
Removing different number of regional lymph nodes affects survival outcomes of operable patients at stage IIA non-small cell lung cancer (according to the 8th edition staging)
Highlight box
Key findings
• Removing different number of RLNs can affect survival outcomes of operable patients at stage IIA NSCLC.
What is known and what is new?
• Surgery combined with chemotherapy is the best treatment for tumor patients at stage I to IIIA.
• The number of RLNs removed can affect survival outcomes of operable patients at stage IIA NSCLC.
What is the implication, and what should change now?
• This means that the benefits of further lymph node dissection are still considerable, and the opinion that excessive lymph node dissection brings too many negative effects should be reassessed.
Introduction
Lung cancer (LC) is one of the top 10 malignant tumors with the highest incidence around the world and it ranked first in the global cancer incidence (11.6%) and mortality (18.4%) in 2018 (1). In the same year, the American Cancer Center reported that approximately 234,030 patients were diagnosed with LC, of which more than 80% were non-small cell lung cancer (NSCLC) patients (2,3). The NSCLC patients have only 15% of 5-year survival rate (4). However, approximately one-third of NSCLC patients who were diagnosed at early stages may have opportunities to be cured by removal of the tumors via surgery (5).
Since staging of LC at the time of initial diagnosis is the most important predictor of survival rate, accurate diagnosis and clinical staging of the LC are the basis for formulating therapy and they form a vital part in the management of these patients (6,7). In 2017, the eighth edition of the tumor, node and metastasis (TNM) classification was published, which provides a higher level of differentiation based on global database, extensive internal validation, sophisticated analysis and multiple evaluations that confirm generalizability (6,8-11). Compared with the 7th edition staging, a significant change in the 8th edition staging is to define stage IIA LC as T2b (tumor size is greater than 4 cm and smaller than or equal to 5 cm) N0M0, and tumor size smaller than or equal to 3 cm with N1M0 (stage IIA in the 7th edition) has been amended to stage IIB. This change provides a more accurate and personalized treatment opportunity for stage IIA NSCLC patients.
According to National Comprehensive Cancer Network Guidelines (Version 1.2020, 2019) in the US, surgery is recommended for suitable patients at stage I to IIIA and this may be the only way to cure NSCLC. Nevertheless, the effect of various surgical methods on survival outcomes of patients is still controversial (12-15). RLN is an important part of radical surgery for patients with limited-stage LC. Martini et al. (16) and Naruke et al. (17) have successively confirmed that removing RLNs in patients with lymph node metastasis can significantly improve the prognosis, which has provided a theoretical basis for the necessity of RLNs removed (18). In addition, extended lymphadenectomy was recommended, and the number of lymph nodes to be removed was 12–22 in the Japanese study (19). However, there are only few studies specifically evaluated the survival benefits of removing different number of RLNs for patients with IIA NSCLC. This study aims at exploring the effect of removing different number of RLNs on survival outcomes in operable patients with IIA NSCLC. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1314/rc).
Methods
Data source
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This retrospective study was conducted to assess the survival outcomes in stage IIA NSCLC surgical patients with different RLNs removed. Data of this study was from the SEER database, which is maintained by the National Cancer Institute in the United States (US). The SEER database includes a population-based cancer registry, established in 1973, which accounts for approximately 10% of the US population (20). According to the SEER RESEARCH DATA RECORD DESCRIPTION CASES DIAGNOSED IN 1973-2011*, scope of Regional Lymph Node Surgery describes the procedure of removal, biopsy, or aspiration of regional lymph nodes performed during the initial work-up or first course of therapy at all facilities.
Study population
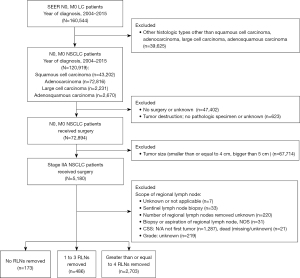
TNM classification was performed according to the criteria of the 8th edition staging of the TNM classification. The cohort was limited to surgical patients at stage IIA NSCLC diagnosed between 2004 and 2015. At the same time, histological type was limited to adenocarcinoma, squamous cell carcinoma, adenosquamous cell carcinoma and large cell carcinoma. Patients with incomplete information or who did not meet the requirements of surgery, pathologic specimen, cancer-specific survival (CSS) (Figure 1) were excluded. The number of RLNs removed was divided into three categories: none RLN removed group, tumor size, RLNs removed 1 to 3 RLNs removed group and greater than or equal to 4 RLNs removed.
Covariates
Baseline clinical characteristics including age, survival time, size of tumor, gender, race, region, year of diagnosis, primary site, grade, laterality, pathology of tumor, stage, radiation, chemotherapy and number of RLNs removed were collected.
Statistical analyses
The Kaplan-Meier analyses and the log-rank test were used to compare LCSS or OS in different groups. Univariate and multivariate Cox regression analyses were performed for the risk factors analysis for survival outcomes: CSS and OS. Predictors (P<0.05) identified in Kaplan-Meier analyses or univariable analyses were entered into a multivariable analysis. Statistical significance was set at a two-tailed P value <0.05. Data were analyzed using IBM SPSS version 21.0 (IBM Corp., Armonk, NY, USA).
Results
Study cohort characteristics
Figure 1 shows a flow diagram of the study for the selection of the appropriate patients from the SEER database. Initially, 160,544 LC patients without lymph nodes and distant metastases were identified. Next, the focus of this study was narrowed down to only four main pathological subtypes: adenocarcinoma, squamous cell carcinoma, adenosquamous cell carcinoma and large cell carcinoma, identifying 120,919 NSCLC patients. Finally, the inclusion criteria were further limited. A total of 3,362 surgical patients at stage IIA NSCLC were included, after excluding the following patients: (I) 47,402 patients without surgery or unknown; (II) 623 patients with tumor destruction but no pathologic specimen or unknown; (III) 67,714 patients with tumor size smaller than or equal to 4 cm or with tumor size greater than 5 cm; (IV) 7 patients with RLNs removed not applicable or unknown; (V) 33 patients with only sentinel lymph node biopsy; (VI) 220 patients with number of RLNs removed unknown; (VII) 31 patients with only biopsy or aspiration of regional lymph node, not otherwise specified; (VIII) 1,287 patients with N/A not first tumor of CSS; (IX) 21 patients with dead (missing/unknown) of CSS; (X) 219 patients with unknown of grade. Patients were divided into three categories as per the number of RLNs removed: no RLNs removed group accounted for 173 patients, 1 to 3 RLNs removed group accounted for 486 patients and greater than or equal to 4 RLNs removed group accounted for 2,703 patients.
A total of 3,362 surgical patients were identified at stage IIA NSCLC, of whom 173 (5.1%), 486 (14.5%) and 2,703 (80.4%) underwent no RLNs removed, 1 to 3 RLNs removed and greater than or equal to 4 RLNs removed, respectively, as a primary treatment from 2004 to 2015. Table 1 shows the based characteristics of all included patients. There were statistically significant differences on LCSS with regard to age (P<0.001), sex (P<0.001), differentiation grade (P<0.001), histologic type (P=0.002), and radiotherapy (RT) (P<0.001) among the three groups by using Kaplan-Meier analyses.
Table 1
| Characteristics | NSCLC, N (%) | P | |||
|---|---|---|---|---|---|
| No RLNs removed | 1 to 3 RLNs removed | Greater than or equal to 4 RLNs removed | Total | ||
| Age (years) | <0.001 | ||||
| Younger than 45 | 3 (1.7) | 4 (0.8) | 35 (1.3) | 42 (1.2) | |
| 45 to 54 | 16 (9.2) | 28 (5.8) | 228 (8.4) | 272 (8.1) | |
| 55 to 64 | 26 (15.1) | 105 (21.6) | 662 (24.5) | 793 (23.6) | |
| 65 to 74 | 61 (35.3) | 192 (39.5) | 1,012 (37.4) | 1,265 (37.6) | |
| Older than or equal to 75 | 67 (38.7) | 157 (32.3) | 766 (28.4) | 990 (29.5) | |
| Sex | <0.001 | ||||
| Female | 69 (39.9) | 210 (43.2) | 1,184 (43.8) | 1,463 (43.5) | |
| Male | 104 (60.1) | 276 (56.8) | 1,519 (56.2) | 1,899 (56.5) | |
| Race | 0.263 | ||||
| White | 143 (82.7) | 414 (85.2) | 2,285 (84.5) | 2,842 (84.5) | |
| Black | 18 (10.4) | 39 (8.0) | 238 (8.8) | 295 (8.8) | |
| Others | 12 (6.9) | 33 (6.8) | 180 (6.7) | 225 (6.7) | |
| Tumor location | 0.252 | ||||
| Upper lobe | 98 (56.6) | 299 (61.5) | 1,598 (59.1) | 1,995 (59.3) | |
| Middle lobe | 7 (4.1) | 22 (4.6) | 89 (3.3) | 118 (3.5) | |
| Lower lobe | 55 (31.8) | 160 (32.9) | 901 (33.3) | 1,116 (33.2) | |
| Not otherwise specified | 5 (2.9) | 2 (0.4) | 23 (0.9) | 30 (0.9) | |
| Overlapping lesion | 3 (1.7) | 2 (0.4) | 59 (2.2) | 64 (1.9) | |
| Main bronchus | 5 (2.9) | 1 (0.2) | 33 (1.2) | 39 (1.2) | |
| Differentiation grade | <0.001 | ||||
| Well differentiated | 20 (11.6) | 44 (9.1) | 259 (9.6) | 323 (9.6) | |
| Moderately differentiated | 68 (39.3) | 211 (43.4) | 1,148 (42.5) | 1,427 (42.4) | |
| Poorly differentiated | 77 (44.5) | 215 (44.2) | 1,225 (45.3) | 1,517 (45.2) | |
| Undifferentiated | 8 (4.6) | 16 (3.3) | 71 (2.6) | 95 (2.8) | |
| Laterality | 0.849 | ||||
| Right-origin of primary | 100 (57.8) | 296 (60.9) | 1,525 (56.4) | 1,921 (57.1) | |
| Left-origin of primary | 73 (42.2) | 190 (39.1) | 1,175 (43.5) | 1,438 (42.8) | |
| One side, unspecified | 0 (0.0) | 0 (0.0) | 2 (0.1) | 2 (0.1) | |
| Paired site | 0 (0.0) | 0 (0.0) | 1 (0.0) | 1 (0.0) | |
| Histologic type | 0.002 | ||||
| Adenocarcinoma | 88 (50.9) | 248 (51.0) | 1,324 (49.0) | 1,660 (49.4) | |
| Squamous cell carcinoma | 69 (39.9) | 208 (42.8) | 1,199 (44.4) | 1,476 (43.9) | |
| Adenosquamous | 4 (2.3) | 15 (3.1) | 103 (3.8) | 122 (3.6) | |
| Large cell carcinoma | 12 (6.9) | 15 (3.1) | 77 (2.8) | 104 (3.1) | |
| Radiotherapy | <0.001 | ||||
| Yes | 36 (20.8) | 67 (13.8) | 251 (9.3) | 354 (10.5) | |
| No | 137 (79.2) | 419 (86.2) | 2,452 (90.7) | 3,008 (89.5) | |
| Chemotherapy | 0.231 | ||||
| Yes | 43 (24.9) | 132 (27.2) | 768 (28.4) | 943 (28.0) | |
| No | 130 (75.1) | 354 (72.8) | 1,935 (71.6) | 2,419 (72.0) | |
NSCLC, non-small cell lung cancer; RLNs, regional lymph nodes.
Univariate analysis showed that there were statistically significant differences on LCSS with regard of age (P<0.001), sex (P<0.001), differentiation grade (P<0.001), histologic type (P=0.002), size (P=0.002), diagnosis (P=0.001), number of RLNs removed (P<0.001), RT (P<0.001) and radiation surgery (P<0.001) with the prognosis of stage IIA NSCLC patients. The outcomes of statistically significant differences on OS were the same as that of LCSS except chemotherapy, whose P value was <0.001 (Table 2).
Table 2
| Variables | Univariable analysis | Multivariable analysisa | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| Age (years) | <0.001 | <0.001 | |||||
| Younger than 45 | Reference | Reference | |||||
| 45 to 54 | 1.544 | 0.801 to 2.979 | 0.195 | 1.592 | 0.824 to 3.077 | 0.209 | |
| 55 to 64 | 1.736 | 0.923 to 3.265 | 0.087 | 1.763 | 0.934 to 3.327 | 0.121 | |
| 65 to 74 | 2.121 | 1.133 to 3.969 | 0.019 | 2.281 | 1.215 to 4.283 | 0.021 | |
| Older than or equal to 75 | 2.518 | 1.344 to 4.719 | 0.004 | 2.728 | 1.451 to 5.131 | 0.005 | |
| Sex | <0.001 | ||||||
| Female | Reference | Reference | |||||
| Male | 1.25 | 1.113 to 1.404 | <0.001 | 1.202 | 1.068 to 1.353 | 0.001 | |
| Differentiation grade | <0.001 | <0.001 | |||||
| Well differentiated | Reference | Reference | |||||
| Moderately differentiated | 1.696 | 1.328 to 2.166 | <0.001 | 1.64 | 1.278 to 2.105 | <0.001 | |
| Poorly differentiated | 1.912 | 1.501 to 2.436 | <0.001 | 1.77 | 1.378 to 2.274 | <0.001 | |
| Undifferentiated | 2.151 | 1.468 to 3.150 | <0.001 | 1.815 | 1.140 to 2.889 | 0.012 | |
| Histologic type | 0.002 | 0.145 | |||||
| Adenocarcinoma | Reference | Reference | |||||
| Squamous cell carcinoma | 1.104 | 0.979 to 1.244 | 0.106 | 0.958 | 0.845 to 1.186 | 0.501 | |
| Adenosquamous | 1.52 | 1.158 to 1.997 | 0.003 | 1.317 | 0.998 to 1.738 | 0.051 | |
| Large cell carcinoma | 1.435 | 1.080 to 1.907 | 0.013 | 1.085 | 0.752 to 1.566 | 0.662 | |
| Size | 0.002 | 0.017 | |||||
| 41 | Reference | Reference | |||||
| 42 | 1.08 | 0.749 to 1.557 | 0.682 | 1.024 | 0.721 to 1.505 | 0.901 | |
| 43 | 1.045 | 0.700 to 1.560 | 0.829 | 0.99 | 0.662 to 1.481 | 0.972 | |
| 44 | 1.069 | 0.680 to 1.682 | 0.772 | 1.029 | 0.653 to 1.622 | 0.882 | |
| 45 | 1.171 | 0.842 to 1.629 | 0.349 | 1.138 | 0.816 to 1.587 | 0.487 | |
| 46 | 0.914 | 0.562 to 1.485 | 0.716 | 0.914 | 0.561 to 1.489 | 0.621 | |
| 47 | 1.223 | 0.810 to 1.846 | 0.339 | 1.168 | 0.773 to 1.766 | 0.468 | |
| 48 | 1.123 | 0.747 to 1.687 | 0.578 | 1.046 | 0.695 to 1.575 | 0.788 | |
| 49 | 1.741 | 1.060 to 2.859 | 0.029 | 1.597 | 0.969 to 2.632 | 0.065 | |
| 50 | 1.481 | 1.066 to 2.057 | 0.019 | 1.378 | 0.990 to 1.919 | 0.063 | |
| Diagnosis | 0.001 | 0.055 | |||||
| 2004 to 2006 | Reference | Reference | |||||
| 2007 to 2009 | 0.907 | 0.785 to 1.047 | 0.181 | 0.959 | 0.830 to 1.108 | 0.572 | |
| 2011 to 2013 | 0.85 | 0.726 to 0.997 | 0.045 | 0.9 | 0.767 to 1.057 | 0.2 | |
| 2013 to 2015 | 0.668 | 0.544 to 0.820 | <0.001 | 0.752 | 0.612 to 0.928 | 0.008 | |
| Number of RLNs removed | <0.001 | <0.001 | |||||
| None | Reference | Reference | |||||
| 1 to 3 | 0.602 | 0.469 to 0.771 | <0.001 | 0.622 | 0.484 to 0.800 | <0.001 | |
| Greater than or equal to 4 | 0.484 | 0.390 to 0.600 | <0.001 | 0.545 | 0.437 to 0.680 | <0.001 | |
| Radiotherapy | <0.001 | ||||||
| Yes | Reference | Reference | |||||
| No | 0.562 | 0.479 to 0.659 | <0.001 | 0.579 | 0.491 to 0.683 | <0.001 | |
| Radiation surgery | <0.001 | ||||||
| Both prior and after | Reference | ||||||
| Radiation prior to surgery | 190.533 | 0.000 to 2.084E+20 | 0.804 | ||||
| Radiation after surgery | 283.097 | 0.000 to 3.094E+20 | 0.79 | ||||
| No radiation surgery | 143.063 | 0.000 to 1.563E+20 | 0.815 | ||||
| Chemotherapy | 0.23 | ||||||
| Yes | Reference | ||||||
| No | 1.08 | 0.952 to 1.226 | 0.233 | ||||
| Race | 0.25 | ||||||
| White | Reference | ||||||
| Black | 0.839 | 0.678 to 1.038 | 0.107 | ||||
| Other | 0.962 | 0.762 to 1.214 | 0.744 | ||||
| Site | 0.268 | ||||||
| Upper lobe | Reference | ||||||
| Middle lobe | 0.79 | 0.563 to 1.110 | 0.174 | ||||
| Lower lobe | 1.057 | 0.933 to 1.196 | 0.383 | ||||
| NOS | 1.092 | 0.602 to 1.982 | 0.772 | ||||
| Overlapping lesion | 1.238 | 0.859 to 1.785 | 0.252 | ||||
| Main bronchus | 1.433 | 0.918 to 2.235 | 0.113 | ||||
| Laterality | 0.873 | ||||||
| Right-origin of primary | Reference | ||||||
| Left-origin of primary | 1.028 | 0.916 to 1.154 | 0.636 | ||||
| Only one side unspecified | 2.078 | 0.292 to 14.779 | 0.465 | ||||
| Paired site | 0.007 | 0.000 to 1.634E+55 | 0.941 | ||||
a, multivariate analysis for age, sex, differentiation grade, histologic type, size, diagnosis, number of RLNs removed, and radiotherapy. LCSS, lung specific survival time; NSCLC, non-small cell lung cancer; HR, hazard ratio; CI, confidence interval; RLNs, regional lymph nodes.
A cox model on LCSS was established by using multivariable analysis of Cox regression. The result showed that there were statistically significant differences (P<0.001) on the overall score of the model coefficients in all the included patients and demonstrated that the prognoses of surgical patients at stage IIA NSCLC were related to the factors such as age, sex, differentiation grade, tumor size, number of RLNs removed and RT. Also, the Cox model on OS showed that there were statistically significant differences (P<0.001) on overall score of the model coefficients in all the included patients and demonstrated that the prognoses of surgical patients at stage IIA NSCLC were related to the factors such as age, sex, differentiation grade, tumor size, number of RLNs removed, RT and CT.
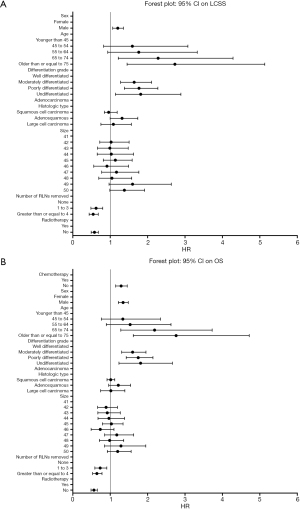
Table 2 also shows the HR and 95% CI on LCSS in the three groups: no RLNs removed group, 1 to 3 RLNs removed group and greater than or equal to 4 RLNs removed group. The result demonstrated that removing different number of RLNs can affect significantly the survival outcomes (LCSS) of surgical patients at stage IIA NSCLC. The result in Table 3 demonstrates the same conclusion of removing different number of RLNs and it affected significantly the survival outcomes (OS) of surgical patients at stage IIA NSCLC. Figure 2 are the Forest Plot of 95% CI in multivariable Cox regression on LCSS and OS, respectively.
Table 3
| Variables | Univariable analysis | Multivariable analysisa | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| Age (years) | <0.001 | <0.001 | |||||
| Younger than 45 | Reference | Reference | |||||
| 45 to 54 | 1.46 | 0.836 to 2.549 | 0.184 | 1.338 | 0.765 to 2.342 | 0.209 | |
| 55 to 64 | 1.741 | 1.020 to 2.970 | 0.042 | 1.533 | 0.896 to 2.624 | 0.121 | |
| 65 to 74 | 2.408 | 1.418 to 4.088 | 0.001 | 2.186 | 1.283 to 3.724 | 0.021 | |
| Older than or equal to 75 | 3.1 | 1.825 to 5.267 | <0.001 | 2.762 | 1.617 to 4.716 | 0.005 | |
| Sex | <0.001 | ||||||
| Female | Reference | Reference | |||||
| Male | 1.376 | 1.250 to 1.514 | <0.001 | 1.344 | 1.219 to 1.482 | 0.001 | |
| Differentiation grade | <0.001 | <0.001 | |||||
| Well differentiated | Reference | Reference | |||||
| Moderately differentiated | 1.67 | 1.371 to 2.034 | <0.001 | 1.598 | 1.306 to 1.956 | <0.001 | |
| Poorly differentiated | 1.852 | 1.523 to 2.252 | <0.001 | 1.749 | 1.427 to 2.143 | <0.001 | |
| Undifferentiated | 1.959 | 1.430 to 2.682 | <0.001 | 1.812 | 1.233 to 2.663 | 0.002 | |
| Histologic type | <0.001 | 0.453 | |||||
| Adenocarcinoma | Reference | Reference | |||||
| Squamous cell carcinoma | 1.199 | 1.088 to 1.322 | <0.001 | 1.013 | 0.915 to 1.122 | 0.803 | |
| Adenosquamous | 1.417 | 1.121 to 1.790 | 0.003 | 1.216 | 0.959 to 1.543 | 0.106 | |
| Large cell carcinoma | 1.317 | 1.033 to 1.678 | 0.026 | 1.017 | 0.743 to 1.392 | 0.917 | |
| Size | <0.001 | 0.004 | |||||
| 41 | Reference | Reference | |||||
| 42 | 0.951 | 0.709 to 1.274 | 0.734 | 0.89 | 0.663 to 1.194 | 0.901 | |
| 43 | 0.981 | 0.714 to 1.374 | 0.904 | 0.922 | 0.670 to 1.269 | 0.972 | |
| 44 | 1.031 | 0.721 to 1.476 | 0.867 | 0.966 | 0.674 to 1.384 | 0.882 | |
| 45 | 1.078 | 0.831 to 1.400 | 0.57 | 1.032 | 0.793 to 1.342 | 0.487 | |
| 46 | 0.752 | 0.503 to 1.125 | 0.165 | 0.734 | 0.490 to 1.100 | 0.621 | |
| 47 | 1.255 | 0.909 to 1.733 | 0.168 | 1.176 | 0.851 to 1.626 | 0.468 | |
| 48 | 1.064 | 0.771 to 1.469 | 0.706 | 0.985 | 0.712 to 1.362 | 0.788 | |
| 49 | 1.469 | 0.971 to 2.220 | 0.068 | 1.287 | 0.849 to 1.952 | 0.065 | |
| 50 | 1.312 | 1.012 to 1.702 | 0,040 | 1.199 | 0.923 to 1.559 | 0.063 | |
| Diagnosis | 0.002 | 0.068 | |||||
| 2004 to 2006 | Reference | Reference | |||||
| 2007 to 2009 | 0.992 | 0.883 to 1.115 | 0.892 | 1.034 | 0.919 to 1.163 | 0.575 | |
| 2011 to 2013 | 0.881 | 0.770 to 1.007 | 0.063 | 0.939 | 0.820 to 1.076 | 0.366 | |
| 2013 to 2015 | 0.738 | 0.620 to 0.878 | 0.001 | 0.827 | 0.693 to 0.986 | 0.035 | |
| Number of RLNs removed | <0.001 | <0.001 | |||||
| None | Reference | Reference | |||||
| 1 to 3 | 0.705 | 0.569 to 0.874 | 0.001 | 0.732 | 0.589 to 0.909 | 0.005 | |
| Greater than or equal to 4 | 0.571 | 0.472 to 0.690 | <0.001 | 0.643 | 0.530 to 0.781 | <0.001 | |
| Radiotherapy | <0.001 | ||||||
| Yes | Reference | Reference | |||||
| No | 0.635 | 0.554 to 0.728 | <0.001 | 0.565 | 0.487 to 0.655 | <0.001 | |
| Radiation surgery | <0.001 | ||||||
| Both prior and after | Reference | ||||||
| Radiation prior to surgery | 156.959 | 0.000 to 1.225E+18 | 0.787 | ||||
| Radiation after surgery | 263.266 | 0.000 to 2.053E+18 | 0.765 | ||||
| No radiation surgery | 148.222 | 0.000 to 1.155E+18 | 0.789 | ||||
| Chemotherapy | <0.001 | ||||||
| Yes | Reference | ||||||
| No | 1.297 | 1.164 to 1.445 | <0.001 | 1.292 | 1.146 to 1.455 | <0.001 | |
| Race | 0.001 | ||||||
| White | Reference | ||||||
| Black | 0.773 | 0.646 to 0.924 | 0.005 | ||||
| Other | 0.762 | 0.617 to 0.940 | 0.011 | ||||
| Site | 0.401 | ||||||
| Upper lobe | Reference | ||||||
| Middle lobe | 0.795 | 0.603 to 1.047 | 0.102 | ||||
| Lower lobe | 1.06 | 0.959 to 1.173 | 0.254 | ||||
| NOS | 0.93 | 0.558 to 1.548 | 0.78 | ||||
| Overlapping lesion | 1.017 | 0.736 to 1.406 | 0.918 | ||||
| Main bronchus | 1.117 | 0.745 to 1.675 | 0.591 | ||||
| Laterality | 0.197 | ||||||
| Right-origin of primary | Reference | ||||||
| Left-origin of primary | 1.03 | 0.938 to 1.132 | 0.536 | ||||
| Only one side unspecified | 1.517 | 0.213 to 10.781 | 0.677 | ||||
| Paired site | 21.018 | 2.942 to 150.170 | 0.002 | ||||
a, multivariate analysis for age, sex, differentiation grade, histologic type, size, diagnosis, number of RLNs removed, radiotherapy and chemotherapy. OS, overall survival; NSCLC, non-small cell lung cancer; HR, hazard ratio; CI, confidence interval; RLNs, regional lymph nodes; NOS, not otherwise specified.
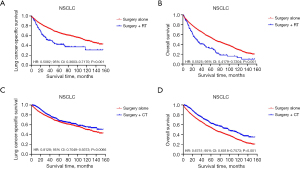
Comparison of survival curve: role of RT and CT on survival outcomes
Survival curves were compared to investigate the effect between RT and CT on survival outcomes in surgical patients at stage IIA NSCLC (Figure 3). Figure 3A,3B shows the effect of RT on LCSS and OS, respectively. 2,419 patients (surgery alone 2,295, surgery + RT 124) were included after excluding 943 patients who received CT in this investigation. The survival curve of surgery alone group was superior to that of surgery + RT group (log rank P<0.001). In Figure 3C,3D, survival curves are compared to study the effect of CT. In this investigation, 3,008 patients (surgery alone 2,295, surgery + CT 713) were included after excluding 354 patients who received RT. The survival curve of surgery + CT group was superior to that of surgery alone group (log rank P=0.0066 on LCSS, P<0.001 on OS). The result shows that CT may contribute to survival outcomes, while RT may harm survival outcomes on surgical patients at stage IIA NSCLC.
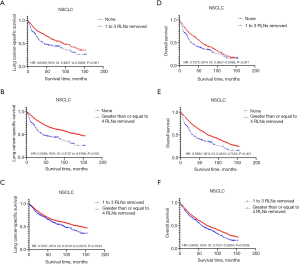
Comparison of survival curves among three groups: no RLNs removed, 1 to 3 RLNs removed and greater than or equal to 4 RLNs removed
The survival curve was compared among three groups: no RLNs removed group, 1 to 3 RLNs removed group and greater than or equal to 4 RLNs removed group, by using Kaplan-Meier analysis. Figure 4 shows that the survival curve on LCSS, OS of the greater than or equal to 4 RLNs removed group was better than that of the others, and the survival curve on LCSS, OS of 1 to 3 RLNs removed group was better than that of no RLNs removed group (log rank P<0.001) in the whole study cohort.
Comparison of OS, LCSS and survival time in the three groups: no RLNs removed, 1 to 3 RLNs removed and greater than or equal to 4 RLNs removed
Table 4 shows the survival time of no RLNs removed group, 1 to 3 RLNs removed group and greater than or equal to 4 RLNs removed group. OS and LCSS were 47.4% and 64.8% in all the included patients, respectively. The OS and LCSS of no RLNs removed group were 32.9% and 47.4%, respectively. The OS and LCSS of 1 to 3 RLNs removed group were 38.5% and 59.3%, respectively. The OS and LCSS of greater than or equal to 4 RLNs removed group were 49.9% and 67.0%, respectively. The results show that the OS and LCSS of greater than or equal to 4 RLNs removed group are better than that of the other groups, and the OS and LCSS of 1 to 3 RLNs removed group are better than that of no RLNs removed group in surgical patients at stage IIA NSCLC.
Table 4
| Variables | Number | Survival (%) | Median survival time (months) | Mean survival time (months) | |
|---|---|---|---|---|---|
| Overall survival | Lung cancer-specific survival | ||||
| Overall patients | 3,362 | 47.4 | 64.8 | 42.0 | 50.8 |
| Number of RLNs removed | |||||
| None | 173 | 32.9 | 47.4 | 24.0 | 38.7 |
| 1 to 3 | 486 | 38.5 | 59.3 | 44.0 | 51.2 |
| Greater than or equal to 4 | 2,703 | 49.9 | 67.0 | 42.0 | 51.5 |
NSCLC, non-small cell lung cancer; RLNs, regional lymph nodes.
Table 4 also shows the median survival time (MDST) and the mean survival time (MST) of the three groups. In all the included patients, the MDST and the MST were 42.0 and 50.8 months, respectively. The MDST and the MST of the no RLNs removed group were 24.0 and 38.7 months, respectively. The MDST and the MST of the 1 to 3 RLNs removed group were 44.0 and 51.2 months, respectively. The MDST and the MST of the greater than or equal to 4 RLNs removed group were 42.0 and 51.5 months, respectively. All in all, the MDST and the MST of greater than or equal to 4 RLNs removed group are better than that of the other groups, and the MDST and the MST of 1 to 3 RLNs removed group are better than that of no RLNs removed group in surgical patients at stage IIA NSCLC.
Discussion
Surgical treatment with lymph node dissection (LND) is the most commonly applied treatment of NSCLC. But there is no specific requirement for the number of LND in the guidelines. At present, the significance of LND in radical surgery of LC mainly includes two aspects: one is to ensure complete tumor resection; another is to ensure the accuracy of lymph node staging (21,22).
Some literatures also reported that the number of LND may impact the accuracy of staging and prognosis. For example, David et al. reported that the OS of patients removed more than 10 lymph nodes was significantly better than that of patients removed smaller than 10 nodes (23). Another study reported that the OS of patients removed greater than 6 lymph nodes was significantly better than that of patients removed lymph nodes smaller than or equal to 6 (24). Liang et al. found that removing more than 16 lymph nodes may influence the survival outcomes of patients at stage I–III LC (25). However, there are currently few researches on whether removing different number of RLNs affects the survival outcomes of surgical patients at stage IIA NSCLC (according the 8th edition staging). The study demonstrated the association between an increased number of dissected RLNs following resection for NSCLC and better long-term survival rates of operable patients at stage IIA NSCLC, and with higher number of removed RLNs, a more positive effect may be obtained as shown by comparing survival curve, HR, OS, LCSS, MDST, MST and survival time of three group: no RLNs removed group, 1 to 3 RLNs removed group and greater than or equal to 4 RLNs removed group. Furthermore, the survival outcomes of greater than or equal to 4 RLNs removed group is also superior to that of 1 to 3 RLNs removed group on the following aspects: survival curve, HR, OS, LCSS. All of our analysis outcomes illustrate that removing different number of RLNs can affect survival outcomes of operable patients at stage IIA NSCLC to different extent, and patients with higher number of RLNs removed may obtain better survival outcomes.
We found that 75.25% of operable patients at stage IIA NSCLC had greater than or equal to 4 RLNs removed, whereas 5.88% had no lymph nodes removed and 18.85% had 1 to 3 RLNs removed from 2004 to 2006. In recent 15 years, there had been a small increase percentage of greater than or equal to 4 RLNs removed, which reached 85.55% in between 2013 to 2015. The above data suggested that both doctors and patients are aware of the benefits of having more RLNs removed for patient prognosis at stage IIA NSCLC (Figure S1).
From the study, the survival outcomes of surgery + CT group were better than that of surgery alone group. Therefore, we compared the survival curves of the three groups in the patients underwent surgery + CT. The result showed that the survival curve of the RLNs removed greater than or equal to 4 group was better than none RLN removed group (log rank P=0.0041), but there was no statistical difference of other survival curves (log rank P>0.05) in patients underwent surgery + CT (Figure S2). It is a known fact that surgery combined with chemotherapy is the best treatment for tumors, but as per the grouping from the study, different number of RLNs removed seems to have little effect on the survival rate. However, the effect of removing different numbers of RLNs on the prognosis of patients at stage IIA NSCLC in result 3.3 is still valid. We think the difference in results may be due to bias caused by discontinuity or unknown on specific number of RLNs removed or location of RLNs, which requires further study.
Currently, there is still lack of a unified definition for LND in LC surgery. The European Society of Thoracic Surgeons (ESTS) has defined the method of LND, which is currently the most recognized version. LND is commonly divided into systematic lymph node dissection (SYLND) or selective LND. At present, SYLND is the gold standard for LND. According to ESTS recommendations, SYLND should remove hilar and interlobular lymph nodes, and at least 3 regions of mediastinal lymph nodes including subcarinal should be removed at the same time. En bloc removal of lymph nodes should be performed to the greatest extent (26). Selective LND refers to the selective dissection of RLNs in the mediastinum according to the location of the tumor (27). This scheme may reduce the complete tumor resection rate and affect the accuracy of staging. Therefore, it has not been widely used, and its feasibility needs further verification (28,29). For better surgical quality control, currently, lymph node atlas of the 8th edition TNM staging provides a standard for the scope of LND. In this study, our findings are consistent with the recommendations of ESTS and may provide a theoretical basis for the necessity of RLNs removed. The results of this study question why the excision of lymph nodes that are not infiltrated by tumor cells is beneficial to the patient prognosis and whether these lymph nodes are really unaffected by the tumor. There are five main reasons to explain this phenomenon: (I) besides the form of tumor cells, tumors may metastasize in the form of free nucleic acid which cannot be detected by common laboratory tests (30); (II) studies have shown that the local tissue microenvironment may have changed before the appearance of tumors, which is conducive to tumorigenesis (31); (III) a greater number of dissected LNs is interpreted as associated with a lower risk of undiscovered positive lymph nodes which increases the staging accuracy (25); (IV) more extensive LN dissection may reflect better technical skill of the treatment team perhaps; (V) negative LNs may reflect the host’s immune response to tumor cells’ survival perhaps (32).
There are still some risks in the LND that are required to be addressed. For example, LND may damage the mediastinal nerve, blood vessel and lymphatic vessel, prolong the time of operation, increase postoperative drainage volume as well as increasing the incidence of chylothorax and injuring laryngeal recurrent nerve (33). Therefore, avoiding damage to important tissues in LND may be beneficial in reducing postoperative complications (34).
RT and CT are discussed here. It can be seen from the results that RT may yield a negative effect on survival outcomes in surgical patients with IIA NSCLC. After excluding patients who received RT, patients underwent surgery + CT had better survival outcomes than patients underwent surgery alone in patients with IIA NSCLC. The results are consistent with the National Comprehensive Cancer Network guidelines (Version 1.2020, 2019) that recommend CT, but not RT, for patients at stage IIA NSCLC. Therefore, this study may provide a theoretical basis for adjuvant therapy in patients with IIA NSCLC.
Admittedly, there are some limitations in the study, mainly owing to its retrospective design. Firstly, patients selected from the SEER database were roughly divided into only three groups based on the number of RLNs removed, not grouping patients according the specific number of RLNs removed. Secondly, the SEER database does not provide specific region and number of RLNs removed which cannot be included in the propensity score matching. These information should be included in future studies. However, with the inclusion of 10 variables and nearly 3,400 patients in our cohort, the present study can represent generalized and credible results by well-balanced analysis of different number of RLNs on survival outcomes. Therefore, our conclusion is a more accurate basis for the surgical approach in the absence of large-scale data from prospective trials, providing useful information for the management of patients at stage IIA NSCLC.
Conclusions
This study illustrates that removing different number of RLNs can affect survival outcomes of operable patients at stage IIA NSCLC. Whether more radical lymphadenectomy is beneficial to patients at stage IIA NSCLC would still need to be further researched.
Acknowledgments
We would like to thank all the staff of the National Cancer Institute for their efforts in the SEER program.
Funding: This work was supported by the National Natural Science Foundation of China (No. 81802262) and Shanghai Tenth Hospital’s improvement plan for NSFC (No. 21JC1402202).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1314/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1314/coif). All authors report that this work was supported by the National Natural Science Foundation of China (No. 81802262) and Shanghai Tenth Hospital’s improvement plan for NSFC (No. 21JC1402202). The authors have no other conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Oser MG, Niederst MJ, Sequist LV, et al. Transformation from non-small-cell lung cancer to small-cell lung cancer: molecular drivers and cells of origin. Lancet Oncol 2015;16:e165-72. [Crossref] [PubMed]
- Barlesi F, Mazieres J, Merlio JP, et al. Routine molecular profiling of patients with advanced non-small-cell lung cancer: results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT). Lancet 2016;387:1415-26. [Crossref] [PubMed]
- Nagasaka M, Gadgeel SM. Role of chemotherapy and targeted therapy in early-stage non-small cell lung cancer. Expert Rev Anticancer Ther 2018;18:63-70. [Crossref] [PubMed]
- Rami-Porta R, Call S, Dooms C, et al. Lung cancer staging: a concise update. Eur Respir J 2018;51:1800190. [Crossref] [PubMed]
- Rami-Porta R, Bolejack V, Giroux DJ, et al. The IASLC lung cancer staging project: the new database to inform the eighth edition of the TNM classification of lung cancer. J Thorac Oncol 2014;9:1618-24.
- Brierley JD, Gospodarowicz MK, Wittekind Ch, editors. UICC TNM Classification of Malignant Tumours. 8th Edn. Oxford: Wiley Blackwell, 2017.
- Amin MB. editor. AJCC Cancer Staging Manual. 8th Edn. Springer, 2017.
- Rami-Porta R. editor. IASLC Staging Manual in Thoracic Oncology. North Fort Myers, Editorial Rx Press, 2016.
- Detterbeck FC, Boffa DJ, Kim AW, et al. The Eighth Edition Lung Cancer Stage Classification. Chest 2017;151:193-203.
- Postmus PE, Kerr KM, Oudkerk M, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28:iv1-iv21. [Crossref] [PubMed]
- Gross JL, Vega MAT, Frenhi GS, et al. Bronchial carcinoid tumors: second primary neoplasms and outcomes of surgical treatment. J Bras Pneumol 2019;45:e20180140. [Crossref] [PubMed]
- Hoy H, Lynch T, Beck M. Surgical Treatment of Lung Cancer. Crit Care Nurs Clin North Am 2019;31:303-13. [Crossref] [PubMed]
- An S, Han GY, Eo W, et al. Comparison of the geriatric nutritional risk index and the prognostic nutritional index in determining survival outcome in patients with non-small cell lung cancer undergoing surgical resection: A cohort study. Medicine (Baltimore) 2022;101:e31591. [Crossref] [PubMed]
- Martini N, Flehinger BJ, Zaman MB, et al. Results of resection in non-oat cell carcinoma of the lung with mediastinal lymph node metastases. Ann Surg 1983;198:386-97. [Crossref] [PubMed]
- Naruke T, Goya T, Tsuchiya R, et al. The importance of surgery to non-small cell carcinoma of lung with mediastinal lymph node metastasis. Ann Thorac Surg 1988;46:603-10. [Crossref] [PubMed]
- Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest 1997;111:1710-7. [Crossref] [PubMed]
- Sasaki E, Nagino M, Ebata T, et al. Immunohistochemically demonstrated lymph node micrometastasis and prognosis in patients with gallbladder carcinoma. Ann Surg 2006;244:99-105. [Crossref] [PubMed]
- Yang J, Zhang Y, Sun X, et al. The prognostic value of multiorgan metastases in patients with non-small cell lung cancer and its variants: a SEER-based study. J Cancer Res Clin Oncol 2018;144:1835-42. [Crossref] [PubMed]
- Rami-Porta R, Wittekind C, Goldstraw P, et al. Complete resection in lung cancer surgery: proposed definition. Lung Cancer 2005;49:25-33. [Crossref] [PubMed]
- Smeltzer MP, Faris NR, Ray MA, et al. Association of Pathologic Nodal Staging Quality With Survival Among Patients With Non-Small Cell Lung Cancer After Resection With Curative Intent. JAMA Oncol 2018;4:80-7. [Crossref] [PubMed]
- David EA, Cooke DT, Chen Y, et al. Does Lymph Node Count Influence Survival in Surgically Resected Non-Small Cell Lung Cancer? Ann Thorac Surg 2017;103:226-35. [Crossref] [PubMed]
- Gajra A, Newman N, Gamble GP, et al. Effect of number of lymph nodes sampled on outcome in patients with stage I non-small-cell lung cancer. J Clin Oncol 2003;21:1029-34. [Crossref] [PubMed]
- Liang W, He J, Shen Y, et al. Impact of Examined Lymph Node Count on Precise Staging and Long-Term Survival of Resected Non-Small-Cell Lung Cancer: A Population Study of the US SEER Database and a Chinese Multi-Institutional Registry. J Clin Oncol 2017;35:1162-70. [Crossref] [PubMed]
- Lardinois D, De Leyn P, Van Schil P, et al. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur J Cardiothorac Surg 2006;30:787-92. [Crossref] [PubMed]
- Watanabe S, Asamura H. Lymph node dissection for lung cancer: significance, strategy, and technique. J Thorac Oncol 2009;4:652-7.
- Adachi H, Sakamaki K, Nishii T, et al. Lobe-Specific Lymph Node Dissection as a Standard Procedure in Surgery for Non-Small Cell Lung Cancer: A Propensity Score Matching Study. J Thorac Oncol 2017;12:85-93. [Crossref] [PubMed]
- Hishida T, Miyaoka E, Yokoi K, et al. Lobe-Specific Nodal Dissection for Clinical Stage I and II NSCLC: Japanese Multi-Institutional Retrospective Study Using a Propensity Score Analysis. J Thorac Oncol 2016;11:1529-37. [Crossref] [PubMed]
- Kumari S, Tewari S, Husain N, et al. Quantification of Circulating Free DNA as a Diagnostic Marker in Gall Bladder Cancer. Pathol Oncol Res 2017;23:91-7. [Crossref] [PubMed]
- Luo Z, Wang Q, Lau WB, et al. Tumor microenvironment: The culprit for ovarian cancer metastasis? Cancer Lett 2016;377:174-82. [Crossref] [PubMed]
- Zhu Z, Chen H, Yu W, et al. Number of negative lymph nodes is associated with survival in thoracic esophageal squamous cell carcinoma patients undergoing three-field lymphadenectomy. Ann Surg Oncol 2014;21:2857-63. [Crossref] [PubMed]
- Wu X, Xing H, Chen P, et al. Lymph Node Dissection Is a Risk Factor for Short-Term Cough after Pulmonary Resection. Curr Oncol 2022;29:294-307. [Crossref] [PubMed]
- Baud G, Jannin A, Marciniak C, et al. Impact of Lymph Node Dissection on Postoperative Complications of Total Thyroidectomy in Patients with Thyroid Carcinoma. Cancers (Basel) 2022;14:5462. [Crossref] [PubMed]


