
Anatomic lung resection after immune checkpoint inhibitors for initially unresectable advanced-staged non-small cell lung cancer: a retrospective cohort analysis
Introduction
With an estimated 2.1 million cases and 1.8 million deaths in 2018, non-small cell lung cancer (NSCLC) is the leading cause of cancer death worldwide (1). Since their introduction in the treatment of NSCLC, immune checkpoint inhibitors (ICIs) have improved the prognosis of advanced NSCLC (2-8). In metastatic stages, ICIs alone or in combination with platinum-based antineoplastic drugs improved overall survival at one year by 15–20% compared to standard chemotherapy (2-8). These encouraging results lead to the approval of different ICIs according to programmed death-ligand 1 (PD-L1) status: patients with tumor expression levels of PD-L1 ≥50% are candidates for single agent pembrolizumab or atezolizumab. Patients with PD-L1 <50% are candidates for immunotherapy with pembrolizumab as a single agent if programmed death 1 (PD-1) >1% (9).
Interestingly, a subset of patients with advanced NSCLC treated with ICI alone or in combination to standard chemotherapy might experience prolonged response and preserved condition. In this infrequent circumstance, the persistence of pulmonary abnormalities might be due to macroscopic residual disease, granulomatosis reaction, or parenchymal fibrosis with no residual tumor (10). Following discussion in Multidisciplinary Tumor Board (MTB), anatomical lung resection is seldomly suggested in order to obtain a full pathological analysis of persisting lung abnormalities and to eradicate any macroscopic residual disease.
However, the feasibility of thoracic surgery after two or three lines of systemic treatment including ICIs remains unknown. Older retrospective studies on pulmonary anatomic resection for advanced initially unresectable NSCLC that have been treated by chemotherapy have outlined the feasibility of such surgery in highly selected patients (11). The objective of the present study is to determine the feasibility and safety of anatomic lung resection for initially unresectable advanced NSCLC (stage IIIB to IVB) with complete or partial response to protocols including ICIs. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-704/rc).
Methods
Study design
We set a retrospective multicentric study including all patients who underwent anatomic lung resection after systemic therapy including ICI for initially unresectable advanced NSCLC in 10 French thoracic centers from January 2016 to December 2020. Inclusion criteria were patients with advanced, unresectable NSCLC upon diagnosis because of solitary or multiple metastases (stage IIIB to IVB), receiving systemic treatments including ICIs (durvalumab, nivolumab, pembrolizumab or atezolizumab), whose surgical management eventually included anatomic lung resection surgery associated with radical mediastinal lymph nodes dissection. Patients already included in prospective clinical trials of ICIs were excluded.
Ethical considerations
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethical Committee of the French Society of Thoracic and Cardiovascular Surgery (Société Française de Chirurgie Thoracique et Cardiovasculaire, SFCTCV #CERC-SFCTCV-2021-07-20-num18_ETHA_salvage_surgery). Since this was a retrospective study, all data was anonymized and direct patients’ consent was waived. The database was declared to Sorbonne-University in order to respect General Data Protection Regulation and authorized by the Assistance Publique-Hôpitaux de Paris (#20210414155455).
Patient management
All patients underwent baseline tumor staging, including contrast-enhanced computed tomography (CT) of brain, chest and abdomen, positron-emission tomography-CT (PET-CT), and pathological evaluation of any enlarged mediastinal lymph nodes by means of bronchoscopy and/or mediastinoscopy. Classification of NSCLC was based on the 8th edition of TNM by the International Association for the Study of Lung Cancer (12). All cases were discussed in multidisciplinary meetings. Decision to initiate systemic treatment was based on advanced unresectable clinical stage IIIB to IVB disease, with treatment protocols varying according to the oncologic center as well as the use of durvalumab “off label”. Introduction of ICI was either at first, second or third line of treatment, depending upon the oncologic center protocols. Radical management of metastases were as followed. Persistent adrenal gland metastases were treated by surgical resection or radiotherapy. Liver metastases and distant lymph nodes metastases disappeared under immunotherapy. Brain and bone metastases were treated by radiotherapy. All patients underwent a complete reassessment of the tumor with CT and PET-CT within 6 weeks before surgery. Upon reassessment, eligibility to surgical resection was suggested in case of well controlled patient and an operable patient. Patients were defined as “well controlled” on the following criteria:
- Partial or complete response to systemic treatment: radiologists determined it on follow-up CT-scan. Target lesions were evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST) or immune-RECIST criteria by the radiologists in order to determine complete response, partial response, confirmed progression or unconfirmed progression (13);
- Well-controlled metastases: absence of any new lesions. The known lesions are limited to one or two that are accessible to radical treatment either by surgery or radiotherapy if they had not disappeared under chemotherapy or ICIs.
Once the patients were defined as well controlled, selection for surgical resection was based on:
- Maintained general condition: patients had a Performance Status of 0 or 1 from the World Health Organization score;
- Residual primary lung tumors amenable to complete surgical resection;
- Operability assessed using the European Society of Thoracic Surgery (ESTS)/European Respiratory Society (ERS) 2009 guidelines (14). Additional testing with stress test and VO2max were selectively performed to refine surgical risk.
Surgery
Surgery consisted of an anatomic lung resection with radical lymph node dissection. Decision concerning type of resection (segmentectomy, lobectomy or pneumonectomy) and surgical approach [conventional open thoracotomy, video-assisted thoracoscopic surgery (VATS) or robotic-assisted thoracoscopic surgery (RATS)] was left at the surgeon’s discretion or experience. Conventional thoracotomy was favored because of potential known dissection difficulties in case of neo-adjuvant chemotherapy. Minimally-invasive surgery was considered in case of residual peripheral tumor of less than 3 cm in diameter and hilar/mediastinal lymph node inferior to 10 mm in diameter on pre-operative computed tomography. When opting for minimally-invasive surgery, decision to conversion into conventional thoracotomy was decided following vascular injury or in presence of intense fibrotic tissue which rendered dissection more difficult and compromised patients’ safety. All patients were managed following a clinical pathway dedicated to enhanced recovery after surgery (ERAS).
Pathologic analysis
All surgical specimens were analyzed to evaluate major and complete pathologic response with a methodology used in prospective trials (15,16). Determination of the pathologic response to therapy was made after review of all tumor slides stained with Hematoxylin and Eosin, by estimating the percentages of (I) viable tumor, (II) necrosis, and (III) stroma, which includes both fibrosis and inflammation, so these three components add up to 100% (17). Major pathological response (MPR) was defined as 10% or less residual tumor following neoadjuvant therapy. Complete pathologic response (CPR) was defined by the lack of all signs of cancer in tissue samples removed during surgery.
Data collection
We gathered the following data: age, gender, clinical and pathologic stage, histology, ICI treatment regimen, surgical procedure, complications, time between the end of treatment and surgical resection, final pathologic analysis, resections margins. Follow-up information was obtained by civil registers and referring to physician interrogation.
Endpoints
Coprimary endpoints were in-hospital morbidity and mortality, defined as any complication requiring specific treatment during initial hospitalization, or death during initial hospitalization. Classification of morbidity was based on the Clavien-Dindo classification of surgical complications (18). Cardiopulmonary complications included respiratory failure, need for re-intubation, prolonged mechanical ventilation >24 h, pneumonia, atelectasis requiring bronchoscopy, pulmonary edema, pulmonary embolism, acute respiratory distress syndrome, arrhythmia, prolonged air leak and broncho-pleural fistula. Secondary endpoints were rate of complete resection of the pulmonary disease, rate of MPR, long-term survival and risk factors associated with in-hospital morbidity.
Statistical analysis
Categorical variables were presented as numbers and proportions and compared using Fisher’s exact test. Given the small sample size and non-normal distribution, continuous variables were expressed as median [interquartile range (IQR)] and compared using Mann-Whitney test. When a significant association was found using this test, the odd ratio was calculated to confirm it. A P value less than 0.05 was considered significant. Overall survival was calculated using Kaplan-Meier analysis. Statistics were performed using Prism GraphPad© (San Diego, CA, USA). Concerning missing values, Mann-Whitney test worked fine with unequal sample size. On survival curves, censoring concerned patients for whom we had information upon date point.
Results
Patients characteristics
The study group included 21 patients (0.43%) among 4,862 patients who had surgery for major lung resection. No patients who were judged able to have the surgical resection refused it. Their clinical characteristics are summarized in Table 1. Most patients were young (median age 64 years old, IQR 51–65 years) with preserved lung function [median percentage of forced expiratory volume in one second in percentage (%FEV1) of 97%, IQR 85–103%; median percentage of diffusing capacity for carbon monoxide (%DLCO) of 67%, IQR 58–90%]. Initial tumor stages were IVA in 16 (76%), IVB in 4 (19.2%) and IIIB in 1 patient (4.8%). Radiotherapy was used on four brain lesions, one bone lesion and one adrenal gland lesion. Detailed of systemic regimens is shown in Table 1. Three patients (14.4%) had a first line treatment with pembrolizumab (200 mg intravenously every three weeks): they were the only patients who didn’t have a second line treatment. Eighteen patients progressed after first line chemotherapy and received second line ICI. Sixteen patients (76%) received a first-line chemotherapy of four cycles involving cisplatin (75 mg/m2) and pemetrexed (500 mg/m2) and two patients had four cycles of cisplatin (75 mg/m2)−paclitaxel (175 mg/m2). ICIs consisted of nivolumab (3 mg/kg every two weeks intravenously) in 12 cases (57%), pembrolizumab (200 mg intravenously every three weeks) in 6 cases (29%), durvalumab (10 mg/kg every two weeks) in 2 cases (10%) and atezolizumab (1,200 mg every three weeks) in 1 case (5%).
Table 1
| Characteristics | Values |
|---|---|
| Sex, n (%) | |
| Male | 8 (38.0) |
| Female | 13 (62.0) |
| Age, years | 64.0 [51.0; 65.0] |
| Performance status, n (%) | |
| 0 | 17 (81.0) |
| 1 | 4 (19.0) |
| Functional respiratory test | |
| %FEV1 | 97.0 [85.0; 103.0] |
| %DLCO | 67.0 [58.0; 90.0] |
| Pathology, n (%) | |
| Adenocarcinoma | 17 (80.8) |
| Squamous cell carcinoma | 3 (14.4) |
| Undifferentiated carcinoma | 1 (4.8) |
| Staging, n (%) | |
| IVA | 16 (76.0) |
| IVB | 4 (19.2) |
| IIIB | 1 (4.8) |
| Metastases localisation, n (%) | |
| Brain | 7 (32.8) |
| Lung | 8 (38.4) |
| Lymph-nodes | 1 (4.8) |
| Bones | 2 (9.6) |
| Liver | 2 (9.6) |
| Adrenal gland | 1 (4.8) |
| Check-point inhibitor and chemotherapy combination, n (%) | |
| CIS + PEM + NIVO | 11 (53.0) |
| CIS + PEM + DUR | 2 (9.6) |
| CIS + PEM + PBL | 3 (14.4) |
| CIS + PX + ATZ | 1 (4.8) |
| CIS + PX + NIVO | 1 (4.8) |
| PBL alone | 3 (14.4) |
Categorical variables are expressed as numbers and proportions. Continuous variables are expressed as median [interquartile range]. %DLCO, diffusing capacity for carbon monoxide in percentage; %FEV1, forced expiratory volume in one second in percentage; CIS, cisplatin; PEM, pemetrexed; NIVO, nivolumab; DUR, durvalumab; PBL, pembrolizumab; PX, paclitaxel; ATZ, atezolizumab.
Surgery
The median time between diagnosis and surgery was 22 months (IQR 18–35 months). This median time between patients who had post-operative complications and patients who didn’t have any post-operative complications wasn’t significantly different [24 months (16–55.5 months) versus 21 months (16.25–31 months), P=0.47]. The operative management is summarized in Table 2. Lobectomy was performed in 17 cases (80.8%), segmentectomy in 3 cases (14.4%) and pneumonectomy in one case (4.8%). Median length of surgery was 180 minutes (130–210 minutes). Conventional thoracotomy was performed in 11 cases (52%), while minimally-invasive approach by VATS or RATS was used in the remaining 10 cases (48%). Even though minimally-invasive surgery tended to be longer than conventional thoracotomy, there was no significant difference in length of surgery between conventional thoracotomy and minimally invasive surgery [161 minutes (120–200 minutes) versus 190 minutes (145–232.5 minutes), P=0.32]. In 5 patients with VATS approach, conversion for open thoracotomy approach was necessary.
Table 2
| Characteristics | Values |
|---|---|
| Median time between diagnosis and surgery (months) | 22.0 [18.0; 35.0] |
| Type of resection, n (%) | |
| Pneumonectomy | 1 (4.8) |
| Lobectomy | 17 (80.8) |
| Segmentectomy | 3 (14.4) |
| Approach, n (%) | |
| Thoracotomy | 11 (52.0) |
| VATS | 7 (33.6) |
| RATS | 3 (14.4) |
| Median duration of surgery (minutes) | 180.0 [130.0; 210.0] |
| Complications, n (%) | 9 (43.2) |
| Grade II, n (%) | |
| Prolonged air leak | 2 (9.6) |
| Pneumonia | 3 (14.4) |
| Arrhythmia | 1 (4.8) |
| Grade IV, n (%) | |
| Broncho-pleural fistula | 2 (9.6) |
| Grade V, n (%) | |
| Broncho-pleural fistula | 1 (4.8) |
| Hospital stay (days) | 8.0 [5.0; 10.0] |
| Pathological staging, n (%) | |
| ypT0N0 | 7 (33.4) |
| ypT1bN1 | 1 (4.8) |
| ypT1cN0 | 3 (14) |
| ypT2aN0 | 3 (14) |
| ypT2bN0 | 1 (4.8) |
| ypT2bN2 | 3 (14) |
| ypT3N0 | 1 (4.8) |
| ypT4N0 | 2 (10.2) |
Categorical variables are expressed as numbers and proportions. Continuous variables are ex-pressed as median [interquartile range]. VATS, video-assisted thoracoscopic surgery; RATS, robotic-assisted thoracoscopic surgery.
Postoperative course
Nine patients (43%) presented early post-operative complications as shown in Table 2. Six patients had grade II complications (2 prolonged air leaks, 3 pneumonia and one arrythmia); 2 patients had a grade IVa complications (2 broncho-pleural fistulas). Prolonged air leak was handled by maintaining the chest tube in place until air leakage stopped. Pneumonia was treated by antibiotics, eventually adapted to the germs identified on the sputum culture or blood culture. One broncho-pleural fistula was treated conservatively with a chest tube and antibiotics. The other broncho-pleural fistula was treated by an intercostal muscle flap and systemic antibiotics. One patient died of broncho-pleural fistula 30 days after surgery (grade V). This last patient had an initial hospital stay of 7 days after surgery without any complication. He was later admitted 30 days after surgery for broncho-pleural fistula, confirmed by bronchoscopy. He died 30 days after initial surgery from multi-organ failure before he could have surgery for a muscle-flap to cover the fistula. The occurrence of post-operative complication was significantly associated with a prolonged hospital stay [10 days (7.0–18.0 days) vs. 5 days (3.75–8.0 days); P=0.01]. In univariable analysis, complications were significantly associated with patients who had lower DLCO values compared to patients who didn’t have post-operative complications [61.0% (57.0–67.0%) vs. 90.5% (72.0–105%); P=0.027] (Figure 1). Odds ratio was 0.095 [95% confidential interval (95% CI): 0.014–0.809; P=0.027]. There was no correlation between the occurrence of postoperative complications and the type of ICI used (Table 3).
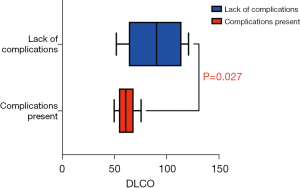
Table 3
| Clinicopathological characteristics | OR | 95% CI | P |
|---|---|---|---|
| Gender | 0.63 | 0.11–3.70 | 0.60 |
| Age | 0.36 | 0.06–2.16 | 0.26 |
| %FEV1 | 0.57 | 0.10–3.27 | 0.53 |
| %DLCO | 0.095 | 0.014–0.809 | 0.027 |
OR, odd ratio; %FEV1, forced expiratory volume in one second in percentage; %DLCO, diffusing capacity for carbon monoxide in percentage.
Oncological outcome
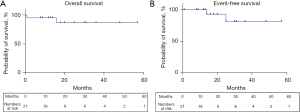
Complete macroscopic and microscopic R0 resection of the pulmonary disease was achieved in all cases. MPR was found in 9 patients (43.2%) and complete pathological response was found in 7 patients (33.4%). Positive hilar/mediastinal lymph node were found in four patients (18.8%) (Table 2). Additional radiotherapy was not applied to N2 patients nor was adjuvant chemotherapy given to persistent N1/N2 positivity. Additional radiotherapy was only administered to one patient who had a cerebral metastasis during follow-up. No patient was lost to follow-up. After a median follow-up of 16 months after surgery (IQR 12–30 months), two patients experienced postoperative recurrence, leading to death in 1 patient (Figure 2). Kaplan-Meier event-free estimate was 81.2% (95% CI: 60.2–100.0%). Nineteen of 21 (90%) patients were still alive at last follow-up. Kaplan-Meier overall survival estimate was 86.6% (95% CI: 70.2–100.0%).
Discussion
Immunotherapy has deeply impacted the therapeutic strategies for advanced NSCLC. ICIs against anti-PD-1 like nivolumab and pembrolizumab, and against anti- PD-L1, like durvalumab or atezolizumab, are now part of the standard protocols offered to patients with advanced unresectable NSCLC of stage III and IV (3). Patients with sustained response to therapeutic protocols including ICIs but persisting parenchymal abnormalities might be seldomly referred to thoracic surgery in order to obtained a complete pathological evaluation of the residual primary tumor and to eradicate any macroscopic residual disease in the lung. Studying postoperative outcomes in these infrequent situations, we found that an open approach was finally performed in 75% of the cases, grade 3 and 4 complications occurred in 24% of the cases, complete thoracic resection was achieved in all cases, and major pathologic response was found in 43% of the cases patients.
The patients in the study were initially judged inoperable. Surgery was not considered an option during multidisciplinary tumour board. Various reasons came into consideration to modify the balance in favor of surgery and therefore explain why surgery was performed very late:
- Significant response to ICI-chemotherapy combination;
- Patient’s operability;
- Resectable primary tumour;
- Metastasis accessible to a local treatment;
- Prolonged survival after ICI-chemotherapy or ICI alone.
This delay may have contributed to difficulty of the resection, including a high rate of conversions from VATS to open resection because of advanced scaring. Intraoperatively, surgical difficulties led to conversion to open thoracotomy in half of the cases initially planned to be operated through minimally invasive approach. Similarly, Bott et al. (19) analyzed the outcomes of 20 patients who underwent lung resections after receiving limited courses of ICIs in the frame of a prospective study. Of the 13 cases that were initiated with minimally invasive approach, the authors reported a conversion rate of 54% (19). It is difficult to objectively assess the difficulties related to surgery after ICIs therapy but conversion rates indirectly outline the difficulty of surgery in those circumstances. Nonetheless, conversions were only seen in VATS surgery cases, which might suggest that RATS offers an increased dexterity in this particular complex situation. In the Neostar study (20), 40% of surgeons have estimated the surgery to be more difficult in patients who had undergone neoadjuvant ICIs.
The morbidity reported in our study was similar to historical studies of anatomical lung resection after induction treatment with chemotherapy (21), prospective studies of anatomical lung resection after induction treatment with ICI (19). There was a non-significant trend towards longer delay in surgery in patients with complications. In the study of Bott et al. (19), 50% of patients experienced any post-operative morbidity and a single patient died of traumatic injury postoperatively. Nonetheless, the complication rate seems high in our study. But the morbidity and mortality reported in our study should be interpreted in the larger context of real-world data of prolonged treatment with a combination of chemotherapy and ICI without being operated, who experienced a rate of grade 3+ complications of 19%, and a rate of adverse-event related mortality of 3.3% (22). Severe adverse events (grade 3 or higher) in our study was lower compared to recent comparative randomized trials with resectable NSCLC. In stage IB to IIIA patients, 83.2% of patients in the nivolumab-plus-chemotherapy group compared to 75.4% in the chemotherapy group alone underwent definite surgery with grade 3–4 adverse events being respectively 33.5% and 36.9% (23). In the Nadim II study which include patients in stage IIIA, definite surgery occurred in 91% patients who received nivolumab and chemotherapy in adjuvant setting compared to 69% patients with neoadjuvant chemotherapy with a moderate increase in grade 3–4 adverse events (24% vs. 10%) respectively (24).
In our study, DLCO was associated with an increased risk of in-hospital morbidity. DLCO is an independent risk factor that is associated with increased morbidity after major pulmonary resection (25,26): it is a key element of evaluation in the ERS/ESTS 2009 guidelines for pre-operative evaluation of patients undergoing lung surgery (14). Unfortunately, we did not assess post-treatment effect on DLCO during pre-operative evaluation. We experienced three broncho-pleural fistulas with one patient subsequently dying from it. Similarly, in the Neostar study, one patient had a broncho-pleural fistula and died from this complication. That patient had pre-operative corticotherapy to treat a pneumonia induced by ICIs (20), suggesting a role of induction treatment in the occurrence of this rare complication.
The low rate of pneumonectomy in our cohort supports the neoadjuvant ICI approach. Many studies are being published or are still ongoing to determine the role and potential benefits of ICIs in neoadjuvant protocol for resectable NSCLC (Table 4). In many of these studies, an important criterion is MPR, defined by persistence of 10% or less viable tumor cells on the surgical specimen. This surrogate criteria has been associated with improved survival in neoadjuvant protocols including cytotoxic agents (15,16). It is now widely used to avoid prolonged follow-up before concluding to the presence or absence of any efficacy of neoadjuvant treatments (16). Table 4 retraces MPR in multiple studies with ICIs in neoadjuvant protocols, with results varying from 0% to 45%, in line with the MPR rate of 43.2% reported in our study.
Table 4
| Authors | NSCLC stages | Interim analysis | Study type | Control group | Patients included | Patients operated | Treatment protocol | Cycles (n) | Delay to surgery (days) | R0 resection (%) | MPR (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Forde et al. (27) | I to IIIa | No | Pilot | No | 21 | 20 | Nivolumab | 2 | 18 | – | 45 |
| Kwiatkowski et al. (28) | Ib to IIIb | Yes | 2 | No | 101 | 90 | Atezolizumab | 2 | – | – | 18 |
| Cascone et al. (29) | I to IIIa | No | 2 | 2 groups | 23; 21 | 21; 16 | Nivolumab; nivolumab + ipilimumab | 3; 3 | 31 | – | 17; 43 |
| Gao et al. (30) | II to IIIb | No | 1b | No | 40 | 37 | Sintilimab | 2 | – | 97.3 | 40.5 |
| Wislez et al. (31) | Ib to IIIa | Yes | 2 | No | 50 | 46 | Durvalumab | 3 | 37 | 90 | 18.6 |
| Besse et al. (32) | Ia to IIIa | No | 2 | No | 30 | 30 | Atezolizumab | 1 | 24 | 96.7 | 0 |
| Provencio et al. (33) | IIIa | No | 2 | No | 46 | 41 | Nivolumab + carboplatin + paclitaxel | 3 | – | 100 | 85 |
| Shu et al. (34) | Ib to IIIa | No | 2 | No | 30 | 29 | Atezolizumab + carboplatin + paclitaxel | 4 | 26.5 | 87 | 57 |
| Forde et al. (23) | Ib to IIIa | No | 3 | Yes | 358 | 358 | Nivolumab and chemotherapy versus chemotherapy alone | 3 | – | 100 | 24 vs. 2 |
ICI, immune checkpoint inhibitor; NSCLC, non-small cell lung cancer; MPR, major pathological response.
The clinical benefit of resecting residual macroscopic primary tumor in initially advanced unresectable NSCLC remains controversial. This multimodal approach of metastatic disease is limited to a highly selected subsets of patients with overall 5-year survival of 38% being reported (35,36). In our study, the median follow-up was 16 months with nineteen of 21 patients alive (90%) at last follow-up; Bott et al. median follow-up is 20 months with 18 of 20 patients (90%) alive at last follow-up (19).
In this highly selected cohort, we focus only on patients who received surgery rather than on those who started treatment with ICIs with the option if secondary resection. There are limited prospective trial on the subject. Faehling et al. reported a prospective study including 35 patients with locally advanced NSCLC (stage III–IVa) who had induction immuno-chemotherapy. Eleven patients had surgery whereas 21 patients had definitive chemoradiotherapy and three patients palliative treatment. Recurrences and tumour-related deaths were lower in patients who had surgery, highlighting the potential benefits of such treatment in patients fit for anatomical lung resection (37). The Increase trial is a phase II prospective trial which has aimed to enroll 29 patients who had ipilimumab and nivolumab with chemoradiotherapy followed by surgery in resectable and borderline resectable T3-4N0-1 NSCLC (38). The absence of a control group is a limit to this study as it is unclear if the patients would have had the same overall survival outcome regardless of the surgery with continued systemic therapy. Finally, the large number of thoracic units, involved in order to gather a significant number of patients in the infrequent situation, introduces potential bias as there is a low number of cases per department and a relatively high number of conversion and complications.
Conclusions
Patients with initially unresectable stage IIIB to IVB NSCLC who had systemic treatment with ICI alone or combined with chemotherapy might undergo thoracic surgery in order to eradicate residual macroscopic disease into the lung with acceptable rates of morbidity, mortality. Minimally invasive techniques have a low applicability. Patients with lower preoperative DLCO value experienced higher risk of postoperative complications. The late timing of surgery may also contribute to complications. Resection should be performed as early in the course of the disease as possible and the timing of resection needs further evaluation. The impact of surgery in the subgroup of patient with prolonged response to ICIs will be scrutinized in the near future.
Acknowledgments
The study was presented at the 29th European Conference on General Thoracic Surgery, 20 June–22 June 2021.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-704/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-704/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-704/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-704/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Ethical Committee of the French Society of Thoracic and Cardiovascular Surgery (Société Française de Chirurgie Thoracique et Cardiovasculaire, SFCTCV #CERC-SFCTCV-2021-07-20-num18_ETHA_salvage_surgery). Since this was a retrospective study, all data was anonymized and direct patients’ consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:1919-29. [Crossref] [PubMed]
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:2078-92. [Crossref] [PubMed]
- Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med 2018;378:2288-301. [Crossref] [PubMed]
- Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2019;381:2020-31. [Crossref] [PubMed]
- Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 2019;393:1819-30. [Crossref] [PubMed]
- Herbst RS, Giaccone G, de Marinis F, et al. Atezolizumab for First-Line Treatment of PD-L1-Selected Patients with NSCLC. N Engl J Med 2020;383:1328-39. [Crossref] [PubMed]
- Nasser NJ, Gorenberg M, Agbarya A. First line Immunotherapy for Non-Small Cell Lung Cancer. Pharmaceuticals (Basel) 2020;13:373. [Crossref] [PubMed]
- Kakimoto T, Sasaki M, Yamamoto T, et al. A Histologically Complete Response to Immunotherapy Using Pembrolizumab in a Patient with Giant Cell Carcinoma of the Lung: An Additional Report and Literature Review. Case Rep Oncol Med 2019;2019:1763625. [Crossref] [PubMed]
- Romero-Vielva L, Viteri S, Moya-Horno I, et al. Salvage surgery after definitive chemo-radiotherapy for patients with Non-Small Cell Lung Cancer. Lung Cancer 2019;133:117-22. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Seymour L, Bogaerts J, Perrone A, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol 2017;18:e143-52. [Crossref] [PubMed]
- Brunelli A, Charloux A, Bolliger CT, et al. ERS/ESTS clinical guidelines on fitness for radical therapy in lung cancer patients (surgery and chemo-radiotherapy). Eur Respir J 2009;34:17-41. [Crossref] [PubMed]
- Hellmann MD, Chaft JE, William WN Jr, et al. Pathological response after neoadjuvant chemotherapy in resectable non-small-cell lung cancers: proposal for the use of major pathological response as a surrogate endpoint. Lancet Oncol 2014;15:e42-50. [Crossref] [PubMed]
- Pataer A, Kalhor N, Correa AM, et al. Histopathologic response criteria predict survival of patients with resected lung cancer after neoadjuvant chemotherapy. J Thorac Oncol 2012;7:825-32. [Crossref] [PubMed]
- Travis WD, Dacic S, Wistuba I, et al. IASLC Multidisciplinary Recommendations for Pathologic Assessment of Lung Cancer Resection Specimens After Neoadjuvant Therapy. J Thorac Oncol 2020;15:709-40. [Crossref] [PubMed]
- Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 2009;250:187-96. [Crossref] [PubMed]
- Bott MJ, Yang SC, Park BJ, et al. Initial results of pulmonary resection after neoadjuvant nivolumab in patients with resectable non-small cell lung cancer. J Thorac Cardiovasc Surg 2019;158:269-76. [Crossref] [PubMed]
- Sepesi B, Cascone T, William W, et al. Surgical Outcomes Following Neoadjuvant Nivolumab or Nivolumab Plus Ipilimumab in Non-Small Cell Lung Cancer - NEOSTAR Study. J Thorac Oncol 2019;14:S241-S242.
- Brunelli A, Rocco G, Szanto Z, et al. Morbidity and mortality of lobectomy or pneumonectomy after neoadjuvant treatment: an analysis from the ESTS database. Eur J Cardiothorac Surg 2020;57:740-6. [Crossref] [PubMed]
- Fujimoto D, Miura S, Yoshimura K, et al. A Real-World Study on the Effectiveness and Safety of Pembrolizumab Plus Chemotherapy for Nonsquamous NSCLC. JTO Clin Res Rep 2022;3:100265. [Crossref] [PubMed]
- Forde PM, Spicer J, Lu S, et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N Engl J Med 2022;386:1973-85. [Crossref] [PubMed]
- Provencio-Pulla M, Larriba J, Martinez-Marti A, et al. Nivolumab + chemotherapy versus chemotherapy as neoadjuvant treatment for resectable stage IIIA NSCLC: Primary endpoint results of pathological complete response (pCR) from phase II NADIM II trial. J Clin Oncol 2022;40:8501.
- Ferguson MK, Vigneswaran WT. Diffusing capacity predicts morbidity after lung resection in patients without obstructive lung disease. Ann Thorac Surg 2008;85:1158-64; discussion 1164-5. [Crossref] [PubMed]
- Brunelli A, Refai MA, Salati M, et al. Carbon monoxide lung diffusion capacity improves risk stratification in patients without airflow limitation: evidence for systematic measurement before lung resection. Eur J Cardiothorac Surg 2006;29:567-70. [Crossref] [PubMed]
- Forde PM, Chaft JE, Pardoll DM. Neoadjuvant PD-1 Blockade in Resectable Lung Cancer. N Engl J Med 2018;379:e14. [Crossref] [PubMed]
- Kwiatkowski D, Chaft J, Johnson B, et al. Neoadjuvant atezolizumab in resectable non-small cell lung cancer (NSCLC): Interim analysis and biomarker data from a multicenter study (LCMC3). J Clin Oncol 2019;37:8503.
- Cascone T, William WN, Weissferdt A, et al. Neoadjuvant nivolumab (N) or nivolumab plus ipilimumab (NI) for resectable non-small cell lung cancer (NSCLC): Clinical and correlative results from the NEOSTAR study. J Clin Oncol 2019;37:8504.
- Gao S, Li N, Gao S, et al. Neoadjuvant PD-1 inhibitor (Sintilimab) in NSCLC. J Thorac Oncol 2020;15:816-26. [Crossref] [PubMed]
- Wislez M, Mazieres J, Lavole A, et al. Neoadjuvant durvalumab in resectable non-small cell lung cancer (NSCLC): Preliminary results from a multicenter study (IFCT-1601 IONESCO). Ann Oncol 2020;31:S794. [Crossref] [PubMed]
- Besse B, Adam J, Cozic N, et al. Neoadjuvant atezolizumab (A) for resectable non-small cell lung cancer (NSCLC): results from the phase II PRINCEPS trial. Ann Oncol 2020;31:S794-S795.
- Provencio M, Insa A, Garcia Campelo R, et al. NADIM Study: Updated Clinical Research and Outcomes. J Thorac Oncol 2019;14:S241.
- Shu CA, Gainor JF, Awad MM, et al. Neoadjuvant atezolizumab and chemotherapy in patients with resectable non-small-cell lung cancer: an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol 2020;21:786-95.
- Tönnies M, Pfannschmidt J, Bauer TT, et al. Metastasectomy for synchronous solitary non-small cell lung cancer metastases. Ann Thorac Surg 2014;98:249-56. [Crossref] [PubMed]
- Mordant P, Arame A, De Dominicis F, et al. Which metastasis management allows long-term survival of synchronous solitary M1b non-small cell lung cancer? Eur J Cardiothorac Surg 2012;41:617-22. [Crossref] [PubMed]
- Faehling M, Fallscheer S, Kramberg S, et al. Prospective trial of immuno(chemo)therapy before resection, definitive chemoradiotherapy or palliative therapy in patients with locally advanced or oligometastatic non-small cell lung cancer without a primary curative option. Eur J Cancer 2021;156:175-86.
- Dickhoff C, Senan S, Schneiders FL, et al. Ipilimumab plus nivolumab and chemoradiotherapy followed by surgery in patients with resectable and borderline resectable T3-4N0-1 non-small cell lung cancer: the INCREASE trial. BMC Cancer 2020;20:764. [Crossref] [PubMed]


