
Preoperative localization of lung nodules: a comparative analysis of hookwire and radio-guided procedures
Introduction
A pulmonary nodule is defined as a round or oval opacity less than 3 cm in size and surrounded by lung (1). There are multiple causes of pulmonary nodules, whether solitary or multiple. The detection of small-sized pulmonary nodules has increased in recent years due to the implementation of computed tomography (CT) devices with lower collimation and reconstruction thicknesses and reconstruction algorithms with maximum intensity projection (MIP) techniques (2). Detecting these nodules is essential in lung cancer screening programs and cancer patients. In the latter, the new targeted therapies or immunotherapy require a molecular determination that may require a histological sample. However, a percutaneous biopsy of these tiny lung lesions has lower performance than in larger volume nodules (3,4), and an excisional biopsy is required. This intervention can be performed by video-assisted thoracoscopic surgery (VATS), which provides a specific diagnosis (5).
Since hookwire marking was published in 1993 (6), multiple localization methods have been described in the literature, with the hookwire being the most widely used. However, the hookwire has a high yield with a morbidity of 13–14%, and the main disadvantage is the migration of the device (7-9). Therefore, in 2000, the marking of pulmonary nodules using radioguided occult lesion localization (ROLL) was described, which consists of injecting a radiotracer inside the nodule and subsequent intraoperative localization using a gamma detection probe (10). This technique had previously been used with great success in other pathologies such as breast nodules (11), allowing the volume of the resection pieces to be reduced (12).
Although some studies compare the effectiveness of different techniques for preoperative localization of pulmonary nodules to date (5,9,13,14), the factors that determine the performance of the ROLL technique compared to the hookwire and how they affect the performance of the ROLL technique, and the volume of the resected lung has not been studied.
The objective of our study was to evaluate the performance and complications of the ROLL technique compared to hookwire marking based on the characteristics of the nodule, the radiological approach, and its impact on the volume of lung parenchyma removed. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-552/rc).
Methods
Our work is a retrospective study in which the series of marking of pulmonary nodules intervened using ROLL has been compared, with prospective inclusion of patients (from November 2015 to September 2018) and the retrospective series of marking with hookwire for resection of pulmonary nodules in our center (from March 2012 to July 2019). In all cases, the need for a presurgical localization was decided by consensus in the multidisciplinary committee of lung neoplasms. The analysis was performed after the approval of the local ethics committee (Comitè d’Ètica de l´Hospital Clínic de Barcelona; HCB/2016/0147). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). In addition, all patients signed the informed consent before the interventional and surgical procedures.
Patient population
Eighty-eight nodules belonging to 76 patients have been studied: 52 resected with hookwire and 36 with ROLL. Table 1 collects the global demographic data grouped by technique, the distribution by lobes of the nodules, and the type of guide used in each group of patients.
Table 1
| Patients | Global (n=76) | Hookwire (n=49) | ROLL (n=27) | Statistical significance |
|---|---|---|---|---|
| Age (years), median (SD) [range] | 64.05 (10.14) [38–82] | 62.47 (10.11) [38–82] | 66.93 (9.89) [40–79] | NS |
| Men:Women | 27:22 | 17:10 | NS | |
| Prior neoplasm (n, %) | 27 (55.1) | 22 (81.5) | P<0.05 | |
| Number of resected nodules | 88 | 52 | 36 | |
| Size (mm), median (SD) [range] | 10.13 (4.59) [3–22] | 10.29 (4.49) [4–22] | 9.89 (4.78) [3–22] | NS |
| Localization, n | ||||
| RUL | 21 | 13 | 8 | – |
| RML | 7 | 6 | 1 | – |
| RLL | 19 | 11 | 8 | – |
| LUL | 23 | 13 | 10 | – |
| LLL | 18 | 9 | 9 | – |
| Type of guidance, n (%) | ||||
| Step by step | 34 (38.6) | 34 (65.4) | 0 (0.0) | – |
| Fluoroscopy-CT | 54 (61.4) | 18 (34.6) | 36 (100.0) | – |
| Final diagnosis, n (%) | ||||
| Malignant | 70 (79.5) | 39 (75.0) | 31 (86.1) | – |
| Benign | 18 (20.5) | 13 (25.0) | 5 (13.9) | – |
ROLL, radioguided occult lesion localization; SD, standard deviation, NS, non-significative; RUL, right upper lobe; RML, right middle lobe; RLL, right lower lobe; LUL, left upper lobe; LLL, left lower lobe; CT, computed tomography; –, non-realized.
Hookwire marking (Figure 1)
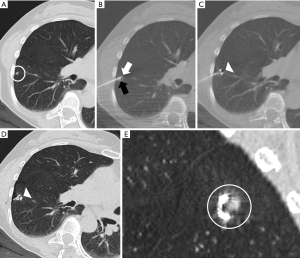
We used a specific marker for the lung (Léleman, Valencia, Spain). The markings were performed using CT fluoroscopy guidance (Sensation 16, Siemens Healthineers, Erlangen, Germany) in 18 cases or step-by-step technique in the remaining 34 cases. The step-by-step technique consists in introducing the needle a few millimeters and taking a CT image to ensure the correct positioning of the needle tip, and so on, consecutively until the needle reaches the nodule. Fluoroscopy-CT guidance consists in advancing the needle while CT is acquiring the image. With fluoroscopy-CT, the needle’s tip’s position can be controlled in real-time. Subsequently, a new CT scan was performed to determine the existence of complications and the exact location of the hookwire.
Marking with ROLL technique (Figure 2)
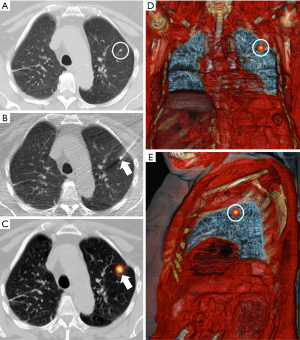
Chiva needles of 22-G and 9 or 16 cm in length (BD, Madrid, Spain) were used for marking. The tip of the needle was placed inside the nodule under CT fluoroscopy guidance, and the radiotracer was injected ([99mTc]Tc-MAA, 37–111 MBq, 0.2 mL; depending on whether the injection was performed on the same-day surgery or the previous one) mixed with 1 mL of iodinated contrast. Next, a new CT was performed to check the iodinated contrast’s location and evaluate potential complications. Subsequently, a single-photon emission CT/CT (SPECT/CT) (InfiniaTM HawkeyeTM 4; GE Healthcare Milwaukee, WI, USA or Symbia Intevo Bold; Siemens Healthineers, Erlangen, Germany) with volumetric reconstruction was performed to verify the location of the radiotracer.
Analysis of the marking procedure
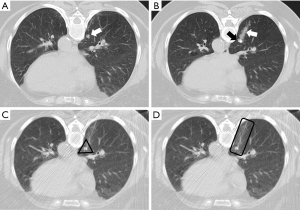
The following measurements were made: the size of the nodule, location according to the lobe, the smallest distance between the pleura and the nodule (pleural-distance; PD), the distance of the intrapulmonary path traveled by the marker (nodule-distance; ND), and marking complications, for each group (Figure 3).
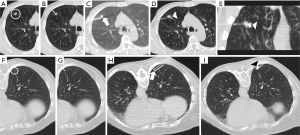
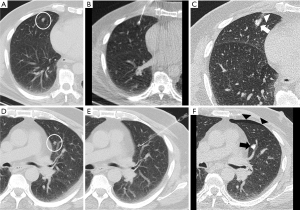
The nodule size was considered the maximum diameter in the lung window (Figure 3A,3F). The shortest distance to the pleura was considered regardless of the route used to mark the lesion (Figure 3B,3G). In both ROLL and hookwire, the ND was considered the path traveled by the needle or the hookwire from its access through the peripheral pleura until it reached the nodule (Figure 3C,3H). The following complications were recorded: pulmonary hemorrhage, pneumothorax, and need for drainage (Figure 4).
Surgical procedure
Patients were referred to the operating room immediately after hookwire localization and between 2 and 18 hours after ROLL marking. In all cases, the procedure was started with VATS. In the hookwire-marked nodules, access to the mechanism was identified at the parietal and visceral pleural levels, and the nodule was resected with safety margins around the distal end and resecting the lung parenchyma that was crossed by the hookwire. For the ROLL technique, a gamma detector probe (Navigator GPS, Dilon Medical Technologies, Newport News, VA, USA) was introduced through one of the VATS accesses. Radiotracer activity was detected inside the lesion, and the absence of significant activity in the surgical bed was assessed. In addition, we collected the following variables: type of resection, VATS success, need for reconversion to thoracotomy, and marker displacement. We consider a successful VATS when the surgeon was able to locate the nodule without any other measure (reconversion to thoracotomy, palpation…).
Pathological evaluation
The presence of the nodule within the surgical piece was verified perioperatively, and the volume of the resected lung was calculated. The volume of the surgical piece (in cubic centimeters) was calculated using a prolate ellipse volume calculation: height × width × length (all in centimeters) × π/6 (15).
Statistical analysis
Results are reported as absolute number and frequency for qualitative variables and as mean and standard deviation (SD) or median and range for continuous variables. Student’s t-test was used to compare continuous variables and the Chi-square test or Fisher’s exact test for qualitative variables. The relationship between the volume of the piece and the distances from the nodule (PD and ND) for each group were determined using a simple linear regression study.
The level of significance was considered at 5%. The analyzes have been carried out with the statistical program StatCrunch (Pearson Education 2020).
Results
Preoperative location
All of the nodules were resected during surgery: 52 nodules, corresponding to 49 patients, by hookwire marking and 36 nodules, in 27 patients, by ROLL. No significant differences were observed in the size of the nodules (Table 1) or the PD (Table 2) between both groups. ND was significantly higher in ROLL-labeled nodules than in hookwire-marked nodules.
Table 2
| Global | Hookwire | ROLL | Statistical significance | |
|---|---|---|---|---|
| Distances related to the nodule, mm | ||||
| PD, median ± SD [range] | 9.36±8.07 [0–43] | 10.13±7.47 [0–29] | 8.25±8.87 [0–43] | NS |
| ND, median ± SD [range] | 19.36±15.57 [0–97] | 16.28±11.46 [0–55] | 23.91±19.51 [0–97] | P=0.042 |
| Complications | ||||
| Pneumothorax | 44 (50.0) | 36 (69.2) | 8 (24.2) | P<0.0001 |
| Pulmonary hemorrhage | 41 (46.6) | 25 (48.1) | 16 (44.4) | NS |
| Volume of the resected lung, cc | ||||
| Global | – | 34.26 (52 nodules) | 20.19 (36 nodules) | P=0.072 |
| PD >0 mm | – | 35.66 (48 nodules) | 20.91 (33 nodules) | P=0.086 |
ROLL, radioguided occult lesion localization; PD, distance to the pleura; SD, standard deviation; ND, distance of the pulmonary path of the needle; NS, non-significative; cc, cubic centimeters; –, non-realized.
Complications
Complications derived from marking are described in Table 2. We found a higher percentage of pneumothorax in the hookwire group, although pneumothorax was asymptomatic in all cases. Hookwire migration occurred in 3/52 hookwire-tagged cases (5.8%) while none of the ROLL cases did. In the cases of migration of the hookwire, pulmonary resection was completed due to the presence of a pulmonary hematoma at the puncture site.
Surgical intervention
All nodules were located and resected that represents a successful CT localization of 100%. Only in one case of ROLL did it have to be converted to thoracotomy because the nodule was very central (1/36: 2.8%), representing a VATS success of 97.2%. There were no other intraoperative complications in any of the cases attributable to the markings.
Pathological study: volume of the surgical piece
The results relative to the volume of the piece are described in Tables 2,3. There is a good correlation between the PD and the volume of the piece in the ROLL group (r=0.53; P=0.001), while there is no correlation for the hookwire group. We found no correlation between the volume of the piece and the ND in either of the two groups (Figure 5, Table 3).
Table 3
| Volume | Global | Hookwire | ROLL |
|---|---|---|---|
| Volume of the resected lung and PD | R=0.16, (P=0.13) | R=0.08, (P=0.56) | R=0.53, (P=0.001) |
| Volume of the resected lung and ND | R=0.05, (P=0.67) | R=0.13, (P=0.37) | R=0.10, (P=0.56) |
ROLL, radioguided occult lesion localization; PD, distance between the nodule and the pleura; ND, distance of the intrapulmonary path of the needle.
In the ROLL group, a smaller piece volume was observed, even for non-subpleural nodules, than in the hookwire group, although without statistically significant differences.
Discussion
Surgical resection of pulmonary nodules is an essential tool in managing primary and metastatic pulmonary lesions. Small nodules require a localization technique for intraoperative identification, such as the hookwire, the most common and first to be used, and the ROLL, with more than 20 years of experience (16,17). Our results demonstrate 100% resection success for both techniques but with a lower rate of complications with ROLL.
Other techniques have been described, such as palpation, ultrasound, or dyes. Palpation is the one that presents the worst detection rates, between 24–28% (14), being useful in solid nodules, larger than one centimeter in size, and located near the anterior or lateral walls of the chest (5,6). Dyes allow the location of lung lesions even by endobronchial puncture, but they present difficulties such as pleural diffusion (4%), confusion with other pigments such as anthracosis (18) and have a lower localization rate (86%). However other series published had demonstrated better results with dye injection that can reach 95% (19-21). Ultrasound, like other techniques, has better results than palpation [100% in some series (5)], although with the difficulty of ultrasound interpretation and the need for the collapse of the lung parenchyma, up to 40 minutes from the pleural incision (5). Hookwire marking achieves better results than palpation, 94% (14), but has some drawbacks such as migration and the appearance of symptomatic pneumothorax. In addition, localization with hookwire determines the volume of resection for the surgeon since the entire intrapulmonary trajectory of the marker must be removed (5). The ROLL technique, which has reported detection rates of 99–100% in series with long experience (16,17), requires a gamma detector probe in the operating room, a specialist in Nuclear Medicine to guide the surgeon during the resection, and a scintigraphy post tracer injection, not available at all centers. One drawback is the possibility of pleural diffusion of the radiotracer, which would prevent the location of the nodule (5). Other localization methods are less used, such as radioactive seeds, lipiodol, or coils (14,22) or combining methods as fluorescent iodized emulsion and hookwire (23). The meta-analysis by Park et al. (14) showed that the best results were obtained with lipiodol labeling compared to hookwire and microcoils. However, lipiodol presented more complications than microcoils, so the latter was considered the best option. Hookwire localization presented a 98% success rate since it was also possible to locate the nodule in cases with migration due to the hemorrhage caused during implantation. The review by Zaman et al. advises against palpation due to its worse results and highlights radiotracers as the best marking method due to their high precision with few complications (9).
In our study, localization using ROLL was more precise than using a hookwire since the hookwire moved in 5.8% of cases. However, the overall resection rate in both cases was 100% since, in the cases in which the hookwire migrated, the hemorrhage in the puncture tract allowed the nodule detection. This percentage is within what was previously published (0.4–9%) (6,7,24,25). The migration of the hookwire has been related to the patient’s movements [especially in nodes anterior to the scapula (26)], cough (27) and the short pleura-node distance (28). Pleural migration of the radiotracer for ROLL (5) has also been described, mainly in subpleural lesions (29), both in peripheral and fissural pleura, as with coils. In our series we did not have any cases of radiotracer migration. We believe that this is due to the injection of the radiotracer inside the nodule and not in its vicinity. This fact probably prevents the tracer from migrating to the pleura even in subpleural nodules.
VATS was converted into a mini-thoracotomy during the resection of a nodule due to central location, representing a 2.8% similar to that described in the literature (16).
Although the volume of resected parenchyma is lower in the ROLL group, the differences are not significant, probably due to the sample size. Nevertheless, the volume of the piece may have an impact in these patients, mainly with a history of oncology, since they could require new lung resections, and preservation of lung parenchyma is important.
In the analysis of the volume of the part, the PD shows a good correlation with the volume of the part. ND does not correlate with volume with either technique. This shows that resection with ROLL allows obtaining a size of the piece that is only affected by the relationship of the nodule with the pleura and not by the lung distance traveled by the needle. Probably, the path traveled by the needle is what determines the volume of parenchyma resected when using the hookwire (5), but not when using ROLL, since it allows the surgeon to choose the best approach, which will be the closest to the pleura. We must point out that, in our series, the distance of the intrapulmonary path of the needle is more significant than that reported in other works with ROLL (30,31). This has allowed us to resect subpleural lesions adjacent to the fissural or mediastinal pleura.
Regarding complications, we detected a high percentage (50%) of pneumothorax in cases marked with a hookwire. The rates published in the literature range between 5.9% (32) and 68.0% (7), in this previous study with 4.6% of pleural drainage prior to surgery. In our series, no patient developed symptomatic pneumothorax or required a pleural drainage tube placement prior to surgery. According to the meta-analysis by Park et al. (14), the mean rate of pneumothorax after placement of a hookwire was 35%, being higher than that identified when using coils (16%) or lipiodol (31%).
We found no significant differences regarding postprocedural pulmonary hemorrhage between both groups. The percentage of hookwires that caused pulmonary hemorrhage is within the range reported in previous publications: 1.3% (33) to 41.6% (34). Postprocedural bleeding seems to be inherent to the marking and not to the type of procedure. In contrast, in the meta-analysis by Park (14), differences were found between the percentage of bleeding between the hookwire (16%), lipiodol (12%), and the coils (6%). According to Ichinose et al. (7), an intrapulmonary needle path greater than 25 mm is a risk factor for developing hemorrhage and hemoptysis.
On the other hand, patients who require marking of several nodules tend to present more complications if the hookwire is used (26). In our series, we only located several nodules in the same patient using ROLL, without any serious associated complications.
Another critical element is the cost of the technique. Localization with ROLL is cheaper, as it had been published, than marking with a hookwire or with coils (22) and, with the new hybrid equipment, it is possible to reduce the time and cost of the examination by being able to perform the procedure in the same Nuclear Medicine department using a SPECT/CT with incorporated fluoroscopy (35) or to assess the margins intraoperatively, using a portable gamma camera (36).
Our work has some limitations. In the first place, it is a comparative analysis of two techniques, in which one of them has been performed prospectively (ROLL) while the evaluation of the data of the hookwire resection is retrospective. However, the same radiologists have carried out the assessment of complications and the measurement of distances for all the cases in the series. Another potential limitation would be the limited number of patients reviewed. It would be expected that, with a more significant number of patients in both techniques, but especially in the ROLL arm, it would be possible to demonstrate that hookwire marking determines more significant resection volumes, which, in our series, is only a trend. Finally, it is a single-center study, which guarantees the homogeneity of the methodology and the data collected but lacks external validity.
Among the strengths of this study, the comparison between the ROLL technique within the same center stands out, therefore without bias from the surgical team. Most of the published articles refer to a single type of marker, and the comparative studies compare the results carried out in different works or compare the hookwire with other localization techniques, but not with ROLL, except for Gonfiotti et al. (13), who also did not find differences in the successful marking between both techniques. In contrast, in our series, we have detected a more significant number of complications with the hookwire. Gonfiotti et al. had 16% of cases of displacement of the hookwire prior to resection that prevented the nodule’s location and only 4% of pleural diffusion of the radiotracer that did not allow detection (13), higher than those observed in our series. Our work demonstrates that both markers offer a high success rate in the resection of pulmonary nodules, but the ROLL technique allows more minor tissue resection and is associated with a lower rate of complications. In addition, it does not limit the patient’s mobility since the chest wall externalizes no element, nor does it condition the volume of tissue that must be removed or the surgical approach. The intrapulmonary path of the needle does not affect the result of the procedure and allows the marking of several lesions in the same lung or both in the same act. In addition, the ROLL technique provides greater flexibility in surgical programming, being able to schedule these patients first thing in the morning, having scheduled the day before. However, prospective, randomized, multicenter studies are needed to compare the different types of markers and to confirm these findings with external validity.
Conclusions
The location of pulmonary nodules with hookwire and using ROLL offer an excellent resection rate with the ROLL technique. The rate of complications is lower with ROLL technique. The intrapulmonary path traveled by the needle does not affect the volume of the surgical piece, and the only important thing is the distance at which the pleural nodule is located, so this technique allows the resection of deep nodules with maximum preservation of healthy lung parenchyma.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-552/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-552/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-552/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The analysis was performed after the approval of the local ethics committee (Comitè d’Ètica de l´Hospital Clínic de Barcelona; HCB/2016/0147). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). In addition, all patients signed the informed consent before the interventional and surgical procedures.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hansell DM, Bankier AA, MacMahon H, et al. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008;246:697-722. [Crossref] [PubMed]
- Gayete Á, Vollmer I. Manejo radiológico de los nódulos pulmonares, solitarios y múltiples. In: del Cura J, Pedraza S, Gayete Á, Rovira Á, editors. Radiología Esencial 2a edición. Editorial Panamericana; 2019. p. 1–24.
- Wallace MJ, Krishnamurthy S, Broemeling LD, et al. CT-guided percutaneous fine-needle aspiration biopsy of small (< or =1-cm) pulmonary lesions. Radiology 2002;225:823-8. [Crossref] [PubMed]
- Lee KH, Lim KY, Suh YJ, et al. Nondiagnostic Percutaneous Transthoracic Needle Biopsy of Lung Lesions: A Multicenter Study of Malignancy Risk. Radiology 2019;290:814-23. [Crossref] [PubMed]
- Daniel TM. A proposed diagnostic approach to the patient with the subcentimeter pulmonary nodule: techniques that facilitate video-assisted thoracic surgery excision. Semin Thorac Cardiovasc Surg 2005;17:115-22. [Crossref] [PubMed]
- McDermott S, Fintelmann FJ, Bierhals AJ, et al. Image-guided Preoperative Localization of Pulmonary Nodules for Video-assisted and Robotically Assisted Surgery. Radiographics 2019;39:1264-79. [Crossref] [PubMed]
- Ichinose J, Kohno T, Fujimori S, et al. Efficacy and complications of computed tomography-guided hook wire localization. Ann Thorac Surg 2013;96:1203-8. [Crossref] [PubMed]
- Kleedehn M, Kim DH, Lee FT, et al. Preoperative Pulmonary Nodule Localization: A Comparison of Methylene Blue and Hookwire Techniques. AJR Am J Roentgenol 2016;207:1334-9. [Crossref] [PubMed]
- Zaman M, Bilal H, Woo CY, et al. In patients undergoing video-assisted thoracoscopic surgery excision, what is the best way to locate a subcentimetre solitary pulmonary nodule in order to achieve successful excision? Interact Cardiovasc Thorac Surg 2012;15:266-72. [Crossref] [PubMed]
- Chella A, Lucchi M, Ambrogi MC, et al. A pilot study of the role of TC-99 radionuclide in localization of pulmonary nodular lesions for thoracoscopic resection. Eur J Cardiothorac Surg 2000;18:17-21. [Crossref] [PubMed]
- Vidal-Sicart S, Rioja ME, Paredes P, et al. Contribution of perioperative imaging to radioguided surgery. Q J Nucl Med Mol Imaging 2014;58:140-60. [PubMed]
- Nadeem R, Chagla LS, Harris O, et al. Occult breast lesions: A comparison between radioguided occult lesion localisation (ROLL) vs. wire-guided lumpectomy (WGL). Breast 2005;14:283-9. [Crossref] [PubMed]
- Gonfiotti A, Davini F, Vaggelli L, et al. Thoracoscopic localization techniques for patients with solitary pulmonary nodule: hookwire versus radio-guided surgery. Eur J Cardiothorac Surg 2007;32:843-7. [Crossref] [PubMed]
- Park CH, Han K, Hur J, et al. Comparative Effectiveness and Safety of Preoperative Lung Localization for Pulmonary Nodules: A Systematic Review and Meta-analysis. Chest 2017;151:316-28. [Crossref] [PubMed]
- Littrup PJ, Williams CR, Egglin TK, et al. Determination of prostate volume with transrectal US for cancer screening. Part II. Accuracy of in vitro and in vivo techniques. Radiology 1991;179:49-53. [Crossref] [PubMed]
- Manca G, Davini F, Tardelli E, et al. Clinical Impact of Radioguided Localization in the Treatment of Solitary Pulmonary Nodule: A 20-Year Retrospective Analysis. Clin Nucl Med 2018;43:317-22. [Crossref] [PubMed]
- Ricciardi S, Davini F, Manca G, et al. Radioguided Surgery, a Cost-Effective Strategy for Treating Solitary Pulmonary Nodules: 20-Year Experience of a Single Center. Clin Lung Cancer 2020;21:e417-22. [Crossref] [PubMed]
- Krimsky WS, Minnich DJ, Cattaneo SM, et al. Thoracoscopic detection of occult indeterminate pulmonary nodules using bronchoscopic pleural dye marking. J Community Hosp Intern Med Perspect 2014; [Crossref] [PubMed]
- Lin MW, Tseng YH, Lee YF, et al. Computed tomography-guided patent blue vital dye localization of pulmonary nodules in uniportal thoracoscopy. J Thorac Cardiovasc Surg 2016;152:535-544.e2. [Crossref] [PubMed]
- Tseng YH, Lee YF, Hsieh MS, et al. Preoperative computed tomography-guided dye injection to localize multiple lung nodules for video-assisted thoracoscopic surgery. J Thorac Dis 2016;8:S666-71. [Crossref] [PubMed]
- Tsai SC, Wu TC, Lai YL, et al. Preoperative computed tomography-guided pulmonary nodule localization augmented by laser angle guide assembly. J Thorac Dis 2019;11:4682-92. [Crossref] [PubMed]
- Lee JW, Park CH, Lee SM, et al. Planting Seeds into the Lung: Image-Guided Percutaneous Localization to Guide Minimally Invasive Thoracic Surgery. Korean J Radiol 2019;20:1498-514. [Crossref] [PubMed]
- Li A, Chan S, Thung KH. Pre-operative CT localization for patients with subsolid opacities expecting video-assisted thoracoscopic surgery-single center experience of fluorescent iodized emulsion and hook-wire localization technique. Br J Radiol 2020;93:20190938. [Crossref] [PubMed]
- Ciriaco P, Negri G, Puglisi A, et al. Video-assisted thoracoscopic surgery for pulmonary nodules: rationale for preoperative computed tomography-guided hookwire localization. Eur J Cardiothorac Surg 2004;25:429-33. [Crossref] [PubMed]
- Chen S, Zhou J, Zhang J, et al. Video-assisted thoracoscopic solitary pulmonary nodule resection after CT-guided hookwire localization: 43 cases report and literature review. Surg Endosc 2011;25:1723-9. [Crossref] [PubMed]
- Yang F, Zhao H, Sui X, et al. Comparative study on preoperative localization techniques using microcoil and hookwire by propensity score matching. Thorac Cancer 2020;11:1386-95. [Crossref] [PubMed]
- Huang W, Ye H, Wu Y, et al. Hook wire localization of pulmonary pure ground-glass opacities for video-assisted thoracoscopic surgery. Thorac Cardiovasc Surg 2014;62:174-8. [PubMed]
- Seo JM, Lee HY, Kim HK, et al. Factors determining successful computed tomography-guided localization of lung nodules. J Thorac Cardiovasc Surg 2012;143:809-14. [Crossref] [PubMed]
- Starnes SL, Wolujewicz M, Guitron J, et al. Radiotracer localization of nonpalpable pulmonary nodules: A single-center experience. J Thorac Cardiovasc Surg 2018;156:1986-92. [Crossref] [PubMed]
- Liu J, Wang X, Wang Y, et al. Comparison of CT-guided localization using hook wire or coil before thoracoscopic surgery for ground glass nodules. Br J Radiol 2020;93:20190956. [Crossref] [PubMed]
- Galetta D, Bellomi M, Grana C, et al. Radio-Guided Localization and Resection of Small or Ill-Defined Pulmonary Lesions. Ann Thorac Surg 2015;100:1175-80. [Crossref] [PubMed]
- Huang HZ, Wang GZ, Xu LC, et al. CT-guided Hookwire localization before video-assisted thoracoscopic surgery for solitary ground-glass opacity dominant pulmonary nodules: radiologic-pathologic analysis. Oncotarget 2017;8:108118-29. [Crossref] [PubMed]
- Klinkenberg TJ, Dinjens L, Wolf RFE, et al. CT-guided percutaneous hookwire localization increases the efficacy and safety of VATS for pulmonary nodules. J Surg Oncol 2017;115:898-904. [Crossref] [PubMed]
- Suzuki K, Shimohira M, Hashizume T, et al. Usefulness of CT-guided hookwire marking before video-assisted thoracoscopic surgery for small pulmonary lesions. J Med Imaging Radiat Oncol 2014;58:657-62. [Crossref] [PubMed]
- Durmo R, Lechiara M, Benetti D, et al. Radioguided lung lesion localization: introducing a fluoroscopy system in a SPECT/CT scan. Nucl Med Commun 2019;40:597-603. [Crossref] [PubMed]
- Vollmer I, Sánchez-Izquierdo N, Martínez D, et al. Role of a portable gamma-camera with optical view for margins assessment of pulmonary nodules resected by radioguided surgery. Eur J Nucl Med Mol Imaging 2021;49:361-70. [Crossref] [PubMed]


