
Clinical effect of total thoracoscopic mitral valve surgery combined with atrial fibrillation radiofrequency ablation in patients with different left atrium diameter—a single center prospective observational cohort study
Introduction
Atrial fibrillation (AF) is the most common type of arrhythmia with increased morbidity and mortality (1). Valvular disease, especially mitral valve disease, is the most common cause of AF, with an incidence of up to 60%. Valvular AF results in the loss of left atrial systolic function and hemodynamic disturbances. Mitral valve surgery combined with atrial fibrillation radiofrequency ablation (AFRA) was listed as a Class I, grade A recommendation in the 2017 European Society of Cardiology (ESC) guidelines for the treatment of AF (2). However, there exists a gap between guideline recommendations and procedure compliance. Only 38% of patients with preoperative AF who underwent mitral valve surgery have concomitant ablation. The slow adoption of the guidelines is in part due to the perceived complexity of the surgical AF ablation procedure (3) and possible increased risks for postoperative permanent pacemakers (PPM) (4). Furthermore, previous studies have shown that the left atrium diameter (LAD) was an important prognostic factor for patients received mitral valve surgery and AFRA with conflicting findings. The prognosis of patients with different LAD is likely to be discrepant (5-7).
Currently, total thoracoscopic mitral valve surgery is a suitable choice for patients with valvular disease due to its advantages of less trauma and pain, fast recovery, and satisfactory results, compared with conventional thoracotomy (8). However, there are relatively few reports on the effect of AFRA combined with total thoracoscopic mitral valve surgery. The present article aims to analyze the clinical data and prognostic results of two cohorts with diverse LADs (LAD >50 and ≤50 mm) who underwent total thoracoscopic mitral valve surgery combined with AFRA to provide basic data and follow-up results for clinical treatment. We present the following article in accordance with the TREND reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1489/rc).
Methods
General data collection
This prospective observational cohort study was performed in the Department of Cardiovascular Surgery, PLA General Hospital. A total of 40 consecutive patients who underwent total thoracoscopic mitral valve surgery with AFRA from January 2021 to June 2022 were enrolled. The same surgeon performed all of the operations. The clinical data of patients were investigated and analyzed. All the included patients had pre-operative long-standing persistent (duration >1 year) AF. The included patients were divided into two groups according to their LAD. There were 24 patients with LAD >50 mm in group A, and 16 patients with LAD ≤50 mm in group B.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Ethics Committee of the First Medical Center of PLA General Hospital (ID: IACUC-DWZX-2021-524). The informed consents were obtained from all the included patients. All personal data were protected in accordance with existing national and international laws.
Study participants
Patients who met the following criteria were included in the study: (I) aged 40–70 years; (II) diagnosed with valvular heart disease and concomitant long-standing persistent AF (>1 year); (III) patients who underwent total thoracoscopic mitral valve surgery; (IV) received pre-operative cardiac color Doppler ultrasound and electrocardiogram examinations; (V) with a left ventricular end-diastolic diameter of ≤70 mm; (VI) patients with a LAD ≤65 mm; and (VII) left ventricular ejection fraction (LVEF) ≥50%. Patients who underwent mitral valve surgery coupled with other operative procedures [e.g. double valve replacement, simple tricuspid valve plasty (TVP)/tricuspid valve replacement (TVR), CABG, aortic valve replacement (AVR), or correction of congenital heart disease] were excluded.
Surgical procedures
Both patient cohorts were intubated with a double-lumen endotracheal tube after anesthesia. A peripheral cardiopulmonary bypass was established, arterial and inferior vena cava cannulation was completed through the femoral artery and femoral vein, and superior vena cava cannulation was achieved via the right internal jugular vein. An incision (length: ~3–4 cm) was made from the mid-clavicular line of the fourth intercostal space on the right side to the anterior axillary line. After single-lung ventilation and cardiopulmonary bypass, a special retractor for the left atrium was inserted into the left atrium via a percutaneous puncture through the inner side of the third rib or the fourth intercostal space near the midclavicular line. Chitwood forceps were placed through the fifth intercostal space of the midaxillary line to clamp the ascending aorta, and cold Histidine-Tryptophan-Ketoglutarate (HTK) cardioplegia was perfused into the coronary artery.
Following successful cardiac arrest, a left atriotomy was performed. A bipolar multifunctional ablation pen (AtriCure Co. Delaware, United States) was used to complete left atrial ablation (counterclockwise ablation from the right inferior pulmonary vein endocardium continuing to the upper edge of the left inferior pulmonary vein, followed by the upper edge of the left upper pulmonary vein, the upper right superior pulmonary vein, and the left atrium incision; subsequently, ablation of the left superior pulmonary vein to the left atrial appendage and the low margin of the left atrium to the posterior mitral valve annulus ablation was completed), and the left atrial appendage was closed with intermittent suturing.
Mitral plastic/replacement was performed. Right atriotomy was performed at the lower 2/3 position of the right atrium, and bipolar radiofrequency ablation forceps and a pen were used to complete right atrium ablation. The Chitwood forceps were released, tricuspid valve plasty was performed according to the De-vega procedure, and the right atrium incision was sutured (Figures 1-3). Subsequently, the peripheral cardiopulmonary bypass was stopped, a thoracic drainage tube was inserted, and the patient was transferred to the intensive care unit (ICU).
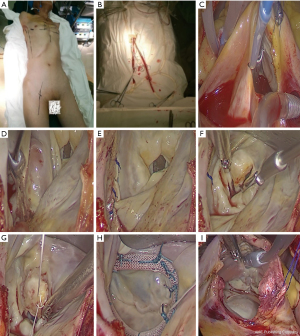
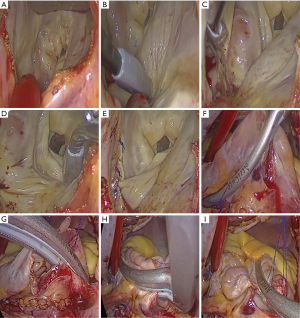
Perioperative treatment
Single-lumen endotracheal intubation was changed immediately after the operation, and the patients were transferred to the ICU. Amiodarone was continuously pumped for maintaining the sinus rhythm (a dose of 1,200 mg was administrated within 24 hours postoperatively) and amiodarone tablets (0.2 mg, 2 times a day) were taken orally following removal of the tracheal intubation on the routine postoperative day. A standard warfarin anticoagulant was also given. After the patients’ conditions stabilized, they were released from the ICU and transferred to the general ward for timely treatment of perioperative complications. We reviewed and recorded the results of chest radiographs, electrocardiograms (ECGs), and echocardiography before discharge from the hospital, and arranged for the patients to be discharged after all inspection and laboratory indicators were normal. After discharge, anticoagulation and amiodarone therapy [0.2 mg, quaque die (qd), 3 months after surgery] were continued.
Observation index
The age, gender, height, body weight, history of smoking and drinking, AF duration, preoperative complications, cardiac function classification, valve regurgitation/stenosis severity, cardiopulmonary bypass (CPB) time, aortic cross-clamp time, AF ablation time, ICU time, postoperative mortality, complication rate, and early sinus rhythm recovery rate of the two groups were observed.
Postoperative follow-up
All patients were re-checked before discharge. Outpatients were followed up at 3 and 6 months timepoints postoperatively with the ECG and echocardiography reports reviewed. The auscultation lasted at least 1 minute; if arrhythmias were found or the patients complained of palpitations, a 24-hour ambulatory electrocardiogram was performed. Asymptomatic patient or those free of arrhythmia received routine 12 lead ECG examination. The parameters of cardiac morphology, cardiac function, and sinus rhythm recovery were documented.
Statistical analysis
Normally distributed continuous variables were expressed as the mean ± standard deviation (mean ± SD) and were compared using the Student’s t-test. Binary variables were expressed as count and percentage and were compared using the χ2 or Fisher Exact Probability tests, as appropriate. The missing data were handled by using mean imputation. A two-tailed P value <0.05 was considered statistically significant. All statistical analyses were performed by using the IBM SPSS software (Version 19.0) (Chicago, United States).
Results
Comparison of the baseline characteristics of the included patients
There were no significant differences between the two groups of patients in terms of age, gender, body weight, preoperative comorbidities, AF duration, preoperative cardiac function, and type of valve disease (P>0.05). The left ventricular diameter and pulmonary artery pressure in group A were significantly higher than those in group B (P<0.05) (Table 1).
Table 1
| Characteristics | Group A (n=24) | Group B (n=16) | F/χ2 | P value |
|---|---|---|---|---|
| Sex (M/F) | 10/14 | 6/103/5 | 0.47 | 0.43 |
| Age (years) | 52±11 | 56±13 | 0.31 | 0.65 |
| BMI (kg/m2) | 23.3±4.1 | 22.5±3.7 | 0.57 | 0.44 |
| Hypertension, n (%) | 8 (33.3) | 6 (37.5) | 0.17 | 0.66 |
| Cerebral infarction, n (%) | 4 (16.7) | 2 (12.5) | 0.08 | 0.72 |
| Peripheral artery embolization | 0 | 0 | – | – |
| AF duration (m) | 16±2 | 13±2 | 0.46 | 0.21 |
| MS, n (%) | 4 (16.7) | 4 (25.0) | 0.13 | 0.56 |
| MR, n (%) | 20 (83.3) | 12 (75.0) | 4.51 | 0.06 |
| LVEF | 0.60±0.05 | 0.64±0.10 | 3.72 | 0.10 |
| sPAP (mmHg) | 51±20 | 43±14 | 7.14 | 0.04 |
| LAD (mm) | 54±2 | 47±3 | 9.87 | 0.02 |
| LVEDD (mm) | 54±10 | 46±8 | 7.54 | 0.03 |
The data are expressed as mean ± SD or number (percentage). BMI, body mass index; AF, atrial fibrillation; MS, mitral stenosis; MR, mitral regurgitation; LVEF, left ventricular ejection fraction; sPAP, pulmonary artery systolic pressure; LAD, left atrial diameter; LVEDD, left ventricular end diastolic diameter; SD, standard deviation.
Comparison of the postoperative indicators in groups A and B
There were no significant differences between the two groups of patients in terms of mitral valve stenosis, regurgitation, or types of procedures. Moreover, there were no significant differences in terms of CPB time, aortic cross-clamp time, and AFRA time (P>0.05) (Table 2).
Table 2
| Surgical types | Group A (n=24) | Group B (n=16) | F/χ2 | P value |
|---|---|---|---|---|
| Mitral stenosis | 4.59 | 0.31 | ||
| Valvular plasty (n) | 2 | 2 | ||
| Replacement (n) | 2 | 1 | ||
| Mitral regurgitation | 5.04 | 0.36 | ||
| Valvular plasty (n) | 20 | 13 | ||
| Replacement (n) | 0 | 0 | ||
| TVP (n) | 14 | 12 | 6.16 | 0.12 |
| CPB time (min) | 150±25 | 141±17 | 7.20 | 0.08 |
| Aortic clamp time (min) | 136±18 | 125±22 | 4.18 | 0.16 |
| AFRA time (min) | 33±10 | 31±11 | 5.58 | 0.21 |
Data are expressed as mean ± SD or count. TVP, tricuspid valve formation; CPB, cardiopulmonary bypass; AFRA, atrial fibrillation radiofrequency ablation.
Comparison of the postoperative data between groups A and B
The LAD of patients in both groups decreased markedly after surgery, and the difference between the two groups was significant (P=0.01). The postoperative intubation time, length of ICU stay, temporary pacemaker time, and duration of intravenous amiodarone administration in group A were longer than those in group B, although the differences did not reach statistical significance. Also, there were no serious adverse complications such as re-thoracotomy for bleeding, permanent pacemaker implantation, or death (Table 3).
Table 3
| Postoperative data | Group A (n=24) | Group B (n=16) | F/χ2 | P value |
|---|---|---|---|---|
| Ventilator time (hours) | 10±4 | 9±5 | 1.47 | 0.59 |
| ICU (days) | 1.7±0.5 | 1.6±0.7 | 4.02 | 0.33 |
| Permanent pacemaker implantation (n) | 0 | 0 | – | – |
| Duration of use of amiodarone (hours) | 22±15 | 20±12 | 3.13 | 0.46 |
| Re-thoracotomy (n) | 0 | 0 | 0 | – |
| Perioperative death (n) | 0 | 0 | 0 | – |
| LVEF | 0.58±0.11 | 0.61±0.10 | 0.87 | 0.41 |
| LAD (mm) | 48±3 | 39±5 | 5.22 | 0.01 |
| LVEDD (mm) | 47±8 | 44±7 | 1.95 | 0.07 |
Data are expressed as mean ± SD or count. ICU, intensive care unit; LVEF, left ventricular ejection fraction; LAD, left atrial diameter; LVEDD, left ventricular end diastolic diameter.
Comparison of sinus rhythm recovery in group A (LAD >45 mm) and group B (LAD ≤45 mm) at 1 week and 6 months postoperatively
The rates of sinus rhythm recovery at 1 week and 6 months in group B (87.5% and 87.5%, respectively) were significantly higher than those in group A (75% and 66.7%, respectively) (P<0.05) (Table 4).
Table 4
| Characteristics of heart rhythm | Group A (n=12) | Group B (n=8) | F/χ2 | P value |
|---|---|---|---|---|
| Heart rhythm at discharge | 4.36 | 0.04 | ||
| Sinus rhythm (n) | 9 | 7 | ||
| AF (n) | 2 | 1 | ||
| Atrial flutter (n) | 1 | 0 | ||
| AF conversion rate (%) | 75.0 | 87.5 | ||
| Heart rhythm in 6 months | 5.48 | 0.02 | ||
| Sinus rhythm (n) | 8 | 7 | ||
| AF (n) | 3 | 1 | ||
| Atrial flutter (n) | 1 | 0 | ||
| AF conversion rate (%) | 66.7 | 87.5 |
AF, atrial fibrillation.
Comparison of the size of the left atrium and ventricle, and the LVEF at 6 months postoperatively
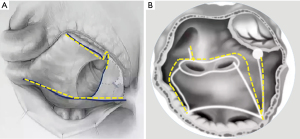
The LAD of patients in groups A (54±2 vs. 48±3 mm) and B (47±3 vs. 39±5) were significantly reduced at 1 week after surgery (P<0.01). After 6 months of follow-up, no significant differences were observed in the LAD, LVEDD and LVEF between the two groups (Figure 4A-4C).
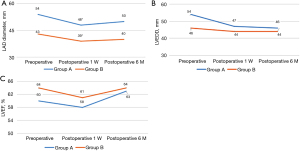
During the 6 months follow-up, no new cerebrovascular events were observed in the two groups. Furthermore, no major anticoagulant-associated complications occurred such as death, heart failure, bleeding, and infarction.
Discussion
AF is the most common arrhythmia in adults. At present, more than 300 million people worldwide are affected by this disease, and this number is expected to more than double over the next 40 years. The increased prevalence of AF may increase the economic burden on families and society, and therefore, AF will become one of the world’s largest epidemics and public health challenges (5).
Mitral valvular AF can cause significant enlargement of the left atrium in some patients due to left atrial pressure or volume overload. A previous study has reported that the increased diameter of the left atrium is an independent factor that causes AF recurrence and leads to late stroke and cardiovascular death (9). However, there are currently fewer clinical reports on whether it increases the risk of surgery and affects the ablation effect compared to patients with valvular AF whose left atrium is not significantly enlarged. The effect of catheter ablation for simple AF ablation is also markedly different due to the difference in the diameter of the left atrium. The current guidelines recommend that catheter ablation with a LAD of 45 mm is effective, but some scholars have suggested that catheter ablation with a LAD of less than 50 mm is effective (10). Recent cardiac ultrasound guidelines also suggest that the assessment of left atrium size is based on multiple indicators, including LAD, left atrium volume, and gender. Female patients with a LAD ≥47 mm and male patients with a LAD ≥53 mm are considered to be severely enlarged (11), and thus, a LAD >50 mm is considered severe enlargement. Our previous report (12) showed that conventional thoracotomy combined with surgical ablation of AF for LAD <45 mm provides good results. Also, the early postoperative and 2-year AF conversion rates can be as high as 91% and 84% respectively, which were 84% and 70% respectively for LAD >45 mm, and these differences were statistically significant. Thus, the enlargement of the left atrium is an important factor for the ablation effect; however, further confirmation is still required. Therefore, the choice of a LAD of 50 mm to compare the two groups has a reference basis and important clinical significance and is based on the LAD range for catheter ablation as well as the definition of severe left atrial enlargement provided by the International Ultrasound Guidelines and our previous research reports.
As reported by Wolf (13), the sinus rhythm conversion rate of minimally invasive maze surgery can be as high as 91.4%. Furthermore, it does not require a median thoracotomy, which greatly reduces surgical trauma, while retaining the efficiency and safety of AF surgical treatment. However, it is not effective for persistent AF and long-standing persistent AF. In the past 10 years, with the rapid development of total thoracoscopy, mitral valve surgery has provided a minimally invasive treatment option for valvular AF. It can achieve total thoracoscopic mitral valve repair or replacement with fantastic effects, and can also be applied for the surgical ablation of AF (14). We used a bipolar multifunctional ablation pen to isolate the left and right atrium according to the standard maze ablation procedure (Figure 3), and simultaneously used bilateral radiofrequency ablation forceps to isolate the right atrium, achieving good conversion effects.
Compared with previous reports on the surgical effect of conventional thoracotomy with mitral valve surgery combined with AF ablation, when the LAD was ≤50 mm, there was no significant difference in the early AF conversion rate between the two groups. However, when the atrium diameter was >50 mm, the early AF conversion rate between the two groups differed markedly. The reasons for this are as follows. Firstly, the bipolar radiofrequency ablation forceps device used in the surgical ablation of AF in conventional thoracotomy has good impedance induction, and the transmural ablation can be accurately determined according to the change of tissue impedance. Also, the transmural ablation line is clearly visible to ensure the continuity of each ablation path. Although cryoablation equipment has not yet been introduced in China, based on our experience, bipolar radiofrequency ablation forceps can be used to clamp the lower edge of the left atrial incision via the coronary sinus and the mitral valve annulus (opposite the P3 area of the posterior mitral valve leaflet) at the same time, and the effect is good. Moreover, although the bipolar radiofrequency ablation pen can evaluate the continuity of the ablation line through the surface eschar during endocardial ablation, the degree of fibrosis in various parts of the left atrium wall is different, and there may be a partially hardened or calcified wall. Since transmural ablation cannot be completed, this results in a reduced ablation effect. Secondly, although all cases in this study had undergone left atrial appendage closure surgery to reduce the risk of left atrial thrombosis, there were no cerebrovascular complications in the postoperative follow-up. However, unlike conventional median thoracotomy, not all cases in our cohort exhibited reduced left atrium volume. According to Laplace’s rule, the larger the left atrium, the greater the pressure on the left atrium wall, which may be the main factor inducing AF.
Mitral valvuloplasty or replacement combined with maze surgery restores hemodynamics and sinus rhythm and helps reduce the diameter of the left atrium. However, the maze surgery itself does not affect the size of the atrium. Therefore, in addition to AF ablation, the recovery of sinus rhythm and the improvement of clinical results may be beneficial for left atrium volume reduction. In a prospective randomized controlled study, Bogachev-Prokophiev et al. (15) divided mitral valvular AF patients into two groups; one group underwent mitral valve surgery combined with maze IV ablation, while the other group underwent mitral valve surgery combined with maze IV ablation, coupled with left atrium volume reduction. After 2 years of follow-up, their results showed that the AF conversion rates of patients who underwent maze ablation combined with left atrial volume reduction during mitral valve surgery were as high as 92.3%, which was much higher than the 78.4% of patients who received maze IV ablation alone. This showed that maze ablation combined with laft atrial volme reduction can help sinus rhythm conversion, which is consistent with our results.
The Marshall ligament has been confirmed as a focal ectopic pacing area (16), and the cutting of the Marshall ligament has also been included in routine surgical ablation treatment. In our study, the minimally invasive total thoracoscopic surgical incision approach was performed through a small right intercostal incision, and the left Marshall ligament could not be cut, which may have led to focal ectopic pacing and AF recurrence.
Fourthly, although the mitral and tricuspid valve isthmus were carefully and adequately ablated to reduce the presence of reentrant areas or the appearance of atrial flutter, the standard ablation method requires coronary sinus ablation and mitral valve annulus in the mitral valve isthmus, as well as the cryoablation of the anterior valve annulus of the tricuspid valve. However, the lack of a cryoablation device was also a factor that affected postoperative sinus rhythm cardioversion. It is believed that with the introduction and updating of the device, the success rate of ablation will further increase.
However, in this study, we found that the ablation rate for LAD <50 mm could be maintained at a high level for 6 months after surgery, and there was no difference in the success rate of ablation with conventional thoracotomy bipolar ablation, which confirms that the LAD size is still one of the most important factors of the ablation effect.
Total thoracoscopic mitral valve surgery combined with maze IV ablation can achieve satisfactory surgical results and a relatively good sinus rhythm cardioversion rate. However, due to the above-mentioned problems, for patients with valvular AF whose left atrium is significantly enlarged, it remains controversial whether to choose minimally invasive mitral valve surgery or conventional thoracotomy for simultaneous ablation. We believe that the best future treatment strategy for valvular AF involves a multidisciplinary teamwork model, using full thoracoscopic minimally invasive valve surgery combined with radiofrequency ablation, and coupled with an intervention catheter supplementary ablation sub-station or a one-stop mode to improve the success rate of continuous and long-standing continuous AF conversion. According to Cox et al. (17), the use of combined hybridization to develop a targeted treatment plan for each AF patient as well as meticulous long-term follow-up of the patient can significantly improve the conversion rates of patients with AF.
Conclusions
Total thoracoscopic mitral valve surgery with AFRA is more effective in maintaining sinus rhythm in patients with LAD ≤50 mm than in those with LAD >50 mm without increased risk of adverse events. Further randomized controlled trials with large sample size are warranted to validate our findings.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the TREND reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1489/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1489/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1489/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Ethics Committee of the First Medical Center of PLA General Hospital (ID: IACUC-DWZX-2021-524). The informed consents were obtained from all the included patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ma J, Chen Q, Ma S. Left atrial fibrosis in atrial fibrillation: Mechanisms, clinical evaluation and management. J Cell Mol Med 2021;25:2764-75. [Crossref] [PubMed]
- Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm 2017;14:e275-444. [Crossref] [PubMed]
- Halas M, Kruse J, McCarthy PM. Concomitant treatment of atrial fibrillation during mitral valve surgery. J Cardiovasc Electrophysiol 2021;32:2873-8. [Crossref] [PubMed]
- DeRose JJ Jr, Mancini DM, Chang HL, et al. Pacemaker Implantation After Mitral Valve Surgery With Atrial Fibrillation Ablation. J Am Coll Cardiol 2019;73:2427-35. [Crossref] [PubMed]
- Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373-498. [Crossref] [PubMed]
- Ye Q, Zhao Y, Liu K, et al. Radiofrequency Ablation in Patients Undergoing Mitral Valve Surgery with or without Giant Left Atria. Ann Thorac Cardiovasc Surg 2022;28:349-58. [Crossref] [PubMed]
- Avdagić H, Sijerčić Avdagić S, Pirić Avdagić M, et al. Effect of the Size of the Left Atrium on Sustained Sinus Rhythm in Patients Undergoing Mitral Valve Surgery and Concomitant Bipolar Radiofrequency Ablation for Atrial Fibrillation. Acta Clin Croat 2017;56:795-802. [PubMed]
- Kim WK, Kim HJ, Kim JB, et al. Concomitant ablation of atrial fibrillation in rheumatic mitral valve surgery. J Thorac Cardiovasc Surg 2019;157:1519-1528.e5. [Crossref] [PubMed]
- Ad N. Surgical Ablation for Atrial Fibrillation During Mitral Valve Surgery: Can We Do More? Ann Thorac Surg 2021;111:34-5. [Crossref] [PubMed]
- Jurin I, Hadžibegović I, Durlen I, et al. Left atrium size and red cell distribution width predict atrial fibrillation progression from paroxysmal or persistent to permanent. Acta Clin Belg 2020;75:205-11. [Crossref] [PubMed]
- Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1-39.e14. [Crossref] [PubMed]
- Yao MH, Ren CL, Zhang L, et al. Short-term and mid-term effects of radiofrequency ablation in mitral valve surgery in patients with different left atrial sizes. J Thorac Dis 2020;12:6030-8. [Crossref] [PubMed]
- Wolf RK. Surgical Treatment of Atrial Fibrillation. Methodist Debakey Cardiovasc J 2021;17:56-64. [Crossref] [PubMed]
- Mehaffey JH, Krebs E, Hawkins RB, et al. Variability and Utilization of Concomitant Atrial Fibrillation Ablation During Mitral Valve Surgery. Ann Thorac Surg 2021;111:29-34. [Crossref] [PubMed]
- Bogachev-Prokophiev AV, Ovcharov MA, Lavinykov SO, et al. Surgical Atrial Fibrillation Ablation With and Without Left Atrium Reduction for Patients Scheduled for Mitral Valve Surgery: A Prospective Randomised Study. Heart Lung Circ 2021;30:922-31. [Crossref] [PubMed]
- Santangeli P, Marchlinski FE. Techniques for the provocation, localization, and ablation of non-pulmonary vein triggers for atrial fibrillation. Heart Rhythm 2017;14:1087-96. [Crossref] [PubMed]
- Cox JL, Churyla A, Malaisrie SC, et al. A Hybrid Maze Procedure for Long-Standing Persistent Atrial Fibrillation. Ann Thorac Surg 2019;107:610-8. [Crossref] [PubMed]
(English Language Editor: A. Kassem)


