
Effect of switching from continuous to bilevel positive airway pressure on sleep quality in patients with obstructive sleep apnea: the prospective POP IN VAuto study
Highlight box
Key findings
• Switching from CPAP/APAP to bilevel PAP could reduce the occurrence of therapy-related side effects, and improved patient-reported outcomes.
What is known and what is new?
• Utilization of CPAP in clinical practice is often limited by tolerability issues, discomfort and poor sleep quality;
• A switch to second-line therapy with bilevel PAP could help to improve sleep quality and patient comfort for a subset of OSA patients.
What is the implication, and what should change now?
• Study results highlight the role of bilevel PAP as a second-line option for patients with OSA that could potentially improve long-term persistence with PAP therapy and improve the patient experience. Results reinforce the importance of a personalized approach to the treatment of sleep apnea.
Introduction
Obstructive sleep apnea (OSA) is the most common form of sleep-disordered breathing. It is highly prevalent worldwide (1), and negatively impacts on neurocognitive, metabolic and cardiovascular health (2-7). The gold standard therapy for moderate to severe OSA is overnight continuous positive airway pressure (CPAP) delivered via a nasal or facial mask (8), which prevents pharyngeal collapse. In addition to markedly reducing the number of apnea and hypopnea events during sleep, treatment of OSA with CPAP has been shown to have several beneficial effects, including improvements in sleepiness, cognition and mood, and reductions in blood pressure (9-12). Treatment with CPAP has also been shown to reduce hospitalization rates and healthcare costs (13), and may also reduce mortality (14).
Despite its widespread usage and documented benefits, utilization of CPAP in clinical practice is often limited by tolerability issues, including mask leak, skin abrasions, discomfort, nasal congestion and poor sleep quality (15-17). The presence of some side effects can contribute to suboptimal device usage (poor compliance) (18), which is common in patients being treated with CPAP (19). It has been suggested that awareness of CPAP-related side effects and the potential strategies that can be used to alleviate them may be critical to improving treatment adherence (17).
Different positive airway pressure (PAP) modalities were developed and proposed as alternatives to CPAP for treating OSA. These include expiratory pressure relief or bilevel modes, in which a different pressure is provided during inspiration vs. expiration (20). However, there is a lack of evidence for the effects of PAP pressure modification strategies on clinical and patient-reported outcomes (21). Therefore, this study investigated the effects of switching to bilevel PAP in VAuto mode on respiratory parameters, device usage, side effects and patient-reported outcomes in patients reporting device comfort and side effects issues during otherwise effective treatment with CPAP or automatic positive airway pressure (APAP). We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-825/rc).
Methods
Study design
This prospective observational pilot study (https://www.clinicaltrials.gov/, NCT02930460) was conducted during routine clinical practice at eight expert sleep centers in France from December 2016 to April 2018. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The protocol was approved by the French National Ethical Committee (CCTIRS) (Approval No. 16-548) on July 7, 2016. All patients provided written informed consent.
Patients
Eligible patients were adults (age ≥18 years) with OSA who had started treatment with CPAP/APAP at least 3 months previously and had issues tolerating device pressures (>10 cmH2O), or persistently reported side effects or discomfort related to CPAP/APAP therapy (without any issues relating to compliance with existing PAP therapy). No patients had been treated with bilevel PAP therapy. Forty-one patients were eligible and 40 were enrolled. Titration of initial therapy was performed according to international guidelines. The treatment pressure for CPAP/APAP was defined as the 95th percentile pressure during a 5- to 10-day titration period with APAP. Patients with severe respiratory disease, nasal obstruction, psychiatric disease or systolic heart failure (left ventricular ejection fraction ≤45%), or who had any contraindications to bilevel PAP were excluded from the study. The median duration of CPAP/APAP treatment after diagnostic was 1.8 years; 25% of patients had started CPAP/APAP <7 months before switching to bilevel PAP and 50% of patients had started CPAP/APAP <22 months before switching to bilevel PAP.
Treatment
All patients were switched from CPAP/APAP to bilevel PAP with an AirCurve 10 VAuto device (ResMed SAS, Saint-Priest, France) (Figure 1). Physicians set the minimum expiratory positive airway pressure (EPAP; not below 4 cmH2O) and maximum inspiratory positive airway pressure (IPAP) based on average values during previous CPAP/APAP, then the device automatically adjusts values within these two limits. The goal was for bilevel PAP to effectively treat obstructive events (apneas and hypopneas). Patients were given oral and written instructions on how to use the device before returning home, and had access to a helpline in case of any problems.
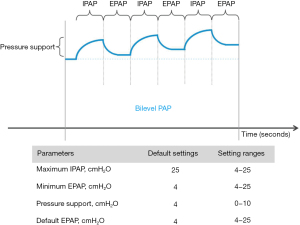
Objectives
This study had a number of objectives. The effectiveness and tolerability of bilevel PAP was determined, along with therapy compliance. In addition, the benefits of bilevel PAP on patient-reported outcomes (sleep quality, daytime sleepiness and fatigue) were assessed. Patient satisfaction with, and preference for, bilevel PAP was also determined.
Assessments and follow-up
Bilevel PAP data was downloaded from the device at 3 and 12 months. Sleep quality was assessed using the Pittsburgh Sleep Quality Index (PSQI) where a score of ≥5 indicates impaired sleep quality and the minimal clinically important difference (MCID) has been defined as ranging from 1.5 to 3 (22,23). Subjective daytime sleepiness was assessed using the Epworth Sleepiness Scale (ESS), where a score of >10 indicates excessive daytime sleepiness and the MCID ranges from 2 to 3 (24). Fatigue was assessed using the Pichot Fatigue Scale, where a score >22 indicates excessive fatigue. Questionnaires were administered and scale scores were determined at baseline prior to initiation of bilevel PAP when patients were still being treated with CPAP/APAP, and then at sleep center visits after 3 and 12 months. The presence or absence of therapy-related side effects was determined by patient interview at each follow-up visit. The presence/severity of side effects was rated on a visual analogue scale from 0 (not at all) to 10 (very important). Patient satisfaction with bilevel PAP was determined based on direct questioning, and asking the question “Do you want to continue treatment?”.
Statistical analysis
Quantitative changes from baseline to the 3- and 12-month follow-up visits are presented as mean values ± standard deviation or median and interquartile range (IQR), and evaluated using paired Student’s t-test or Wilcoxon-Mann-Whitney nonparametric test depending on the normality of distribution and group comparison. Qualitative changes were described using paired frequency distribution and compared using the Chi-squared Mantel-Haenszel test. The McNeymar paired test was used to evaluate binomial changes. All statistical tests were two-sided, and P values <0.05 were considered statistically significant. All analyses were performed using IBM SPSS statistics software (Version 22.0, IBM Corp., Armonk, New York).
Results
Population
A total of 41 patients were assessed at baseline, one of whom was excluded for not meeting study inclusion criteria. Therefore, 40 patients were started on bilevel PAP. Two patients stopped therapy before the 3-month follow-up and another eight stopped before the final assessment at 12 months (Figure 2). Just over two-thirds of patients were male, mean age was 64±11 years, and nearly half of all patients were classified as obese based on the body mass index (Table 1). The most common comorbidity was hypertension (53% of patients), followed by dyslipidemia (40%) and diabetes (22.5%) (Table 1). Based on the mean AHI, patients had severe OSA (Table 1). Four patients changed their mask at the time of starting bilevel PAP: two from a nasal mask to a full-face mask, and two from a full-face mask to a nasal mask. No patients had a mask change during the follow-up period after bilevel PAP initiation.
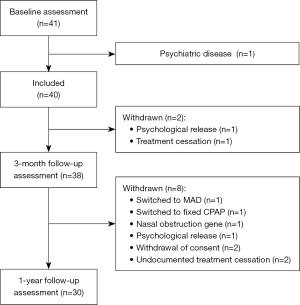
Table 1
| Variables | Patients (n=40) |
|---|---|
| Age, years | 64±11 |
| Male | 27 (67.5) |
| Body mass index, kg/m2 | 30.7±5.8 |
| Current smoker | 12 (30.0) |
| Hypertension | 21 (52.5) |
| Dyslipidemia | 16 (40.0) |
| COPD | 1 (2.5) |
| Type 2 diabetes | 9 (22.5) |
| Baseline AHI, /h | 46.6±19.5 |
| Mean SpO2, % | 91.9±2.6 |
| Nadir SpO2, % | 77.6±7.8 |
| Time spent with SpO2 <90%, min | 60.8±89.3 |
| Oxygen desaturation >3%, /h | 36.3±24.1 |
Values are mean ± standard deviation, or number of patients (%). AHI, apnea-hypopnea index; COPD, chronic obstructive pulmonary disease; SpO2, oxygen saturation.
Device parameters and treatment efficacy
Before switching to bilevel PAP, 25/40 patients (62.5%) were using APAP and 15/40 (37.5%) were using CPAP. Mean pressure during treatment with CPAP/APAP was 11.5±2.1 cmH2O, 52.5% of patients used a heated humidifier and 50% used a nasal mask. After switching from CPAP/APAP to bilevel PAP, residual AHI remained below 5/h (4.0±3.6/h at 1 year vs. 4.9±3.1/h at baseline; P=0.14) and device usage remained good based on usage per night (6.0±2.0 at 1 year vs. 6.1±2.0 at baseline; P=0.31) and the proportion of nights with device usage of >4 h (83.5%±22.5% at 1 year vs. 84.1%±23.6% at baseline; P=0.76) (Table 2). The proportion of patients using a heated humidifier at 3 months and 1 year after switching to bilevel PAP was 45% and 33%, respectively. At 3-month follow-up, a switch to bilevel PAP was associated with significant reductions in EPAP, 95th percentile pressure, leak and 95% percentile leak (Table 2, Figure 3). These reductions were maintained at the 1-year follow-up, although the change in 95th percentile leak was no longer statistically significant compared with baseline on CPAP/APAP (Table 2).
Table 2
| Variable | CPAP/APAP | Bilevel positive airway pressure | |||||
|---|---|---|---|---|---|---|---|
| 3 months | Mean difference | P value | 1 year | Mean difference | P value | ||
| Residual AHI, /h | 4.9±3.1, 4.5 [2.7–7.2] | 4.6±4.0, 4 [2–5.3] | 0.3±3.8 | 0.89 | 4.0±3.6, 3.7 [1.8–5] | 0.9±4.0 | 0.14 |
| Device usage, h/night | 6.1±2.0, 6.4 [4.9–7.3] | 6.3±2.0, 7 [4.9–7.5] | 0.2±1.1 | 0.85 | 6.0±2.0, 6.4 [4.4–7.3] | 0.1±1.1 | 0.31 |
| Nights with usage >4 h, % | 84.1±23.6, 98 [80–100] | 82.9±26.3, 98 [79–100] | 1.2±22.3 | 0.76 | 83.5±22.5, 97 [78–100] | 0.6±25.5 | 0.76 |
| EPAP, cmH2O* | 12.0±2.2, 12 [11–13] | 8.7±2.8, 8 [7–9] | 3.3±2.8 | <0.001 | 9.8±3.4, 9 [8–12] | 2.2±3.4 | 0.005 |
| 95th percentile pressure, cmH2O* | 13.4±2.1, 14 [12–14] | 10.6±2.6, 10 [9–11] | 2.8±2.6 | <0.001 | 10.6±3.1, 10 [8–11] | 2.8±2 .9 | 0.001 |
| Leak, L/min* | 5.5±11.3, 1 [0–6] | 1.9±4.2, 0 [0–1] | 3.6±10.2 | 0.049 | 1.6±3.2, 0 [0–2] | 3.9±1.1 | 0.033 |
| 95th percentile leak, L/min | 15.1±15.3, 13 [3–20] | 10.0±14.2, 5 [0–13] | 5.1±13.9 | 0.017 | 10.3±11.0, 8 [0–16] | 4.8±13.8 | 0.082 |
Values are mean ± standard deviation and median [interquartile range]. *, device-measured mean median values on the period. AHI, apnea-hypopnea index; APAP, automatic positive airway pressure; CPAP, continuous positive airway pressure; EPAP, end-expiratory positive airway pressure.

Patient-reported outcomes
At baseline, patients showed significantly impaired sleep quality based on the PSQI score (7.2±4.0). Switching to bilevel PAP was associated with significant reductions in the PSQI score at both 3- and 12-month follow-up such that the score at 12 months was below the level that indicated impaired sleep quality (4.5±2.7; P<0.001 vs. baseline) (Table 3). Based on the MCID, the proportion of patients had a clinically relevant reduction in PSQI score after switching to bilevel PAP was 55% at 3 months and 70% at 1 year. Patients did not have excessive daytime sleepiness at baseline (ESS score 5.3±3.3), but the ESS score did decrease significantly from baseline to the 12-month follow-up (to 4.1±2.8; P=0.02) (Table 3). The proportion of patients with a clinically relevant change in ESS score at 1 year (based on the MCID) was 46%. The Pichot Fatigue Scale score did not indicate the presence of fatigue at baseline (7.4±5.8) and there was no significant change at 3 months or 1 year after switching to bilevel PAP (6.3±6.1 and 6.4±6.2, respectively; P=0.17 and P=0.48) (Table 3).
Table 3
| Questionnaire Score | CPAP/APAP | Bilevel positive airway pressure | |||||
|---|---|---|---|---|---|---|---|
| 3 months | Difference | P value | 1 year | Difference | P value | ||
| PSQI score (0–21) | 7.2±4.0 | 5.0±3.2 | 2.2±3.4 | 0.005 | 4.5±2.7 | 2.7±2.7 | <0.001 |
| ESS score (0–24) | 5.3±3.3 | 5.2±3.9 | 0.1±4.0 | 0.42 | 4.1±2.8 | 1.2±3.0 | 0.02 |
| Pichot Fatigue Scale score (0–32) | 7.4±5.8 | 6.3±6.1 | 1.2±4.2 | 0.17 | 6.4±6.2 | 1.0±6.1 | 0.48 |
Values are mean ± standard deviation. APAP, automatic positive airway pressure; CPAP, continuous positive airway pressure; ESS, Epworth Sleepiness Scale; PSQI, Pittsburgh Sleep Quality Index.
Side effects and patient satisfaction
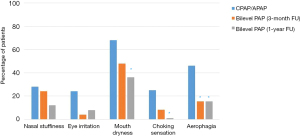
Compared with baseline, the proportion of patients reporting a number of common PAP side effects was reduced at 1 year after switching to bilevel PAP: nasal stuffiness (28% down to 12%; P=0.29); irritated eyes (24% to 8%; P=0.29); dry mouth (68% to 36%; P=0.021); choking sensation (25% to 0%; P=0.031); and aerophagia/bloating (46% to 15%; P=0.008) (Figure 4).
Nearly all patients (96%) preferred the bilevel PAP device over their previous CPAP/APAP device, and 96% stated that they wanted to continue using bilevel PAP.
Discussion
This prospective pilot study investigated the impact of a switch to bilevel PAP in patients reporting side effects during effective treatment with CPAP/APAP. The bilevel PAP VAuto mode provides auto-adjusting pressure between the minimum EPAP and the maximum IPAP to maintain upper airway patency. In combination with the auto EPAP, a fixed pressure support (4 cmH2O) is used to facilitate patient inspiration. Current results showed that the change in therapy mode significantly reduced the occurrence of several problematic side effects and had positive effects on patient-reported outcomes such as sleep quality and daytime sleepiness. Patient satisfaction with bilevel PAP was high, and nearly all patients expressed a preference to continue with this therapy. The proportion of patients remaining on bilevel PAP therapy after 12 months in the current study (74%) compares favorably with that recently reported based on analysis of a large French dataset (77%) (25).
Despite the occurrence of problematic side effects, the patients enrolled in our study were still achieving good levels of CPAP/APAP device usage and therapeutic effectiveness (based on residual AHI) before switching to bilevel PAP. This contrasts with previous studies where patients had low compliance to CPAP/APAP therapy, high pressures and high residual AHI prior to switching to bilevel PAP (26-28). Therefore, the previous studies documented improvements in compliance and residual AHI after the change in PAP mode, but this was not the case in our study where previously good levels of device usage and residual AHI were maintained after the change in PAP therapy mode. Also, in contrast to the previous shorter-term studies (26-28), we showed persistently good adherence and effectiveness of bilevel PAP for up to 1 year. This shows, for the first time to our knowledge, that switching from CPAP/APAP to bilevel PAP has no effect on therapy compliance while decreasing the required expiratory pressure and treatment-related side effects, thus improving patient comfort.
Although patients enrolled in our study were not showing issues with compliance to CPAP/APAP therapy at the time of enrolment into the study, they were reporting significant issues and side effects that had a negative impact on the PAP therapy experience. PAP therapy-related side effects may not be stable over time, but some (such as dry mouth and number of awakenings) have been shown to be significantly associated with worse adherence to therapy over 1 year of follow-up, both in terms of stopping treatment and objective hours of device usage (18). Choking is another side effect that has been reported to have a significant impact on patient compliance (29). In this study, one-quarter of all patients reported a choking sensation while using CPAP/APAP, but the frequency of this side effect had decreased 3 months after switching to bilevel PAP and was almost nonexistent at the 1-year follow-up. This is likely due to the significantly lower EPAP during bilevel PAP compared with previous CPAP/APAP.
It has previously been reported that a switch from CPAP to APAP was associated with a reduction in bloating symptoms (30). This was thought to be due primarily to significantly lower median and 95th centile pressures during APAP vs. CPAP (30). Changing from CPAP to bilevel PAP was also associated with resolution of aerophagia in the majority of patients in another study (31). Similar results were observed in our study, with a significant reduction in the proportion of patients reporting aerophagia at 1 year after switching from CPAP/APAP to bilevel PAP (46.2% to 15.4%; P=0.008). As noted previously, this was likely a result of the significantly lower EPAP at both 3-month and 1-year follow-up in our study.
Switching from CPAP to APAP did not have any effect on the occurrence of dry mouth and mask leaks in an earlier study (32). In contrast, mouth dryness was markedly reduced three months after switching from CPAP/APAP in our study, and the number of patients reporting this side effect was significantly lower after 1 year of bilevel PAP compared with during previous CPAP/APAP. Contributing factors to these findings include the significant reduction in EPAP resulting in less mask leak.
Evaluation of patient-reported outcomes was another important part of the current study. Symptoms associated with OSA, such as daytime sleepiness, can impact on patient quality of life (as well as physical health), and CPAP treatment can have a negative impact on sleep quality (33-35). This means that determining the effects of treatments for OSA on patient-reported outcomes is important. Our results showed significant improvements in daytime sleepiness and sleep quality after patients were switched to bilevel PAP, even though compliance with previous CPAP/APAP was adequate. Similar effects were reported in a previous study, although patients in that study were noncompliant with CPAP prior to switching to bilevel PAP (28). In contrast, data from a meta-analysis failed to show any clinically significant differences in patient outcomes (sleepiness and sleep-related quality of life) with bilevel or APAP compared with CPAP (16). However, the quality of evidence supporting these findings was low (16). Although our data are preliminary, the findings suggest that this is an area that requires additional research because a positive impact of bilevel PAP on patient-reported outcomes could be an important contributor to continuation and optimal usage of PAP therapy in patients with OSA.
A personalized medicine approach to the management of OSA recognizes that there is individual variability and the presentation and course of disease, resulting in different patient phenotypes (36). An OSA phenotype has been broadly defined as “a category of patients with OSA distinguished from others by a single or combination of disease features, in relation to clinically meaningful attributes (symptoms, response to therapy, health outcomes, quality of life) (37). Thus, a “one size fits all” approach to treatment is not appropriate. Therefore, while bilevel PAP is unlikely to represent a first-line treatment option for OSA, the results of the current study suggest that it could be a good option for patients experiencing pressure-related side effects during treatment with CPAP/APAP. The current findings suggest that a switch to second-line therapy with bilevel PAP could help to improve sleep quality and patient comfort for a subset of OSA patients, thus potentially allowing a higher proportion to experience the benefits associated with adequate levels of PAP therapy usage.
Study limitations
Key strengths of this study were its prospective design and the fact that it was conducted at several expert sleep centers; the latter increases the external validity of the study findings. However, the observational design did not include a separate control group, although patients acted as their own control based on comparisons before and after the switch to bilevel PAP. In addition, this pilot study enrolled a limited number of patients. This lack of power could have contributed to a lack of significance in changes from baseline to the 3-month follow-up. Therefore, a randomized study enrolling on a larger number of patients is needed to better determine the effects of switching to bilevel PAP in patients with problematic side effects during CPAP/APAP.
Conclusions
Switching from CPAP/APAP to bilevel PAP maintained therapy efficacy and compliance, reduced the occurrence of therapy-related side effects, and improved patient-reported outcomes. This highlights the role of bilevel PAP as a second-line option for patients with OSA that could potentially improve long-term persistence with PAP therapy and improve the patient experience, thus reinforcing the importance of a personalized approach to the treatment of sleep apnea.
Acknowledgments
Medical writing and editing assistance was provided by Nicola Ryan, independent medical writer, funded by ResMed.
Funding: This study was funded by ResMed.
Footnote
Provenance and Peer Review: This article was a standard submission to the series “Sleep Section” published in the Journal of Thoracic Disease. The article has undergone external peer review.
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-825/dss
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-825/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-825/coif). Alain Palot has received investigator fees for clinical trials funded by ResMed. SL has received investigator or co-investigator fees for clinical trials funded by Nyxoah, Oniris, ResMed, Orthosom, Isis Médical, Asten, has received consulting fees from Nyxoah, Bioprojet, travel grants from Oxyvie, Vitalaire, S2A Santé, UCB Pharma, Bioprojet and Jazz Pharmaceuticals, Speaker’s fees from Les Ateliers D’Arcachon, Cidelec, Philips, ResMed, Bioprojet, Jazz Pharmaceutical, and was an employee of Bioserenity from 2019 to 2021. Arnaud Prigent has received consulting fees from ResMed. AG, EA, and FL are employees of ResMed. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The protocol was approved by the French National Ethical Committee (CCTIRS) (approval No. 16-548) on July 7, 2016. All patients provided written informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Benjafield AV, Ayas NT, Eastwood PR, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med 2019;7:687-98. [Crossref] [PubMed]
- Alomri RM, Kennedy GA, Wali SO, et al. Association between nocturnal activity of the sympathetic nervous system and cognitive dysfunction in obstructive sleep apnoea. Sci Rep 2021;11:11990. [Crossref] [PubMed]
- Farrell PC, Richards G. Recognition and treatment of sleep-disordered breathing: an important component of chronic disease management. J Transl Med 2017;15:114. [Crossref] [PubMed]
- Jennum P, Coaquira Castro J, Mettam S, et al. Socioeconomic and humanistic burden of illness of excessive daytime sleepiness severity associated with obstructive sleep apnoea in the European Union 5. Sleep Med 2021;84:46-55. [Crossref] [PubMed]
- Legault J, Thompson C, Martineau-Dussault MÈ, et al. Obstructive Sleep Apnea and Cognitive Decline: A Review of Potential Vulnerability and Protective Factors. Brain Sci 2021;11:706. [Crossref] [PubMed]
- Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA 2000;283:1829-36. [Crossref] [PubMed]
- Peppard PE, Young T, Palta M, et al. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 2000;342:1378-84. [Crossref] [PubMed]
- Patil SP, Ayappa IA, Caples SM, et al. Treatment of Adult Obstructive Sleep Apnea with Positive Airway Pressure: An American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med 2019;15:335-43. [Crossref] [PubMed]
- Labarca G, Saavedra D, Dreyse J, et al. Efficacy of CPAP for Improvements in Sleepiness, Cognition, Mood, and Quality of Life in Elderly Patients With OSA: Systematic Review and Meta-analysis of Randomized Controlled Trials. Chest 2020;158:751-64. [Crossref] [PubMed]
- Labarca G, Schmidt A, Dreyse J, et al. Efficacy of continuous positive airway pressure (CPAP) in patients with obstructive sleep apnea (OSA) and resistant hypertension (RH): Systematic review and meta-analysis. Sleep Med Rev 2021;58:101446. [Crossref] [PubMed]
- Shirahama R, Tanigawa T, Ida Y, et al. Long-term effect of continuous positive airway pressure therapy on blood pressure in patients with obstructive sleep apnea. Sci Rep 2021;11:19101. [Crossref] [PubMed]
- Walker A, Naughton MT, Shaw L, et al. Depression scores improve with continuous positive airway pressure in specialized sleep clinics: real-world data. J Clin Sleep Med 2021;17:1201-9. [Crossref] [PubMed]
- Cai Q, Tan H, Singer J. Impact of positive airway pressure among obstructive sleep apnea patients. Am J Manag Care 2012;18:e225-33.
- Woehrle H, Schoebel C, Oldenburg O, et al. Low long-term mortality in patients with sleep apnoea and positive airway pressure therapy: analysis of a large German healthcare database. Somnologie 2020;24:151-8.
- Hoffstein V, Viner S, Mateika S, et al. Treatment of obstructive sleep apnea with nasal continuous positive airway pressure. Patient compliance, perception of benefits, and side effects. Am Rev Respir Dis 1992;145:841-5. [Crossref] [PubMed]
- Patil SP, Ayappa IA, Caples SM, et al. Treatment of Adult Obstructive Sleep Apnea With Positive Airway Pressure: An American Academy of Sleep Medicine Systematic Review, Meta-Analysis, and GRADE Assessment. J Clin Sleep Med 2019;15:301-34. [Crossref] [PubMed]
- Ghadiri M, Grunstein RR. Clinical side effects of continuous positive airway pressure in patients with obstructive sleep apnoea. Respirology 2020;25:593-602. [Crossref] [PubMed]
- Ulander M, Johansson MS, Ewaldh AE, et al. Side effects to continuous positive airway pressure treatment for obstructive sleep apnoea: changes over time and association to adherence. Sleep Breath 2014;18:799-807. [Crossref] [PubMed]
- Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc 2008;5:173-8. [Crossref] [PubMed]
- Sanders MH, Kern N. Obstructive sleep apnea treated by independently adjusted inspiratory and expiratory positive airway pressures via nasal mask. Physiologic and clinical implications. Chest 1990;98:317-24. [Crossref] [PubMed]
- Kennedy B, Lasserson TJ, Wozniak DR, et al. Pressure modification or humidification for improving usage of continuous positive airway pressure machines in adults with obstructive sleep apnoea. Cochrane Database Syst Rev 2019;12:CD003531. [Crossref] [PubMed]
- Kim SH, Jeong JH, Lim JH, et al. Acupuncture using pattern-identification for the treatment of insomnia disorder: a systematic review and meta-analysis of randomized controlled trials. Integr Med Res 2019;8:216-26. [Crossref] [PubMed]
- Shergis JL, Ni X, Jackson ML, et al. A systematic review of acupuncture for sleep quality in people with insomnia. Complement Ther Med 2016;26:11-20. [Crossref] [PubMed]
- Patel S, Kon SSC, Nolan CM, et al. The Epworth Sleepiness Scale: Minimum Clinically Important Difference in Obstructive Sleep Apnea. Am J Respir Crit Care Med 2018;197:961-3. [Crossref] [PubMed]
- Pépin JL, Bailly S, Rinder P, et al. CPAP Therapy Termination Rates by OSA Phenotype: A French Nationwide Database Analysis. J Clin Med 2021;10:936. [Crossref] [PubMed]
- Benjafield AV, Pépin JL, Valentine K, et al. Compliance after switching from CPAP to bilevel for patients with non-compliant OSA: big data analysis. BMJ Open Respir Res 2019;6:e000380. [Crossref] [PubMed]
- Carlucci A, Ceriana P, Mancini M, et al. Efficacy of Bilevel-auto Treatment in Patients with Obstructive Sleep Apnea Not Responsive to or Intolerant of Continuous Positive Airway Pressure Ventilation. J Clin Sleep Med 2015;11:981-5. [Crossref] [PubMed]
- Gentina T, Fortin F, Douay B, et al. Auto bi-level with pressure relief during exhalation as a rescue therapy for optimally treated obstructive sleep apnoea patients with poor compliance to continuous positive airways pressure therapy--a pilot study. Sleep Breath 2011;15:21-7. [Crossref] [PubMed]
- Borel JC, Tamisier R, Dias-Domingos S, et al. Type of mask may impact on continuous positive airway pressure adherence in apneic patients. PLoS One 2013;8:e64382. [Crossref] [PubMed]
- Shirlaw T, Hanssen K, Duce B, et al. A Randomized Crossover Trial Comparing Autotitrating and Continuous Positive Airway Pressure in Subjects With Symptoms of Aerophagia: Effects on Compliance and Subjective Symptoms. J Clin Sleep Med 2017;13:881-8. [Crossref] [PubMed]
- Tran KA, Doghramji K. Does transition from CPAP to BIPAP improve symptoms of aerophagia Sleep 2018;41:A203. (abstract 0544).
- Bloch KE, Huber F, Furian M, et al. Autoadjusted versus fixed CPAP for obstructive sleep apnoea: a multicentre, randomised equivalence trial. Thorax 2018;73:174-84. [Crossref] [PubMed]
- Lacasse Y, Godbout C, Sériès F. Health-related quality of life in obstructive sleep apnoea. Eur Respir J 2002;19:499-503. [Crossref] [PubMed]
- Akashiba T, Kawahara S, Akahoshi T, et al. Relationship between quality of life and mood or depression in patients with severe obstructive sleep apnea syndrome. Chest 2002;122:861-5. [Crossref] [PubMed]
- Baldwin CM, Griffith KA, Nieto FJ, et al. The association of sleep-disordered breathing and sleep symptoms with quality of life in the Sleep Heart Health Study. Sleep 2001;24:96-105. [Crossref] [PubMed]
- Edwards BA, Redline S, Sands SA, et al. More Than the Sum of the Respiratory Events: Personalized Medicine Approaches for Obstructive Sleep Apnea. Am J Respir Crit Care Med 2019;200:691-703. [Crossref] [PubMed]
- Zinchuk AV, Gentry MJ, Concato J, et al. Phenotypes in obstructive sleep apnea: A definition, examples and evolution of approaches. Sleep Med Rev 2017;35:113-23. [Crossref] [PubMed]


