
Clinical features of recurrent spontaneous pneumomediastinum
Highlight box
Key findings
• Recurrence of spontaneous pneumomediastinum may have a similar or less aggressive clinical presentation than its new onset.
• The presence of preexisting lung diseases may increase the risk of spontaneous pneumomediastinum recurrence.
What is known and what is new?
• Spontaneous pneumomediastinum recurrence is rare, and its clinical presentation is unclear.
• Clinicoradiological findings in patients with spontaneous pneumomediastinum recurrence revealed its relatively mild clinical presentation and potential association with respiratory comorbidities.
What is the implication, and what should change now?
• In patients with spontaneous pneumomediastinum and preexisting lung diseases, care should be taken about recurrent spontaneous pneumomediastinum.
Introduction
Spontaneous pneumomediastinum (SPM) is a rare entity defined as the presence of free air in the mediastinum that is not associated with any noticeable cause such as chest trauma. It has been proposed that the pathophysiology of SPM involves rupture of the alveoli caused by a rapid increase in alveolar pressure, which has been referred to as the Macklin effect (1). The Macklin effect refers to the phenomenon of a large pressure gradient between the alveoli and lung interstitium inducing alveolar rupture and the subsequent release of free air along the pulmonary vessels and bronchus in the limited area of the lungs coursing toward the mediastinum. Studies have suggested that SPM frequently presents with the sudden onset of symptoms, such as chest pain, in young adults with or without events, such as physical exercise, that can trigger a rapid increase in intrathoracic pressure (2,3). SPM is generally considered to be associated with a relatively benign course. However, data pertaining to long-term clinical outcomes, such as SPM recurrence, are limited.
The recurrence of SPM is considered rare, with a reported incidence of 1.2% (4). Because of its rarity, predisposing factors for SPM recurrence have not been investigated. In this study, we retrospectively investigated 30 consecutive patients with new-onset SPM who had been treated at our institution for 10 years, including five patients who subsequently experienced SPM recurrence. To assess the predisposing factors for SPM recurrence, we specifically evaluated patient background characteristics including body habitus and preexisting lung diseases, clinical presentation including trigger actions, radiological extent of SPM, and inflammatory response to SPM evaluated by laboratory data. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1141/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This single-institution, retrospective study was approved by the institutional review board of the National Hospital Organization Shinshu Ueda Medical Center (IRB No: 03-06). We utilized an opt-out approach instead of obtaining written informed consent from each patient. Using the institutional database of the National Hospital Organization Shinshu Ueda Medical Center, we identified 30 patients who were diagnosed with and treated for SPM between January 2012 and December 2021. Patient demographic information and data regarding clinical variables, including age, smoking history, body mass index (BMI), medical history, trigger action, symptoms, and radiological and blood test findings, were obtained from the medical records to perform this study. SPM was defined as the presence of free air in the mediastinum that was not associated with chest trauma, mechanical ventilation, surgical or medical procedures, or specific diseases known to cause pneumomediastinum, such as esophageal perforation. The radiological extent of SPM was assessed using a previously reported grading system (5). Using this grading system, the extent of subcutaneous emphysema was primarily evaluated based on chest radiography (CXR) findings (CXR grades 0–4), and the extent of mediastinal air was evaluated based on chest computed tomography (CT) scans (CT grades A-C) (Figure 1). CXR grading was performed as follows: grade 0, no abnormal findings; grade 1, air space present only in the mediastinum; grade 2, air space in the mediastinum plus mild subcutaneous emphysema that was only identifiable by careful observation using the magnification function; grade 3, subcutaneous emphysema clearly revealed without using the magnification function; or grade 4, marked subcutaneous emphysema with outlined muscle fibers of the pectoralis major. The CT grading was performed as follows: grade A, free air confined to the superior mediastinum that was defined as the mediastinum superior to the level of the tracheal carina; grade B, free air extending from the superior mediastinum to the middle mediastinum that was defined as the mediastinum between the level of the tracheal carina and that of the caudal end of the inferior pulmonary vein; and grade C, free air extending from the superior to the inferior mediastinum that was defined as the mediastinum inferior to the inferior pulmonary vein.
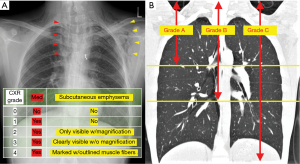
We evaluated the inflammatory response to SPM by assessing the maximum body temperature, white blood cell (WBC) count, and serum C-reactive protein (CRP) level during hospitalization, including the time in the emergency department. All patients were hospitalized and treated conservatively. If the general condition of a patient was stable, the blood test showed acceptable results, and the CXR did not show worsening of the pneumomediastinum, then the patient was discharged. We usually followed-up patients at our outpatient clinic after hospitalization, but we did not perform periodic follow-up thereafter. Instead, we contacted all patients via telephone to ask them whether they had been diagnosed and treated for recurrent SPM specifically for the purpose of this study.
Statistical analyses
Data are shown as median [interquartile range (IQR)] or number (%). Comparisons between groups were evaluated using the chi-square test for categorical variables and the Mann-Whitney U test for continuous variables. Significant differences in clinical data for cases of recurrence and non-recurrence of SPM were analyzed using the Mann-Whitney U test. All statistical analyses were conducted using IBM SPSS Statistics 27 (IBM, Armonk, NY, USA). All statistical tests were two-sided, and P<0.05 was considered statistically significant.
Results
Patient characteristics and clinical course of SPM
Table 1 shows the demographic data of the 30 patients who were diagnosed with and treated for SPM. Most of these patients were male (87%). The median age of the patients was 16 years (range, 12–26 years; IQR, 13–18 years). The median BMI was 17.9 kg/m2 (IQR, 16.6–20.1 kg/m2). Five patients had a relevant medical history. Of these five patients, three had preexisting lung diseases (two patients had bronchial asthma and one patient had congenital pulmonary atresia). The remaining two patients had a history of pneumothorax. All patients had experienced at least one symptom during the first visit. The acute onset of chest pain (70%), dyspnea (23%), and throat symptoms (neck and pharyngeal pain) (50%) were the most frequently reported symptoms. In terms of trigger factors, 16 patients (53%) had performed physical activity that could have increased the intrathoracic pressure at the initiation of SPM. The most common trigger was a ball game exercise (9 patients), followed by swimming/diving (2 patients), other exercise (2 patients), vomiting (2 patients), and using a loud voice (1 patient). In contrast, 14 patients (47%) had no specific triggers for the initiation of SPM.
Table 1
| Patient characteristics | Recurrent SPM | |||
|---|---|---|---|---|
| All (n=30) | No (n=25) | Yes (n=5) | P value | |
| Background | ||||
| Age (years) | 16 [13–18] | 15 [13–19] | 16 [16–18] | 0.59 |
| Sex | 1 | |||
| Male | 26 (87%) | 21 (84%) | 5 (100%) | |
| Female | 4 (13%) | 4 (16%) | 0 | |
| BMI (kg/m2) | 17.9 [16.6–20.1] | 18.0 [16.7–20.1] | 17.6 [16.6–19.4] | 0.83 |
| Smoking | 2 (7%) | 2 (8%) | 0 | 0.51 |
| MH | 5 (17%) | 2 (8%) | 3 (60%) | 0.022 |
| CPA | 1 (3%) | 0 | 1 (20%) | |
| Asthma | 2 (7%) | 0 | 2 (40%) | |
| Pneumothorax | 2 (7%) | 2 (8%) | 0 | |
| Bullae or blebs on CT | 9 (30%) | 6 (24%) | 3 (60%) | 0.14 |
| Triggersa | ||||
| Without trigger | 14 (47%) | 10 (40%) | 4 (80%) | 0.15b |
| With trigger | 16 (53%) | 15 (60%) | 1 (20%) | |
| Ball games | 9 (30%) | 9 (36%) | 0 | |
| Football | 6 (25%) | 6 (24%) | 0 | |
| Volleyball | 2 (7%) | 2 (8%) | 0 | |
| Basketball | 1 (3%) | 1 (4%) | 0 | |
| Other triggers | 7 (23%) | 6 (24%) | 0 | |
| Swimming/diving | 2 (7%) | 2 (8%) | 0 | |
| Other exercise | 2 (7%) | 2 (8%) | 0 | |
| Vomiting | 2 (7%) | 2 (8%) | 0 | |
| Shouting | 1 (3%) | 0 | 1 (20%) | |
| Symptoms | ||||
| Any symptoms | 30 (100%) | 25 (100%) | 16 (100%) | - |
| Chest pain | 21 (70%) | 18 (72%) | 3 (60%) | |
| Back pain | 2 (7%) | 2 (8%) | 0 | |
| Dyspnea | 7 (23%) | 6 (24%) | 1 (20%) | |
| Pharyngeal pain | 7 (23%) | 6 (24%) | 1 (20%) | |
| Neck pain | 8 (27%) | 7 (28%) | 1 (20%) | |
| Hoarseness | 1 (3%) | 1 (4%) | 0 | |
Data are shown as the number (%) or median [25–75 percentiles]. a, Physical activity with the potential for increased intrathoracic pressure; b, comparison between patients with and without triggers. SPM spontaneous pneumomediastinum; BMI, body mass index; MH, medical history; CPA, congenital pulmonary atresia; CT, computed tomography.
Table 2 shows the radiological extent of SPM and clinical data during hospitalization. Twenty-four patients had radiographic signs suggestive of subcutaneous or mediastinum emphysema (CXR grade 1–3). However, SPM in six patients could not be diagnosed by CXR alone, and pneumomediastinum was confirmed by chest CT (CXR grade 0). Nine patients had bullae or blebs on chest CT. None of the patients underwent bronchoscopy, esophagogastroduodenoscopy, or esophagoscopy. All patients were admitted to the hospital because of the initial SPM. The median duration of hospitalization was 8 days (range, 6–9 days). The vital signs of all patients were evaluated, and all patients underwent blood tests during hospitalization and their time in the emergency department. The median maximum values were 37.2 ℃ (range, 37.0–37.3 ℃) for body temperature, 9.7×103/µL (range, 6.3×103–12.3×103/µL) for the WBC count, and 0.2 mg/dL (range, 0.1–0.7 mg/dL) for the CRP level. The patients did not show worsening of the general condition or emphysema; therefore, they were discharged from the hospital.
Table 2
| Clinicoradiologic findings | All (n=30) | Recurrent SPM | ||
|---|---|---|---|---|
| No (n=25) | Yes (n=5) | P value | ||
| Radiologic extent of SPM | ||||
| Subcutaneous emphysema | ||||
| Negative | 12 (40%) | 9 (36%) | 3 (60%) | 0.36 |
| Positive | 18 (60%) | 16 (64%) | 2 (40%) | |
| Chest radiography grade | ||||
| Grade 0 | 6 (20%) | 5 (20%) | 1 (20%) | 0.49 |
| Grade 1 | 6 (20%) | 4 (16%) | 2 (40%) | |
| Grade 2 | 6 (20%) | 6 (24%) | 0 (0%) | |
| Grade 3 | 12 (40%) | 10 (40%) | 2 (40%) | |
| Grade 4 | 0 (0%) | 0 (0%) | 0 (0%) | |
| Chest CT grade | ||||
| Grade A | 1 (3%) | 1 (4%) | 0 (0%) | 0.29 |
| Grade B | 5 (17%) | 3 (12%) | 2 (40%) | |
| Grade C | 24 (80%) | 21 (84%) | 3 (60%) | |
| Clinical data during hospitalization | ||||
| Initial oxygen saturation (%) | 98 [97–99] | 98 [97–99] | 98 [97–100] | 0.78 |
| Oxygen requirement | ||||
| Yes | 8 (27%) | 8 (32%) | 0 (0%) | 0.28 |
| No | 22 (73%) | 17 (68%) | 5 (100%) | |
| Maximum body temperature (℃) | 37.2 [37.0–37.3] | 37.2 [37.0–37.3] | 37.3 [37.0–37.3] | 0.87 |
| Maximum WBC count (103/µL) | 9.7 [6.3–12.3] | 10.1 [6.6–12.5] | 7.0 [5.6–10.9] | 0.35 |
| Maximum CRP count (mg/dL) | 0.2 [0.1–0.7] | 0.4 [0.2–0.7] | 0.1 [0.1–0.6] | 0.20 |
| Length of hospital stay (days) | 8 [6–9] | 8 [6–8] | 8 [6–9] | 0.77 |
Data are shown as the number (%) or median [25–75 percentiles]. SPM, spontaneous pneumomediastinum; CT, computed tomography; WBC, white blood cell; CRP, C-reactive protein.
Clinical features of five patients with SPM recurrence
Table 3 presents the clinical features of the five patients who experienced SPM recurrence. All these patients were male and had experienced at least one symptom at the time of recurrence. The interval between the date of the initial SPM and that of recurrence was 32 to 315 days. One patient experienced two episodes of recurrence 315 days and 372 days after the initial onset. The median patient age was 16 years (range, 16–18 years). The median BMI was 17.6 kg/m2 (range, 16.6–19.4 kg/m2). Among the five patients, two (40%) had a history of asthma and three (60%) had bullae or blebs on chest CT. In terms of trigger factors, only one patient had performed strenuous physical activity (shouting) at the time of the initial SPM episode, and none of the patients reported a specific trigger at the time of recurrence. The extent of pneumomediastinum at the time of recurrence was similar to or less aggressive than that at the time of the initial SPM for all patients except for one patient with the same CXR grade but an increased CT grade (case 1) (Figure 2).
Table 3
| Case | Age/sex | BMI | MH | Bullae or blebs on CT | Interval (days)a | Clinical presentation at the initial SPM | Clinical presentation at the recurrence | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trigger factor | Symptom | CXR grade | CT grade | LOS (days) | Trigger factor | Symptom | CXR grade | CT grade | LOS (days) | |||||||
| 1 | 18/male | 19.4 | Asthma | Yes | 32 | None | Chest pain | 1 | 2 | 6 | None | Chest pain | 1 | 3 | 6 | |
| 2 | 16/male | 16.6 | CPA | Yes | 39 | None | Chest pain | 3 | 3 | 8 | None | Chest pain | 0 | 3 | Outpatient | |
| 3 | 16/male | 16.6 | None | No | 96 | None | Chest pain, dyspnea | 3 | 3 | 9 | None | Neck pain | 1 | 2 | 6 | |
| 4 | 18/male | 17.6 | None | Yes | 126 | None | Neck pain | 1 | 3 | 9 | None | Neck pain, dyspnea | 1 | 3 | 8 | |
| 5b | 14/male | 20.5 | Asthma | No | 315 | Loud voice | Pharyngeal pain | 0 | 2 | 5 | None | Neck pain | 0 | 2 | 5 | |
a, interval between the date of onset of the initial SPM and that of recurrence; b, this case involved multiple recurrences; however, the data shown in this table are those of the first recurrence. BMI, body mass index; MH, medical history; SPM, spontaneous pneumomediastinum; CXR, chest radiography; CT, computed tomography; LOS, length of hospital stay; CPA, congenital pulmonary atresia.
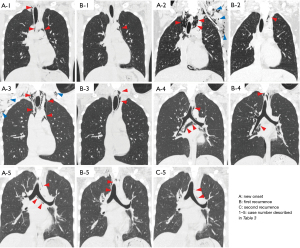
Predisposing factors for SPM recurrence
A comparison of patients with and without SPM recurrence (Tables 1,2) showed no statistically significant difference in terms of patient background characteristics, triggers for SPM, symptoms, radiologic extent of SPM, and clinical data during hospitalization; however, there was a statistically significant difference in medical history, with preexisting lung diseases (congenital pulmonary atresia and bronchial asthma) only observed in patients with recurrent SPM (P=0.002). In terms of triggers for SPM, only one of the five patients with SPM recurrence (20%) had an apparent trigger at the onset of the initial SPM, whereas the majority of patients without recurrence (15/25; 60%) had performed trigger activities at the onset of SPM (no statistically significant difference; P=0.157). Ball game exercise was the most frequent trigger activity (9/16; 56%); however, it was only observed in patients without recurrence (Table 2).
Representative image of SPM and the suggested Macklin effect
Figure 3 shows the representative chest CT images at the onset of SPM, with free air spreading widely to the mediastinum. However, lung parenchymal free air was only seen in a limited area of the lungs; in this case, it was in the lower lobe. This finding suggests that the Macklin effect is a potential mechanism underlying SPM. The mechanism of the Macklin effects involves a large pressure gradient between the alveoli and lung interstitium inducing alveolar rupture, which is followed by the release of free air along the pulmonary vessels and bronchus in the limited area of the lungs coursing toward the mediastinum (1,6).
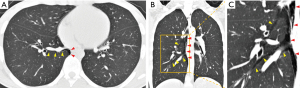
Discussion
In this study, we examined the clinical features of 30 patients with SPM, including five patients with SPM recurrence. This novel study had some strengths. For example, this is the first study to provide comparative data and radiological images of the initial SPM and SPM recurrence (Figure 2, Table 3), thereby demonstrating that the clinical presentation during recurrence is similar to or less aggressive than that at the time of the initial onset. Furthermore, this is the first study to statistically analyze the predisposing factors for SPM recurrence by using various factors, including the radiologic extent of SPM and laboratory data, suggesting that the presence of preexisting lung diseases, such as bronchial asthma, is a potential predisposing factor for recurrence (Tables 1,2).
SPM was first described by Hamman in 1939 (7). Since then, many case reports and literature have been published, and the potential causes of SPM have been discussed. Childhood asthma is one of the known predisposing conditions for SPM (8-10). Among patients with childhood asthma visiting emergency departments, the reported incidence of SPM was 0.3% (8), which was higher than the general incidence of SPM of 0.001% to 0.01% (11). However, many reports suggest that SPM recurrence is rare. According to a literature review of SPM, only 5 (1.2%) of the 389 cases were identified as recurrences (4). However, many of these studies did not clearly document the clinical characteristics of patients with recurrent SPM (3,12-15). In our study, SPM recurrence was observed in five patients (16.7%) within 1 year of the initial onset. Regarding trigger factors, none of the patients with SPM recurrence performed strenuous exercise at the onset of the initial or at the onset of recurrent SPM. However, patients with SPM recurrence frequently had bullae or blebs on chest CT and a history of preexisting lung diseases including asthma. These findings indicate that SPM recurrence could be related to intrinsic factors such as alveolar fragility rather than extrinsic factors, which increase intrathoracic pressure, such as strenuous exercise.
In this study, the median length of the hospital stay was 8 days, which was relatively longer than that reported by other studies. During the study period, we treated patients in the hospital setting until we confirmed that their emphysema mostly disappeared, which might have contributed to the relatively longer hospital stay. Additionally, the relatively lower medical care costs in Japan compared with those in other developed countries might have contributed to the relatively longer hospital stay. However, none of the patients had any exacerbating conditions, including emphysema, during hospitalization. Moreover, none of the patients required invasive treatments such as surgery, radiological interventions, or chest tube drainage. Therefore, outpatient treatment or short-term hospitalization for 1 or 2 days should be sufficient unless the patients have coexisting pneumothorax or are suspected of having organ rupture, such as that associated with Boerhaave syndrome. Moreover, because recurrent SPM demonstrated similar or less aggressive clinical presentations compared with the initial SPM, hospitalization might not be required for SPM recurrence.
This study had several limitations. First, this was a single-center, retrospective investigation with a relatively small sample size. Second, we did not have a unified treatment algorithm for SPM, and the treatment policy and evaluations of clinical presentations were dependent on the attending physicians, which may have affected our retrospective findings. Third, we did not perform regular follow-up evaluations after the initial SPM. Although we conducted telephone interviews of all patients who did not have SPM recurrence to assess the potential for SPM recurrence, we may have missed some patients with SPM recurrence that was not recognized and healed spontaneously. Fourth, according to a published literature review of spontaneous pneumomediastinum, the mean age range is generally between 18 and 27 years (4). In a report with the largest number of cohort (n=62), the reported median age was 30 years (range, 18–84 years) years old. In comparison, the range of age of our patient cohort (median, 16 years; and range, 12–26 years) is relatively lower. Additionally, despite their young ages, 17 % of the patients had a medical history of respiratory-related illness and 30% of patients had radiological findings of bulla or bleb on CT scan (Table 1). Patient characteristics in our cohort mentioned above might have affected the higher recurrence rate in our study cohort compared with previously published studies.
Conclusions
In comparison with the initial onset of SPM, recurrent SPM had a similar or less aggressive clinical presentation. The extent of pneumomediastinum, triggers, and the clinical presentations of the initial SPM were not associated with recurrence; however, preexisting lung diseases may affect the risk of SPM recurrence.
Acknowledgments
We would like to thank Editage (www.editage.com) for English editing.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1141/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1141/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1141/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1141/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board of National Hospital Organization Shinshu Ueda Medical Center (IRB No. 03-06). We utilized an opt-out approach instead of obtaining written informed consent from each patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Macklin MT, Macklin CC. Malignant interstitial emphysema of the lungs and mediastinum as an important occult complication in many respiratory diseases and other conditions: interpretation of the clinical literature in the light of laboratory experiment. Medicine 1944;23:281-358.
- Koullias GJ, Korkolis DP, Wang XJ, et al. Current assessment and management of spontaneous pneumomediastinum: experience in 24 adult patients. Eur J Cardiothorac Surg 2004;25:852-5. [Crossref] [PubMed]
- Macia I, Moya J, Ramos R, et al. Spontaneous pneumomediastinum: 41 cases. Eur J Cardiothorac Surg 2007;31:1110-4. [Crossref] [PubMed]
- Takada K, Matsumoto S, Hiramatsu T, et al. Spontaneous pneumomediastinum: an algorithm for diagnosis and management. Ther Adv Respir Dis 2009;3:301-7. [Crossref] [PubMed]
- Kaneki T, Kubo K, Kawashima A, et al. Spontaneous pneumomediastinum in 33 patients: yield of chest computed tomography for the diagnosis of the mild type. Respiration 2000;67:408-11. [Crossref] [PubMed]
- Eguchi T, Takasuna K, Matsubara M, et al. Pneumomediastinum and retropneumoperitoneum due to the Macklin effect. Ann Thorac Surg 2012;94:298. [Crossref] [PubMed]
- Hannman L. Spontaneous mediastinal emphysema. Bull Johns Hopkins Hosp 1939;64:1-21.
- Stack AM, Caputo GL. Pneumomediastinum in childhood asthma. Pediatr Emerg Care 1996;12:98-101. [Crossref] [PubMed]
- Tortajada-Girbés M, Moreno-Prat M, Ainsa-Laguna D, et al. Spontaneous pneumomediastinum and subcutaneous emphysema as a complication of asthma in children: case report and literature review. Ther Adv Respir Dis 2016;10:402-9. [Crossref] [PubMed]
- Porpodis K, Zarogoulidis P, Spyratos D, et al. Pneumothorax and asthma. J Thorac Dis 2014;6:S152-61. [Crossref] [PubMed]
- Takada K, Matsumoto S, Hiramatsu T, et al. Management of spontaneous pneumomediastinum based on clinical experience of 25 cases. Respir Med 2008;102:1329-34. [Crossref] [PubMed]
- Gerazounis M, Athanassiadi K, Kalantzi N, et al. Spontaneous pneumomediastinum: a rare benign entity. J Thorac Cardiovasc Surg 2003;126:774-6. [Crossref] [PubMed]
- Mihos P, Potaris K, Gakidis I, et al. Sports-related spontaneous pneumomediastinum. Ann Thorac Surg 2004;78:983-6. [Crossref] [PubMed]
- Abolnik I, Lossos IS, Breuer R. Spontaneous pneumomediastinum. A report of 25 cases. Chest 1991;100:93-5. [Crossref] [PubMed]
- Iyer VN, Joshi AY, Ryu JH. Spontaneous pneumomediastinum: analysis of 62 consecutive adult patients. Mayo Clin Proc 2009;84:417-21. [Crossref] [PubMed]


