
A cross-sectional study: analysis of anatomical variation in the right upper lung intersegmental vein V2a based on a 3D reconstruction technique
Highlight box
Key findings
• This study, supported by 3D reconstruction technology, preliminarily summarized the V2a typology.
What is known and what is new?
• The position and common variation of V2a were clearly defined for the first time in the “Surgical Anatomy of Bronchopulmonary Segments”, edited by Jiayuan Jiang. Since then, most of the understanding of intersegmental veins in relevant books has been based on anatomical specimens, and the progress is very slow;
• This study, with the help of 3D reconstruction and accurate reduction of anatomical structure, summarized the return of V2a to different vein and anatomical proximity.
What is the implication, and what should change now?
• Mastering the V2a anatomic variation type of intersegmental vein is very helpful for segmental lung surgery. More data are needed to confirm this finding.
Introduction
Due to the widespread prevalence of low-dose spiral computed tomography (CT) screening, the detection rate of pulmonary grinding glass nodules has been increasing year by year (1). Data from multiple studies show that the oncological effect of anatomical pulmonary segment resection of pulmonary grinding glass nodules is no less than that of lobectomy (2-5), and it can protect more lung functions (6,7). Pulmonary segment surgery can be used as the main treatment for early lung grinding glass nodules (8,9). The key to anatomical pulmonary segment resection is the correct identification of intersegmental veins; however, intersegmental venous anatomy is relatively complex and variable and there are absolute individual differences. Thus, it is particularly important to be familiar with the intersegmental veins. Okami et al. showed that nearly one-third of lung cancers occur in the right upper lung (10), so the need for refined anatomy of the right upper lung segment is further increased.
In recent years, several centers have reported analyses of the anatomy of the right upper lobe of the lung based on 3-dimensional (3D) reconstruction (11-14), which has greatly assisted in achieving accurate and effective anatomical segmental lung resection. The position and common variation of V2a were clearly defined for the first time in the “Surgical Anatomy of Bronchopulmonary Segments”, edited by Jiayuan Jiang (15). Due to the conditions at that time, no further study was carried out. Since then, most of the understanding of intersegmental veins in relevant books has been based on anatomical specimens, and the progress is very slow. With the introduction of 3D reconstruction, thoracic surgeons have made progress in understanding the anatomy of pulmonary segments, but there are still few articles on the intersegmental veins of the lung. The position of V2a in the right upper pulmonary intersegmental vein is deep and there are many variations, which brings great difficulties to the identification during operation. This study, with the help of 3D reconstruction and accurate reduction of anatomical structure (16,17), summarized the return of V2a to different vein and anatomical proximity, which can help thoracic surgeons better understand the anatomy of the right upper pulmonary segment and open pulmonary segment surgery. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1454/rc).
Methods
Clinical data
Patients admitted to our hospital between January 2020 and December 2020 due to right upper pulmonary nodules were selected. A total of 157 patients were included in this study, and with no history of right pulmonary resection Their age and sex were collected, while smoking and drinking history, pulmonary function and basic disease status were not included or excluded. All selected patients completed enhanced CT examination before surgery, and based on the examination data, 3D reconstruction of the right upper lobe arteries, veins, and bronchial structures was performed. The study was undertaken at the Department of Thoracic Oncology Surgery, Fujian Cancer Hospital, Clinical Oncology School of Fujian Medical University.
CT images and 3D reconstruction
Our hospital uses a 256-channel multilayer spiral CT (Philips, Tokyo, Japan) and the interactive qualitative and quantitative analysis (IQQA) intelligent 3D imaging method. The patient lay in the supine position on the spiral CT bed, hands above the head, and 50 mL of ioversol (Jiangsu Hengrui Pharmaceutical Co., Ltd., Jiangsu, China) was injected into the elbow vein with a high-pressure syringe at a speed of 5 mL/s. Arterial and venous enhanced scanning was performed with a scanning range from the thoracic and venous phases. The scan parameters were as follows: tube voltage 120 V, tube current 150 mA, reconstruction layer thickness 0.6 mm, layer spacing 1 mm, and complete pulmonary artery CT angiography. The scanned data were imported into the IQQA system, and the 3D reconstruction system was used to reconstruct anatomical structures such as bronchi and blood vessels at the location of the lung nodules in 3D. The anatomical accuracy of IQQA reconstruction has been proven in many aspects and fields (16,17). The software simulation system was used to quantitatively assess the location and size of the lung segments to be resected, and finally, intersegmental and subsegmental veins were identified according to their relationship to the bronchi and arteries.
Definition of pulmonary segmental veins
The nomenclature of the pulmonary segmental veins in this study was based on the Surgical Anatomy of Bronchopulmonary Segments by Jiang (15), “Illustrated Anatomical Segmentectomy for Lung Cancer” by Nomori and Okada (18), and the descriptions by Nagashima et al. (14), among others. The pulmonary veins were defined as follows: (I) the apical segmental vein V1, which is mostly superficial and runs down the mediastinal surface under the pleura and is divided into the intrasegmental vein V1a and the intrasegmental vein V1b in the apical segment. (II) The posterior segmental vein can be divided into the central part, a deep vein that usually runs between the segmental bronchi B1 and B2, and the interlobular part, which is a superficial vein that joins B2b and B3a. The 2 branches join B2b and B3a and merge with the superior pulmonary vein. (III) The anterior segmental veins are divided into the intersegmental vein V3a and the anterior intrasegmental veins V3b and V3c. Nagashima et al. (14) further simplified these as follows: (I) anterior vein (V. ant): the V. ant originates from V1b and descends anteriorly into the bronchi of the upper lung lobes, finally joining the upper pulmonary vein from the mediastinal pleural side; and (II) central vein: the central vein originates in V2a, descends between B2 and B3 and joins the upper pulmonary vein from the interlobular side.
Definition of anatomical variations in intersegmental vein V2a
Jiang (15) defined V2a (which is written as V2ap in the original) between B2 and B3, which is mainly the terminal branch of the central part of V2. Nomori and Okada (18) describe V2a as being located posterior to B1, travelling between S1 and S2 and being mainly the terminal branch of V2. Therefore, the above definition was followed in the present study, and all other cases of V2a travel patterns and anatomical positions were defined as variation.
Statistical analysis
In this study, chi-square test was used to compare the type of right upper pulmonary vein with the data in the anatomical books of pulmonary segments. All statistical analyses were conducted using SPSS software, version 22 (SPSS, Inc., Chicago, IL, USA).
Statistical analysis of the pulmonary vein
In this study, data related to the right upper pulmonary apical segmental vein V1 and central vein V2 were reconstructed and broadly classified into 3 types with reference to the method of right upper pulmonary vein staging described by Shimizu et al. and Nomori and Okada (13,18). A total of 117 (117/157, 74.5%) patients had a right upper pulmonary vein draining into the central vein and apical segmental vein, and 15 (15/157, 9.6%) patients had a right upper pulmonary vein draining only into the central vein, 25 (25/157, 15.9%) patients had a right upper pulmonary vein draining into the apical segment vein only (Table 1).
Table 1
| Classification | Type of right upper pulmonary vein | ||
|---|---|---|---|
| Apical segmental vein and central vein | Apical segmental vein only, no central vein | Central vein only, no apical segmental vein | |
| “Illustrated Anatomical Segmentectomy for Lung Cancer” by Hiroaki Nomori and Morihito Okada (18) | 70% | 22% | 8% |
| Nagashima et al. (14) | 81% | 12% | 7% |
| This study | 74.5% | 15.9% | 9.6% |
After validation of the data for right upper pulmonary vein type in this study with the data from articles on anatomical segmental lung resection and the atlas of lung cancer by Nomori and Okada (18) and Nagashima et al. (14), a chi-square test (χ2=2.26 <5.991) showed 95% certainty that the proportion of right upper pulmonary veins classified in the cases used in this study was close to the proportions in the abovementioned studies. Based on the similarity of the right upper pulmonary vein type proportions, this study further examined right upper intersegmental pulmonary vein V2a reflux with the aid of 3D reconstruction techniques to analyze anatomical variations in its course and location.
Ethical statement
This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of the Fujian Cancer Hospital, Clinical Oncology School of Fujian Medical University (No. K20220-017-01), and individual informed consent was waived for this retrospective analysis.
Results
A total of 157 patients (70 males and 87 females) were enrolled in the present study between January 2020 and December 2020. The median age was 56 years (range, 32–85 years). All patients underwent segmentectomy or subsegmentectomy after 3D reconstruction.
Summary of V2a reflux types under 3D reconstruction
Based on the data obtained from 3D reconstruction, V2a could be classified into types A, B, C, and D depending on the anatomical location where it returned to different veins and converged (Figure 1). Type A was observed in 139 cases (88.5%), with V2a returning to the posterior segmental veins of the right upper lung, including the terminal branch as the central part of V2 and converging at the interlobular part of V2. Type B was observed in 15 cases (9.6%), with V2a originating from the most terminal vein and returning to V1 and eventually through V1 to the intersegmental plane. Type C was observed in 2 cases (1.3%), where no V2a was found in the intersegmental plane, and the intersegmental vein was absent. Type D was observed in 1 case (0.6%), where V2a flowed directly back into the right atrium.
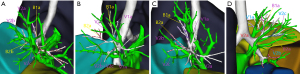
In type A, there were 3 anatomical subtypes (A1, A2, A3) depending on the different vessels (posterior segment central, posterior segment interlobular, and central vein) to which V2a returned and the converging anatomical locations (Figure 2). In subtype A1 (110/139 cases, 79.1%), V2a returned to the posterior segment central vein or V2a and V1a converged at the posterior segment central vein. In subtype A2 (8/139 cases, 5.8%), V2a flowed from the B2 mediastinal surface down to the interlobular part of the posterior segmental vein. V2b and V2c flowed below the B2 bronchus, both of which were interlobular veins. In subtype A3 (21/139 cases, 15.1%), V2a flowed between B1a and B2a and back to the central vein at the junction of the B2 and B3 bronchus. V2a became the posterior central vein, while V2b and V2c, both of which are interlobular veins, flowed below the B2 bronchus.
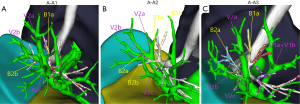
Type B was divided into 3 subtypes (B1, B2, B3) depending on the location of V2a in relation to the bronchi of the apical segment (Figure 3). In B1 subtype (1/15 cases, 6.7%), V2a continued from the mediastinal surface of B1 back down to V1, and with V1a on the mediastinal side of B1 back to the right upper pulmonary vein. In B2 subtype (7/15 cases, 46.7%), V2a continued from the medial side of the B1 lung back down to V1, usually travelling between the B1 and B2 trachea. In subtype B3 (7/15 cases, 46.7%), V2a flowed back into the central part of the posterior segmental vein, with V2b, V2c, and V1a joining V1, travelling above the B2 and B3 bronchi, and joining V1 at the junction of B1a and B1b.
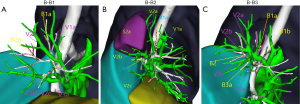
The specific subtyping percentages of V2a are shown in Table 2.
Table 2
| Type | N, % |
|---|---|
| Type A V2a (peripheral type) | 139/157, 88.5% |
| A1 | 110/139, 79.1% |
| A2 | 8/139, 5.8% |
| A3 | 21/139, 15.1% |
| Type B V2a (tip) | 15/157, 9.6% |
| B1 | 1/15, 6.7% |
| B2 | 7/15, 46.7% |
| B3 | 7/15, 46.7% |
| Type C V2a (deletion) | 2/157, 1.3% |
| Type D V2a (atrial type) | 1/157, 0.6% |
Discussion
There are many anatomical variants of pulmonary segmental vessels and segmental bronchi. 3D reconstruction technology can recreate pulmonary arteries, pulmonary veins, and bronchi into 3D geometric images, which can be used to observe the anatomical structures from multiple levels, angles, and adjustable fields of view in all directions so that anatomical variants, the anatomical alignment, and the locations of arterioles and veins can be detected in advance and precise target segmental resection can be better achieved. Nagashima et al. and He et al. used 3D reconstruction to describe the various types of variation of pulmonary segmental arteries and bronchi and also indirectly confirmed the accuracy of 3D reconstruction and its importance in pulmonary segmental surgery (14,19,20). The correct identification of intersegmental veins is central to anatomical segmental lung resection, and misinterpretation may lead to serious complications such as postoperative hemoptysis (21), so it is particularly important to fully understand the morphology of the intersegmental vessels, including rare anatomical variants. In the anatomy of the right upper pulmonary intersegmental vein, V1a and V2c can be identified with the help of the corresponding segmental bronchial and vascular pathways, whereas V2a usually travels deeper in the lung and is more difficult to identify intraoperatively than the other intersegmental veins because of its variable anatomical location and degree of variability.
Based on the importance of the intersegmental vein V2a and its complex and variable anatomy, this study summarized and analyzed the anatomical structures associated with the right upper lung intersegmental vein V2a in 157 patients by means of 3D reconstruction techniques based on imaging data. V2a could be classified into different types according to the veins to which it refluxed. The subtypes were further subdivided by the differences in their vascular course and segmental bronchial location, thus deepening the understanding of their anatomical location. At the same time, a rare anatomical variation of 2a flowing directly into the right atrium was identified in this study, which effectively prevented missed cuts, incorrect cuts, and anatomical control mismatches during surgery. Combining the V2a vein typing and 3D reconstruction techniques presented in this study, the anatomical structure of the lung segment could be rapidly identified preoperatively, thus improving the efficiency of precise resection of the target segment intraoperatively.
V2a is mainly the terminal branch of the central part of V2, accounting for 76% of cases, with another 12% as the source of the interlobular part, and the remaining 12% merging with V1a anteriorly. In this study, subtypes A1+B3 corresponded to the central terminal branch, accounting for 75% of cases (110+7/157). Subtypes A2+A3 corresponded to the interlobular geniculate branch, accounting for 18% of cases (8+21/157), and subtype B corresponded to injected V1, accounting for 9.6% of cases (15/157). The comparisons showed that the data were generally similar, and with the aid of 3D reconstruction, V2a types could be observed more easily and the anatomical differences between the subtypes could be further refined, providing complementary meaning and reference value to the anatomical data of V2a. In the present study, a few cases were found to lack intersegmental venous V2a. This finding was different from previous anatomical reports of the lung segments, and more data are expected to validate this finding.
This study had several limitations. First, changes in the pulmonary segmental veins are closely related to changes in the pulmonary segmental arteries and bronchi, which were not integrated in this study. Second, we exclusively used 3D reconstructed (IQQA) data, and there may have been discrepancies with the true intraoperative anatomical data. Third, the sample size of this study was small, and more data are needed to corroborate the findings.
Conclusions
This study, supported by 3D reconstruction technology, preliminarily summarized the V2a typology and further refined the anatomical differences of each subtype. The results of this study are convenient for thoracic surgeons to learn the anatomy of the right upper lung segment and perform segmental lung surgery.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1454/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1454/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1454/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics board of Fujian Cancer Hospital, Clinical Oncology School of Fujian Medical University (No. K20220-017-01) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N Engl J Med 2020;382:503-13. [Crossref] [PubMed]
- Yendamuri S, Sharma R, Demmy M, et al. Temporal trends in outcomes following sublobar and lobar resections for small (≤ 2 cm) non-small cell lung cancers--a Surveillance Epidemiology End Results database analysis. J Surg Res 2013;183:27-32. [Crossref] [PubMed]
- Altorki NK, Wang X, Wigle D, et al. Perioperative mortality and morbidity after sublobar versus lobar resection for early-stage non-small-cell lung cancer: post-hoc analysis of an international, randomised, phase 3 trial (CALGB/Alliance 140503). Lancet Respir Med 2018;6:915-24. [Crossref] [PubMed]
- Kamigaichi A, Tsutani Y, Fujiwara M, et al. Postoperative Recurrence and Survival After Segmentectomy for Clinical Stage 0 or IA Lung Cancer. Clin Lung Cancer 2019;20:397-403.e1. [Crossref] [PubMed]
- Kodama K, Higashiyama M, Okami J, et al. Oncologic Outcomes of Segmentectomy Versus Lobectomy for Clinical T1a N0 M0 Non-Small Cell Lung Cancer. Ann Thorac Surg 2016;101:504-11. [Crossref] [PubMed]
- Tanvetyanon T, Keenan RJ. Recovery of lung function after segmentectomy versus lobectomy for early-stage lung cancer. J Thorac Dis 2018;10:S2144-6. [Crossref] [PubMed]
- Charloux A, Quoix E. Lung segmentectomy: does it offer a real functional benefit over lobectomy? Eur Respir Rev 2017;26:170079. [Crossref] [PubMed]
- Suzuki K, Saji H, Aokage K, et al. Comparison of pulmonary segmentectomy and lobectomy: Safety results of a randomized trial. J Thorac Cardiovasc Surg 2019;158:895-907. [Crossref] [PubMed]
- Aokage K, Saji H, Suzuki K, et al. A non-randomized confirmatory trial of segmentectomy for clinical T1N0 lung cancer with dominant ground glass opacity based on thin-section computed tomography (JCOG1211). Gen Thorac Cardiovasc Surg 2017;65:267-72. [Crossref] [PubMed]
- Okami J, Shintani Y, Okumura M, et al. Demographics, Safety and Quality, and Prognostic Information in Both the Seventh and Eighth Editions of the TNM Classification in 18,973 Surgical Cases of the Japanese Joint Committee of Lung Cancer Registry Database in 2010. J Thorac Oncol 2019;14:212-22.
- Boyden EA, Scannell JG. An analysis of variations in the bronchovascular pattern of the right upper lobe of 50 lungs. Am J Anat 1948;82:27-73. [Crossref] [PubMed]
- Cory RA, Valentine EJ. Varying patterns of the lobar branches of the pulmonary artery. A study of 524 lungs and lobes seen at operation of 426 patients. Thorax 1959;14:267-80. [Crossref] [PubMed]
- Shimizu K, Nagashima T, Ohtaki Y, et al. Analysis of the variation pattern in right upper pulmonary veins and establishment of simplified vein models for anatomical segmentectomy. Gen Thorac Cardiovasc Surg 2016;64:604-11. [Crossref] [PubMed]
- Nagashima T, Shimizu K, Ohtaki Y, et al. An analysis of variations in the bronchovascular pattern of the right upper lobe using three-dimensional CT angiography and bronchography. Gen Thorac Cardiovasc Surg 2015;63:354-60. [Crossref] [PubMed]
- Jiang JY. Surgical anatomy of bronchopulmonary segment. Shanghai: Shanghai Scientific & Technical Publishers, 1960;22-5.
- Zhi XY, Hu J, Liu LX, et al. Expert consensus on 3D visual localization and surgical planning of pulmonary nodules with artificial intelligence platform. Chinese Journal of Clinical Thoracic and Cardiovascular Surgery 2019;26:1161-6.
- Luo Y, Zhang M, Zhou T, et al. Three-dimensional visualisation techniques in paediatric living liver transplantation. Zhonghua Wai Ke Za Zhi 2016;54:700-3. [PubMed]
- Nomori H, Okada M. Illustrated Anatomical Segmentectomy for Lung Cancer. 1st edition. Tokyo: Springer; 2012.
- He H, Chen P, Chen X, et al. Analysis of anatomical variations of the lingular artery of the left upper lobe using 3D computed tomography angiography and bronchography. J Thorac Dis 2021;13:5035-41. [Crossref] [PubMed]
- He H, Wang F, Wang PY, et al. Anatomical analysis of variations in the bronchus pattern of the left upper lobe using three-dimensional computed tomography angiography and bronchography. Ann Transl Med 2022;10:305. [Crossref] [PubMed]
- Gossot D, Seguin-Givelet A. Anatomical variations and pitfalls to know during thoracoscopic segmentectomies. J Thorac Dis 2018;10:S1134-44. [Crossref] [PubMed]
(English Language Editor: A. Muylwyk)


