
Clinical outcomes of immune checkpoint inhibitor therapy for advanced lung adenosquamous carcinoma
Highlight box
Key findings
• Immune checkpoint inhibitors (ICIs) show considerable potential as a treatment option for lung adenosquamous carcinoma (ASC).
What is known and what is new?
• Immunotherapy has demonstrated marked efficacy against non-small cell lung cancer. However, owing to lung ASC rarity, the clinical benefits of ICIs on ASC have not been explored in detail.
• This is the first study to focus on the efficacy of ICIs for ASC.
What is the implication, and what should change now?
• ICIs may be employed as a treatment option for lung ASC. Further large-scale studies are warranted to explore the relationship between programmed death-ligand 1 expression and ICIs efficacy in lung ASC.
Introduction
Adenosquamous carcinoma (ASC) is a relatively uncommon lung cancer subtype, accounting for 0.4% to 4% of all non-small cell lung cancer (NSCLC) cases (1). ASC is defined as a tumor type containing components of both squamous cell carcinoma (SCC) and adenocarcinoma (ADC), with each component comprising at least 10% of the tumor (2).
In general, ASC is highly aggressive with poorer prognosis than ADC and SCC (1,3). In a study by Maeda et al. (4), the 5-year survival rate at all stages was 23.3% for ASC, 40.8% for SCC, and 58.0% for ADC. Gawrychowski et al. (5) analyzed data from 96 ASC patients, which revealed cumulative postoperative survival rates at 5 and 10 years of 25.4% and 19.2%, compared to 42.5% and 39.1% for contemporaneous ADC cases, respectively. However, owing to its rarity, this disease has received limited research attention with regard to clinical diagnosis and prognosis. Poor prognosis and limited treatment options remain a significant clinical challenge for patients with lung ASC.
The rapid development of immune checkpoint inhibitors (ICIs) has effectively revolutionized the management of numerous cancer types. Along with advances in cancer immunotherapy, the discovery of programmed death 1 (PD-1) and programmed death-ligand 1 (PD-L1) inhibitors has led to significant changes in standard treatment regimens for NSCLC. PD-1 inhibitor monotherapy and combination of PD-1 inhibitors with platinum-based regimens have been established as first-line standard treatment for NSCLC (6,7). In an earlier study on 5 ASC patients receiving ICIs, Manglaviti et al. (8) reported an objective response rate (ORR) and a disease control rate (DCR) of 60.0% and 60.0% and overall median progression-free survival (PFS) and overall survival (OS) of 7.7 months and 8.8 months, respectively. However, due to the limited patient numbers, no retrospective analyses or case series reports on the efficacy of PD-1/PD-L1 inhibitors for ASC have been published to date.
To our knowledge, the clinical benefits of ICIs on ASC have not been explored in detail. The current study focused on evaluating the relationship between PD-L1 expression status and efficacy of ICIs in patients with pulmonary ASC in order to optimize treatment outcomes. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1011/rc).
Methods
Study design
This study involved a retrospective analysis of patients diagnosed with lung ASC at Zhejiang Cancer Hospital from November 2017 to October 2021. All patients were pathologically confirmed with ASC according to the criteria set by the 2021 World Health Organization classification of lung tumors (2). All diagnoses were validated via immunohistochemical (IHC) analysis. In addition, stages IIIB, IIIC, or IV ASC were confirmed in patients according to the tumor, node, metastasis staging system (version 8) (9) with an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0–2. PD-L1 expression was assessed by experienced pathologists at our institution using the 22C3 companion diagnostic test (22C3 PharmDx; Agilent Technologies, Santa Clara, CA, USA), with expression of at least 1% considered positive. Molecular analysis of mutations was performed using next-generation sequencing (NGS) or amplification refractory mutation system (ARMS) technology. All patients received ICI treatment. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The protocol was approved by the Institutional Ethics Committee of Zhejiang Cancer Hospital (No. IRB-2022-187) and written informed consent was waived due to the retrospective nature of the study.
Treatment and response assessments
Clinical information was obtained from patient medical records. ICI regimens and doses for all patients were compliant with the National Comprehensive Cancer Network (NCCN) guidelines or clinical trials until disease progression or unacceptable toxicity was confirmed. Specifically, patients were treated with sintilimab (200 mg), tislelizumab (200 mg), pembrolizumab (2 mg/kg), or other PD-1 inhibitors every 2 or 3 weeks until confirmation of disease progression or unacceptable toxicity. Prior to the analysis, treatment efficacy was evaluated by two oncologists based on tumor response according to the Response Evaluation Criteria in Solid Tumors (RECIST 1.1) via chest computed tomography and/or brain magnetic resonance imaging every two cycles or early on following the appearance of key signs of progression. Objective tumor responses included complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). The ORR was defined as the sum of CR and PR and DCR assessed as the sum of objective response and stabilization rate (CR + PR + SD).
Evaluation of adverse reactions
Toxicity was monitored based on Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. Immune-related adverse events (irAEs) were defined as those with a potential immunological basis that required more frequent monitoring and intervention with immune suppression and/or endocrine replacement therapy. The diagnosis and severity of irAEs were based on clinical examinations along with biological and imaging data. IrAEs occurred during or after immunotherapy. The severity of adverse reactions was determined by the requirement of dosage reduction or discontinuation of ICIs. Scores of 1 to 5 were used for the analysis of irAE grade by two or more independent medical professionals.
Follow-up and statistical analysis
The final follow-up time was May 1, 2022, over a median follow-up period of 17.93 (14.47–21.40) months. The rate of follow-up was 100%. All patients were evaluated for PFS and OS. Categorical data were compared using Fisher’s exact test. PFS encompassed the time from day one of immunotherapy to documented progression or death from any cause, or until the date of the last follow-up visit for patients who were still alive and had not progressed. OS was defined as the time from day one of immunotherapy to death or last follow-up. Kaplan-Meier survival analysis was used for evaluation of patient survival and the log-rank test was performed to compare the survival rates regarding different prognostic factors. Univariate and multivariate analyses were performed using the Cox regression model. Statistical significance was set at two-sided P<0.05 for all tests. Statistical analyses were performed using Statistical Package for Social Sciences, version 25.0 (Chicago, IL, USA).
Results
Patient characteristics and treatments
A total of 38 patients who met the inclusion criteria were enrolled. The baseline characteristics of patients are listed in Table 1. Our study population consisted of 27 (71.1%) men and 11 (28.9%) women with the median age at diagnosis of 62 years (range, 37–78 years). The majority of patients were smokers (57.9%, 22/38). At enrollment, 9 (23.7%) and 29 (76.3%) patients had stage III and IV disease, respectively. Five patients (13.2%) had ECOG PS of 2, with the remaining classified as 0 to 1 (86.8%). We identified 11 (28.9%) patients with epidermal growth factor receptor (EGFR) mutation, 4 (10.5%) with Kirsten rat sarcoma (KRAS) mutation, 1 (2.6%) with mesenchymal-epithelial transition factor (MET) mutation, 1 (2.6%) with MET amplification, and 2 (5.3%) with anaplastic lymphoma kinase (ALK) rearrangement. In the EGFR mutation-positive group, 6 (15.8%) patients had exon 19 deletions, 3 (7.9%) had exon 21 L858R, and 2 (5.2%) had uncommon mutations. In total, 17 patients were successfully evaluated for PD-L1, among whom 11 (28.9%) were positive. Additionally, all patients received immunotherapy. Within our patient group, 17 (44.7%) received first-line immunotherapy, 9 (23.7%) received ICI monotherapy and 29 (76.3%) received combination ICI therapy. The detailed baseline characteristics of patients administered ICI monotherapy and combination therapy are described in Table S1. The most commonly used immunotherapy drugs were sintilimab (31.6%, 12/38), pembrolizumab (15.8%, 6/38), and tislelizumab (13.2%, 5/38), followed by other PD-1 inhibitors (39.5%, 15/38).
Table 1
| Characteristics | n (%) |
|---|---|
| Sex | |
| Male | 27 (71.1) |
| Female | 11 (28.9) |
| Median age [range], years | 62 [37–78] |
| Smoking history | |
| Yes | 22 (57.9) |
| No | 16 (42.1) |
| ECOG PS | |
| 0–1 | 33 (86.8) |
| 2 | 5 (13.2) |
| TNM staging | |
| III | 9 (23.7) |
| IV | 29 (76.3) |
| Diagnosis method | |
| Surgery | 26 (68.4) |
| Percutaneous lung biopsy | 8 (21.1) |
| Bronchial biopsy | 4 (10.5) |
| Mutation status | |
| EGFR mutation | 11 (28.9) |
| KRAS mutation | 4 (10.5) |
| MET mutation | 1 (2.6) |
| MET amplification | 1 (2.6) |
| ALK rearrangement | 2 (5.3) |
| EGFR mutation type | |
| Wild-type | 27 (71.1) |
| Exon 19 deletions | 6 (15.8) |
| Exon 21 L858R mutations | 3 (7.9) |
| Exon 20ins | 1 (2.6) |
| 21L861Q | 1 (2.6) |
| PD-L1 status | |
| Positive | 11 (28.9) |
| Negative | 6 (15.8) |
| Unknown | 21 (55.3) |
| Liver metastasis | |
| Yes | 4 (10.5) |
| No | 34 (89.5) |
| Brain metastasis | |
| Yes | 7 (18.4) |
| No | 31 (81.6) |
| Bone metastasis | |
| Yes | 14 (36.8) |
| No | 24 (63.2) |
| Previous surgery | |
| Yes | 24 (63.2) |
| No | 14 (36.8) |
| Previous radiotherapy | |
| Yes | 18 (47.4) |
| No | 20 (52.6) |
| Previous chemotherapy | |
| Yes | 17 (44.7) |
| No | 21 (55.3) |
| Previous targeted therapy | |
| Yes | 10 (26.3) |
| No | 28 (73.7) |
| Line of ICI treatment | |
| First line | 17 (44.7) |
| Second or further line | 21 (55.3) |
| ICI regimen | |
| Monotherapy | 9 (23.7) |
| Combination treatment | 29 (76.3) |
ECOG PS, Eastern Cooperative Oncology Group Performance status; TNM staging, tumor node metastasis staging; EGFR, epidermal growth factor receptor; KRAS, Kirsten rat sarcoma; MET, mesenchymal-epithelial transition factor; ALK, anaplastic lymphoma kinase; PD-L1, programmed death-ligand 1; ICI, immune checkpoint inhibitor.
Efficacy of ICIs
During the study period, 9 patients (23.7%) showed PR, 24 (63.2%) had SD, and 5 (13.2%) had PD, resulting in ORR of 23.7% and DCR of 86.8%. Six patients (21.1%) continued to receive immunotherapy. The median PFS with ICI therapy was 5.47 months [95% confidence interval (CI), 4.46–6.48], with a 1-year PFS rate of 10.5% (4/38). The median OS of patients administered ICI was 24.10 months (95% CI, 5.39–42.82), with a 1-year survival rate of 34.2% (13/38).
Seventeen patients (44.7%) received first-line treatment and 21 (55.3%) received second- or further-line treatment. The ORR value of patients receiving ICIs as first-line relative to second- or further-line treatment was 23.5% (4/17) vs. 23.8% (5/21; P=1.000). The DCR of ICIs as first-line and second- or further-line treatment was 100% (17/17) and 76.2% (16/21), respectively (P=0.053). The median PFS rates of patients from the two treatment groups were 5.77 months (95% CI, 4.28–7.26) and 5.00 months (95% CI, 2.80–7.20), respectively (P=0.938, Figure 1A). The median OS of the first-line treatment group was not reached while that of the second- or further-line treatment groups was 11.30 months (95% CI, 8.45–14.15; P=0.032, Figure 1B).
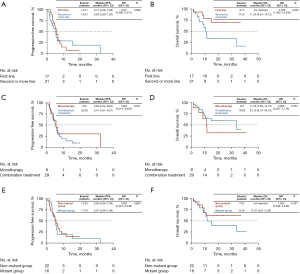
The relative efficacies of monotherapy and combination ICI therapy were further explored. ORR and DCR of the monotherapy group were 11.1% (1/9) and 88.9% (8/9) while the corresponding values of the combination treatment group were 27.6% (8/29) and 86.2% (25/29), respectively, indicating no significant differences between the two groups (ORR: P=0.298, DCR: P=1.000). The median PFS rates of the monotherapy and combination treatment groups were 5.63 months (95% CI, 2.04–9.22 months) and 5.47 months (95% CI, 4.37–6.57 months), respectively (P=0.531, Figure 1C). The median OS rate of the monotherapy group was 11.30 months (95% CI, 7.90–14.70 months) and that of the combination treatment group was 24.10 months (95% CI, 8.10–40.10 months; P=0.357, Figure 1D).
Patients were divided into two subgroups: mutant and non-mutant (with and without EGFR/ALK/KRAS/MET alterations, respectively). Overall, 16 (42.1%) patients were in the mutant and 22 (57.9%) in the non-mutant group, with ORR of 25.0% (4/16) and 22.7% (5/22; P=1.000) and DCR of 93.8% (15/16) and 81.8% (18/22), respectively (P=0.374). The median PFS rates of the mutant and non-mutant groups were 5.07 months (95% CI, 2.69–7.46 months) and 5.63 months (95% CI, 4.24–7.03 months), respectively (P=0.880, Figure 1E). The median OS rates of the mutant and non-mutant groups were 11.67 months (95% CI, 5.39–17.95 months) and not reached, respectively (P=0.421, Figure 1F).
PD-L1 expression
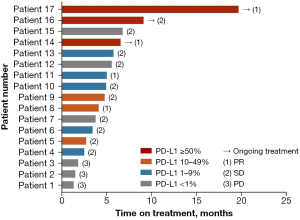
In total, 17 patients were successfully evaluated for PD-L1 expression status, among whom 11 (64.7%) were identified as PD-L1-positive. A Swimmer plot was generated to describe in detail the treatment outcomes of patients evaluated for PD-L1 expression status (Figure 2). The ORR for PD-L1-positive patients was 36.4% (4/11) and DCR was 100% (11/11). The ORR for PD-L1-negative patients was 0 (0/6) and DCR was 50% (3/6). No significant differences in ORR and DCR were observed between the two groups (ORR: P=0.275; DCR: P=0.055). The median PFS of PD-L1-positive and PD-L1-negative patient groups was 5.00 months (95% CI, 4.03–5.97 months) and 1.90 months (95% CI, 0.00–4.61 months), respectively (P=0.166, Figure 3A). The median OS of PD-L1-positive and PD-L1-negative patient groups was 11.30 months (95% CI, 10.23–12.38 months) and not reached, respectively (P=0.966, Figure 3B).

Univariate analysis of clinical features and prognosis
In univariate analysis of PFS and OS, the following criteria were assessed: sex, age, smoking history, ECOG PS score, stage, liver metastasis, brain metastasis, bone metastasis, surgery, previous radiotherapy, previous chemotherapy, EGFR/ALK/KRAS/MET alterations, PD-L1 status, and ICI regimens. Univariate analysis showed that PFS was significantly correlated with age [hazard ratio (HR): 1.047; 95% CI, 1.006–1.089; P=0.024] and PS (HR: 6.657; 95% CI, 1.919–23.091; P=0.003) (Figure 4A) while OS was significantly correlated with PS (HR: 6.970; 95% CI, 1.699–28.593; P=0.007) (Figure 4B). Factors (age and PS) identified as significant in univariate analysis of PFS were selected for Cox multivariate regression analysis, which validated PS as an independent prognostic factor of PFS in ASC patients administered ICI. Poor PS scores were associated with shorter PFS (HR: 4.509; 95% CI, 1.219–16.675; P=0.024).

Toxicity of ICI treatment
Evaluation of toxicity in all 38 patients revealed an incidence of irAEs of 52.6% (20/38), which were mainly classified as Grade 1–2 (16/38, 42.1%). The incidence of Grades 3–4 irAEs was 13.2% (5/38). The most common irAE was malaise in 8 patients (21.1%), including 1 patient with Grade 2. The next most frequently identified irAE was pneumonitis, which was associated with death in one patient. Other common toxicities included elevated liver function tests (LFT; two cases), pruritus (two cases), hypothyroidism (two cases), and renal abnormality (two cases). Patients discontinued ICI treatment due to irAEs, including pneumonitis, hypothyroidism and elevated LFTs. IrAEs occurred in 6 (66.7%) patients in the monotherapy group and 14 (48.3%) patients in the combination therapy group. However, these differences were not statistically significant (P=0.560; Table 2).
Table 2
| Adverse event | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| Malaise | 7 | 1 | ||
| Pneumonitis | 4 | 1 | 1 | |
| Elevated LFTs | 2 | |||
| Pruritus | 1 | 1 | ||
| Hypothyroidism | 2 | |||
| Renal abnormality | 2 | |||
| Skin rash | 1 | |||
| Dry skin | 1 | |||
| Anemia | 1 | |||
| Anorexia | 1 | |||
| Edema limbs | 1 | |||
| Sinus tachycardia | 1 | |||
| Thyroid stimulating hormone increase | 1 | |||
| Hypertension | 1 |
NCI-CTCAE, National Cancer Institute-Common Terminology Criteria for Adverse Events; LFT, liver function test.
Discussion
To our knowledge, this is the first study to focus on the efficacy of ICIs for ASC. In our population of patients with lung ASC receiving ICI therapy, ORR and DCR were 23.7% and 86.8% and overall median PFS and OS were 5.47 and 24.10 months, respectively.
The rapid development of ICIs has transformed the management of numerous cancers and marks the beginning of a new era in cancer therapy (10,11). In recent years, immunotherapy has demonstrated marked efficacy against NSCLC. The clinical efficacy of ICIs for advanced NSCLC is significant, with 3-year OS of 19% in previously treated patients and 26.4% in treatment-naïve patients, and >18 months of PFS (12). In the KEYNOTE-042 study (13), the benefit-to-risk profile suggests that pembrolizumab monotherapy can be extended as first-line therapy to patients with locally advanced or metastatic NSCLC without sensitive EGFR or ALK alterations and low PD-L1 expression. Immunochemotherapy may also have an enhanced effect. For instance, in an earlier study, addition of tislelizumab to chemotherapy led to significantly prolonged PFS, higher ORR, and a manageable safety/tolerability profile in patients with advanced squamous NSCLC, regardless of PD-L1 expression (14). However, immunotherapy is reported to be less effective for patients carrying mutations in specific genes (15,16). In CheckMate 012, nivolumab monotherapy showed poor efficacy in patients with EGFR mutations compared to those with wild-type EGFR (ORR: 14% vs. 30%; mPFS: 1.8 vs. 8.8 months; mOS: 18.8 months vs. not reached) (17). However, owing to the relative rarity of ASC, the efficacy of ICIs for this disease has not been established to date. In this study, overall median PFS and OS in 38 patients with lung ASC receiving PD-1 inhibitors were 5.47 and 24.10 months, respectively. ASC patients receiving ICIs as first-line treatment had similar PFS as those administered ICIs as the second line or beyond. The OS of patients receiving ICIs as first-line treatment was longer than that of patients receiving ICIs as second-line or further treatment. ASC patients administered PD-1 inhibitors as monotherapy had similar PFS and OS as those treated with a combination of specific targeted treatments or chemotherapy. Additionally, median PFS and OS were comparable in patients with and without alterations in EGFR/ALK/KRAS/MET. The lack of meaningful results in our study may be attributed to the small sample size. Univariate and multivariate analyses consistently identified PS as an independent prognostic factor for PFS in ASC patients administered ICIs.
Immunological biomarkers, such as PD-L1, and high tumor mutational burden (TMB) are associated with a good response to ICIs (18). The KEYNOTE-010 study (19) demonstrated that pembrolizumab prolonged OS and had a favorable benefit-to-risk profile in patients with previously treated PD-L1-positive advanced NSCLC. However, high PD-L1 expression does not always reflect good efficacy of ICIs. For example, although the positive PD-L1 expression rate is relatively low (24%, 181/756) in renal cell carcinoma, PD-1/PD-L1 inhibitors are reported to exert significant survival benefits compared with chemotherapy, irrespective of PD-L1 expression (20,21). Few cases of ASC expressing PD-L1 have been documented. In a previous study, PD-L1 expression in ASC was determined as 48.6% at the 5% cutoff (22). Another study reported PD-L1 expression of 21.43% and 39.29% in glandular and squamous components of lung ASC samples, respectively (23). Shi et al. (24) showed similar PD-L1 expression rates in the squamous cell component of ASCs and lung SCC (38.89% vs. 28.92%, P=0.293) as well as the adenocarcinoma component of ASCs and lung adenocarcinomas (11.11% vs. 13.53%, P=1.000). PD-L1 inhibitors may thus present a potentially effective treatment choice for ASC patients with high PD-L1 expression. In our investigation, 17 patients were successfully evaluated for PD-L1 expression status, with 64.7% identified as PD-L1-positive. Notably, expression of PD-L1 was higher relative to previous studies, which could be attributable to the small sample size. ASC patients in the PD-L1-positive group had similar PFS and OS rates as those in the PD-L1-negative group. Further studies with larger sample sizes are required to comprehensively explore the relationship between expression of PD-L1 and efficacy of immunotherapy in ASC.
Finally, ICI treatment for irAEs in ASC should be noted. The incidence of irAEs was 52.6%, with malaise identified as the most frequently observed adverse event. The second most common irAE was pneumonitis, which contributed to the death of one patient. Patients discontinued ICI treatment due to the development of a number of irAEs, including pneumonitis as well as elevated thyroid stimulating hormone and LFTs. No significant differences were observed in the incidence of adverse events between monotherapy and combination therapy groups.
This study had some inherent limitations that should be taken into consideration. Firstly, the sample size of ASC patients treated with ICIs was relatively small and further studies on expanded patient cohorts are essential to validate these data. Secondly, the retrospective nature of the current study may have influenced the results. Thirdly, heterogeneous ICI regimens were used in this research. However, given the lack of relevant clinical studies to date, our findings provide a valuable set of guidelines for further exploring the utility of ICIs as a treatment option for lung ASC.
Conclusions
ICIs may be employed as a treatment option for lung ASC. Further large-scale studies are warranted to explore the relationship between PD-L1 expression and ICI efficacy in ASC. Additionally, prospective studies are necessary in the future to evaluate the efficacy and safety of ICIs as a therapeutic strategy for advanced ASC.
Acknowledgments
We thank International Science Editing (http://www.internationalscienceediting.com) for editing this manuscript.
Funding: This study was funded by the Medical Scientific Research Foundation of Zhejiang Province (No. 2022KY653). Zhengbo Song was sponsored by Zhejiang Provincial Program for the Cultivation of High-level Innovative Health Talents.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1011/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1011/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1011/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1011/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Ethics Committee of Zhejiang Cancer Hospital (No. IRB-2022-187) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Li C, Lu H. Adenosquamous carcinoma of the lung. Onco Targets Ther 2018;11:4829-35. [Crossref] [PubMed]
- Nicholson AG, Tsao MS, Beasley MB, et al. The 2021 WHO Classification of Lung Tumors: Impact of Advances Since 2015. J Thorac Oncol 2022;17:362-87. [Crossref] [PubMed]
- Cooke DT, Nguyen DV, Yang Y, et al. Survival comparison of adenosquamous, squamous cell, and adenocarcinoma of the lung after lobectomy. Ann Thorac Surg 2010;90:943-8. [Crossref] [PubMed]
- Maeda H, Matsumura A, Kawabata T, et al. Adenosquamous carcinoma of the lung: surgical results as compared with squamous cell and adenocarcinoma cases. Eur J Cardiothorac Surg 2012;41:357-61. [Crossref] [PubMed]
- Gawrychowski J, Bruliński K, Malinowski E, et al. Prognosis and survival after radical resection of primary adenosquamous lung carcinoma. Eur J Cardiothorac Surg 2005;27:686-92. [Crossref] [PubMed]
- Nasser NJ, Gorenberg M, Agbarya A. First line Immunotherapy for Non-Small Cell Lung Cancer. Pharmaceuticals (Basel) 2020;13:373. [Crossref] [PubMed]
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:2078-92. [Crossref] [PubMed]
- Manglaviti S, Brambilla M, Signorelli D, et al. Immune-Checkpoint Inhibitors in Advanced Non-Small Cell Lung Cancer With Uncommon Histology. Clin Lung Cancer 2022;23:e17-28. [Crossref] [PubMed]
- Kay FU, Kandathil A, Batra K, et al. Revisions to the Tumor, Node, Metastasis staging of lung cancer (8(th) edition): Rationale, radiologic findings and clinical implications. World J Radiol 2017;9:269-79. [Crossref] [PubMed]
- Baxi S, Yang A, Gennarelli RL, et al. Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: systematic review and meta-analysis. BMJ 2018;360:k793. [Crossref] [PubMed]
- Wilky BA. Immune checkpoint inhibitors: The linchpins of modern immunotherapy. Immunol Rev 2019;290:6-23. [Crossref] [PubMed]
- Remon J, Reguart N, Auclin E, et al. Immune-Related Adverse Events and Outcomes in Patients with Advanced Non-Small Cell Lung Cancer: A Predictive Marker of Efficacy? J Thorac Oncol 2019;14:963-7. [Crossref] [PubMed]
- Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 2019;393:1819-30. [Crossref] [PubMed]
- Wang J, Lu S, Yu X, et al. Tislelizumab Plus Chemotherapy vs Chemotherapy Alone as First-line Treatment for Advanced Squamous Non-Small-Cell Lung Cancer: A Phase 3 Randomized Clinical Trial. JAMA Oncol 2021;7:709-17. [Crossref] [PubMed]
- Hellmann MD, Rizvi NA, Goldman JW, et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol 2017;18:31-41. [Crossref] [PubMed]
- Zhang L, Bai L, Liu X, et al. Factors related to rapid progression of non-small cell lung cancer in Chinese patients treated using single-agent immune checkpoint inhibitor treatment. Thorac Cancer 2020;11:1170-9. [Crossref] [PubMed]
- Gettinger S, Rizvi NA, Chow LQ, et al. Nivolumab Monotherapy for First-Line Treatment of Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2016;34:2980-7. [Crossref] [PubMed]
- Havel JJ, Chowell D, Chan TA. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat Rev Cancer 2019;19:133-50. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Rini BI, Battle D, Figlin RA, et al. The society for immunotherapy of cancer consensus statement on immunotherapy for the treatment of advanced renal cell carcinoma (RCC). J Immunother Cancer 2019;7:354. [Crossref] [PubMed]
- Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med 2015;373:1803-13. [Crossref] [PubMed]
- Liu Y, Dong Z, Jiang T, et al. Heterogeneity of PD-L1 Expression Among the Different Histological Components and Metastatic Lymph Nodes in Patients With Resected Lung Adenosquamous Carcinoma. Clin Lung Cancer 2018;19:e421-30. [Crossref] [PubMed]
- Hlaing AM, Furusato B, Udo E, et al. Expression of phosphatase and tensin homolog and programmed cell death ligand 1 in adenosquamous carcinoma of the lung. Biochem Biophys Res Commun 2018;503:2764-9. [Crossref] [PubMed]
- Shi X, Wu S, Sun J, et al. PD-L1 expression in lung adenosquamous carcinomas compared with the more common variants of non-small cell lung cancer. Sci Rep 2017;7:46209. [Crossref] [PubMed]


