapy) and arm C (bevacizumab + chemotherapy) were comparable in most subgroups, except those with high PD-L1 expression. In this study, most patients treated with rh-endostatin did not supply PD-L1 test data, and while we can speculate that those with high expression of PD-L1 may benefit more from PD-1/PD-L1 inhibitors or PD-1/PD-L1 inhibitors + rh-endostatin, it remains unconfirmed by validated randomized trials.
Our study had some limitations. The retrospective and nonrandomized nature of this study could weaken the conclusions. In addition, the relatively long duration of patient enrollment for data analysis may have introduced treatment bias. These limitations need to be considered when interpreting the results.
In summary, the extended use of rh-endostatin (≥4 cycles) combined with chemotherapy in patients with metastatic NSCLC resulted in significantly longer survival without more adverse events, particularly for patients with squamous cell NSCLC, which merits further evaluation in a larger prospective randomized study.
Acknowledgments
The abstract with modification was presented at the 55th Annual Meeting of the American Society for Clinical Oncology, 2019.
Funding: This work was supported in part by the 2020 Natural Science Foundation of Hubei Province (No. 2020CFB592 to Yi Cheng).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1292/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1292/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1292/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7-30. [Crossref] [PubMed]
- Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018;29:iv192-237. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Aisner DL, et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 2.2021. J Natl Compr Canc Netw 2021;19:254-66. [Crossref] [PubMed]
- Wu YL, Planchard D, Lu S, et al. Pan-Asian adapted Clinical Practice Guidelines for the management of patients with metastatic non-small-cell lung cancer: a CSCO-ESMO initiative endorsed by JSMO, KSMO, MOS, SSO and TOS. Ann Oncol 2019;30:171-210. [Crossref] [PubMed]
- Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol 2002;29:15-8. [Crossref] [PubMed]
- Skobe M, Rockwell P, Goldstein N, et al. Halting angiogenesis suppresses carcinoma cell invasion. Nat Med 1997;3:1222-7. [Crossref] [PubMed]
- Shahneh FZ, Baradaran B, Zamani F, et al. Tumor angiogenesis and antiangiogenic therapies. Hum Antibodies 2013;22:15-9. [Crossref] [PubMed]
- Sandler A, Gray R, Perry MC, et al. Paclitaxel carboplatin alone or with bevacizumab for non-small cell lung cancer. N Engl J Med 2006;355:2542-50. [Crossref] [PubMed]
- Novello S, Kaiser R, Mellemgaard A, et al. Analysis of patient-reported outcomes from the LUME-Lung 1 trial: a randomised, double-blind, placebo-controlled, Phase III study of second-line nintedanib in patients with advanced non-small cell lung cancer. Eur J Cancer 2015;51:317-26. [Crossref] [PubMed]
- Garon EB, Ciuleanu TE, Arrieta O, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet 2014;384:665-73. [Crossref] [PubMed]
- Johnson DH, Fehrenbacher L, Novotny WF, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol 2004;22:2184-91. [Crossref] [PubMed]
- O'Reilly MS, Boehm T, Shing Y, et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell 1997;88:277-85. [Crossref] [PubMed]
- Sun Y, Wang JW, Liu Y, et al. Results of phase III trial of rh-endostatin (YH-16) in advanced non-small lung cancer (NSCLC) patients. 2005 ASCO Annual Meeting Proceedings. J Clin Oncol 2005; 23: Abstract 7138.
- Wang Z, Zhang H, Zhou C, et al. Real-world outcomes of various regimens of recombinant human endostatin combined with chemotherapy in non-driver gene mutation advanced non-small cell lung cancer. Cancer Med 2019;8:1434-41. [Crossref] [PubMed]
- Qin ZQ, Yang SF, Chen Y, et al. Continuous intravenous infusion of recombinant human endostatin using infusion pump plus chemotherapy in non-small cell lung cancer. World J Clin Cases 2022;10:1164-71. [Crossref] [PubMed]
- Ge W, Cao DD, Wang HM, et al. Endostar combined with chemotherapy versus chemotherapy alone for advanced NSCLCs: a meta-analysis. Asian Pac J Cancer Prev 2011;12:2705-11. [PubMed]
- Zhou S, Zuo L, He X, et al. Efficacy and safety of rh-endostatin (Endostar) combined with pemetrexed/cisplatin followed by rh-endostatin plus pemetrexed maintenance in non-small cell lung cancer: A retrospective comparison with standard chemotherapy. Thorac Cancer 2018;9:1354-60. [Crossref] [PubMed]
- Hu W, Fang J, Nie J, et al. Efficacy and safety of extended use of platinum-based doublet chemotherapy plus endostatin in patients with advanced non-small cell lung cancer. Medicine (Baltimore) 2016;95:e4183. [Crossref] [PubMed]
- Yuan M, Zhai Y, Men Y, et al. Endostar (rh-endostatin) improves efficacy of concurrent chemoradiotherapy for locally advanced non-small cell lung cancer: A systematic review and meta-analysis. Thorac Cancer 2021;12:3208-15. [Crossref] [PubMed]
- Li K, Shi M, Qin S. Current Status and Study Progress of Recombinant Human Endostatin in Cancer Treatment. Oncol Ther 2018;6:21-43. [Crossref] [PubMed]
- Rong B, Yang S, Li W, et al. Systematic review and meta-analysis of Endostar (rh-endostatin) combined with chemotherapy versus chemotherapy alone for treating advanced non-small cell lung cancer. World J Surg Oncol 2012;10:170. [Crossref] [PubMed]
- Wang J, Sun Y, Liu Y. Results of randomized, multicenter double-blind phase III trial of rh-endostatin (YH-16) in advanced non-small lung cancer (NSCLC) patients. Zhongguo Fei Ai Za Zhi 2005;8:283-90. [PubMed]
- Fabrice B, Arnaud S, Achim R, et al. Randomized Phase III Trial of Maintenance Bevacizumab With or Without Pemetrexed After First-Line Induction With Bevacizumab, Cisplatin, and Pemetrexed in Advanced Non-squamous Non–Small-Cell Lung Cancer: AVAPERL (MO22089). J Clin Oncol 2013;31:3004-11. [Crossref] [PubMed]
- Patel JD, Socinski MA, Garon EB, et al. PointBreak: a randomized phase III study of pemetrexed plus carboplatin and bevacizumab followed by maintenance pemetrexed and bevacizumab versus paclitaxel plus carboplatin and bevacizumab followed by maintenance bevacizumab in patients with stage IIIB or IV nonsquamous non-small-cell lung cancer. J Clin Oncol 2013;31:4349-57. [Crossref] [PubMed]
- Zhou C, Wu YL, Chen G, et al. BEYOND: A randomized, double-blind, placebo-controlled, multicenter, phase III study of first-line carboplatin/paclitaxel plus bevacizumab or placebo in Chinese patients with advanced or recurrent nonsquamous non-small-cell lung cancer. J Clin Oncol 2015;33:2197-204. [Crossref] [PubMed]
- Scagliotti G, Novello S, von Pawel J, et al. Phase III study of carboplatin and paclitaxel alone or with sorafenib in advanced non-small-cell lung cancer. J Clin Oncol 2010;28:1835-42. [Crossref] [PubMed]
- Herbst RS, Sun Y, Eberhardt WE, et al. Vandetanib plus docetaxel versus docetaxel as second-line treatment for patients with advanced non-small-cell lung cancer (ZODIAC): a double-blind, randomised, phase 3 trial. Lancet Oncol 2010;11:619-26. [Crossref] [PubMed]
- Scagliotti GV, Krzakowski M, Szczesna A, et al. Sunitinib plus erlotinib versus placebo plus erlotinib in patients with previously treated advanced non-small-cell lung cancer: a phase III trial. J Clin Oncol 2012;30:2070-8. [Crossref] [PubMed]
- Reck M, Kaiser R, Mellemgaard A, et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a phase 3, double-blind, randomised controlled trial. Lancet Oncol 2014;15:143-55. [Crossref] [PubMed]
- Sun Y, Wang JW, Liu YY, et al. Long-term results of a randomized, double-blind, and placebo-controlled phase III trial: Endostar (rh-endostatin) versus placebo in combination with vinorelbine and cisplatin in advanced non-small cell lung cancer. Thorac Cancer 2013;4:440-8. [Crossref] [PubMed]
- Han B, Xiu Q, Wang H, et al. A multicenter, randomized, double-blind, placebo-controlled study to evaluate the efficacy of paclitaxel-carboplatin alone or with endostar for advanced non-small cell lung cancer. J Thorac Oncol 2011;6:1104-9. [Crossref] [PubMed]
- Garcia J, Hurwitz HI, Sandler AB, et al. Bevacizumab (Avastin) in cancer treatment: A review of 15 years of clinical experience and future outlook. Cancer Treat Rev 2020;86:102017. [Crossref] [PubMed]
- Rosen LS, Jacobs IA, Burkes RL. Bevacizumab in Colorectal Cancer: Current Role in Treatment and the Potential of Biosimilars. Target Oncol 2017;12:599-610. [Crossref] [PubMed]
- Rugo HS, Barry WT, Moreno-Aspitia A, et al. Randomized Phase III Trial of Paclitaxel Once Per Week Compared With Nanoparticle Albumin-Bound Nab-Paclitaxel Once Per Week or Ixabepilone With Bevacizumab As First-Line Chemotherapy for Locally Recurrent or Metastatic Breast Cancer: CALGB 40502/NCCTG N063H (Alliance). J Clin Oncol 2015;33:2361-9. [Crossref] [PubMed]
- Gridelli C, Bennouna J, de Castro J, et al. Randomized phase IIIb trial evaluating the continuation of bevacizumab beyond disease progression in patients with advanced non-squamous non-small-cell lung cancer after first-line treatment with bevacizumab plus platinum-based chemotherapy: treatment rationale and protocol dynamics of the AvaALL (MO22097) trial. Clin Lung Cancer 2011;12:407-11. [Crossref] [PubMed]
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:2078-92. [Crossref] [PubMed]
- Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N Engl J Med 2018;379:2040-51. [Crossref] [PubMed]
- Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med 2018;378:2288-301. [Crossref] [PubMed]
(English Language Editor: A. Muylwyk)


