Diagnostic yield of chest radiograph in management of adults with difficult-to-treat chronic cough—retrospective study
Highlight box
Key findings
• CXR shows a limited diagnostic yield in adults with difficult-to- treat CC, who are referred to cough clinic. In this group thorax CT may add important data, which might change either diagnostic or therapeutic approach.
What is known and what is new?
• CXR is a mandatory diagnostic procedure in adults with CC, while the utility of thorax CT is a matter of discussion.
• The results of this study show the added value of thorax CT scanning in adults with difficult-to-treat CC managed in cough clinic.
What is the implication, and what should change now?
• Thorax CT should be considered as a component of the diagnostic algorithm in adults with difficult-to-treat CC.
Introduction
Chronic cough (CC), defined as cough lasting over 8 weeks, is a frequent complaint affecting about 5–10% of the adult population and resulting in a significant impairment of quality of life (1-6).
Since lung cancer, tuberculosis and other serious pulmonary diseases are common causes of CC, the diagnostic work-up should begin with exclusion of alarming symptoms such as hemoptysis, dyspnea, voice disturbances, chest pain, fever and weight loss. In active smokers, smoking related bronchitis is the most common cause of CC and the management should start with smoking cessation. In contrast, asthma (A), gastroesophageal reflux disease (GERD) and upper airway cough syndrome (UACS) (2,7) are considered to be the most common triggers of CC in non-smoking patients. If causal treatment is ineffective and cough persists, refractory chronic cough (RCC) is diagnosed. In case of thorough diagnostics is inconclusive in the identification of CC cause, unexplained CC (UCC) should be diagnosed (8). Both RCC and UCC are commonly associated with hypersensitivity of cough reflex, which is a key component in the pathomechanism of CC (9).
Based on the available data, plain chest radiograph (CXR) is considered to be the mandatory imaging test in the diagnostic pathway in patients with CC, while the value of thoracic computed tomography (CT) in these patients remains a matter of discussion (5,10). According to American College of Chest Physicians (ACCP) guidelines thoracic CT should be considered if inadequate response to optimal treatment of the most common causes of CC is documented (10,11). Similarly, the American College of Radiology (ACR) guidelines suggest usefulness of chest CT in patients with CC, especially in these with increased risk for lung cancer (12). On the contrary, recent European Respiratory Society (ERS) guidelines on the management of CC suggest against the routine use of chest CT scan when both CXR and physical examination are normal (conditional recommendation) (5). Although a few previous studies revealed significant CT findings in 6.5 to 58% patients with CC and normal CXR, there was inadequate data about the impact of these findings on further CC management (13-16). It should be noted that the use of CXR as the only imaging test limits the possibility of exclusion of rare, but relevant causes of CC such as bronchiectasis or interstitial lung disease (ILD) (17,18). In our earlier study, the negative predictive value (NPV) of CXR in diagnosing the causes of CC reached only 64% (16). Since the number of patients in our previous study was not numerous, we decided to analyze the diagnostic accuracy of CXR and value of chest CT in the diagnosis and management of larger group of patients with difficult to treat CC diagnosed in our cough clinic. We present the following article in accordance with the STARD reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-111/rc).
Methods
General study design
A retrospective analysis of chest imaging tests was performed in all patients, who were referred to the Department of Internal Medicine, Pulmonary Diseases and Allergy of the Medical University of Warsaw due to CC as a main or isolated symptom between 2015 and 2019. The primary measures used in the study were CXR and chest CT scans. Plain CXR was performed in all patients, either during the diagnostic work-up in our institution or earlier, during the initial evaluation, but not longer than 6 months prior to admission. All patients with CC were carefully diagnosed and treated according to the recommendations of the ERS (1), British Thoracic Society (BTS) (2) and ACCP (3,10). Detailed diagnostic algorithm is shown in Figure S1. Thoracic CT scan was performed not in all patients, but if any of the following cases were present:
- if any abnormalities were found in either chest X-ray or physical examination;
- in these patients who did not respond to earlier treatment of CC;
- if any risk factors for lung cancer were identified (detailed indications are given below).
The results of CT scan were used as the “gold standard” to assess the diagnostic yield of CXR.
The result of CXR or chest CT was regarded as positive if it had influenced either the diagnosis or management of CC. This study was a part of a larger project on the effectiveness of management of adults with CC referred to our cough center. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of the Medical University of Warsaw (No. KB/101/2009). Patients were asked to sign an informed consent to include data on the results of their diagnostic tests and management in research analysis and publication.
Outcome measures
The following outcome measures were analyzed:
- Primary outcome: Diagnostic yield of CXR in management of patients with difficult-to-treat CC.
- Secondary outcome: The impact of CT scanning on the diagnosis of underlying diseases and management of patients with CC.
Patients and methods
Chest imaging tests of all adult patients referred to our department due to CC were included in the analysis. CC was defined as cough lasting longer than 8 weeks and being the main or isolated patients’ ailment. Patients who had both the results of CXR and CT scan available were included in the final analysis. The median time-interval between performing CXR and CT was 11 weeks (Figure S1). CXRs and chest CT scans were assessed by a specialist in radiology (MZ) and subsequently assigned by a pulmonary specialist (MD, EMG) to one of the two categories: (I) significant abnormalities or (II) non-significant changes in terms of identifying causal diagnosis or modification of further CC diagnostics or management. The assignment was discussed and a consensus opinion was noted in the database. The radiologist was blinded to the results of the management of CC, while pulmonary specialists were not.
Plain chest radiograph
Only digital CXRs which included at least postero-anterior (PA) view were submitted for analysis. The results of CXRs were defined as significant in terms of diagnosis of CC cause when at least one of the abnormalities listed in Table 1 was revealed. To better assess the significance of the current findings, all available previous CXRs were also analyzed.
Table 1
| In chest radiograph | In chest computed tomography |
|---|---|
| Pulmonary nodules >10 mm | Solitary pulmonary nodule >10 mm with malignant features (e.g., heterogeneous density, part-solid nodule, spiculated or lobulated margins) |
| Multiple pulmonary nodules | |
| Pulmonary mass | Pulmonary mass |
| Lobar or pulmonary atelectasis | Subsegmental, segmental, lobar or pulmonary atelectasis |
| Pulmonary consolidations | Parenchymal opacities (both ground glass and consolidations) |
| Tree in bud pattern | |
| Interstitial pattern | Interstitial pattern |
| Bronchiectasis | Bronchiectasis |
| Bronchial wall thickening | |
| Significant bronchial deformation | |
| Mucoid impaction | |
| Significant tracheal stenosis | |
| Pleural effusion | Pleural effusion |
| Pneumothorax | Pneumothorax |
| Abnormal diaphragm position | Diaphragm dysfunction |
| Hiatal hernia | Hiatal hernia |
| Widened mediastinum | Mediastinal lymphadenopathy (lymph node short axis >10 mm) |
| Enlarged pulmonary hilum | Hilar lymphadenopathy |
| Pulmonary trunk dilatation (>32 mm) | |
| Enlarged heart silhouette | Enlarged heart silhouette |
Abnormalities defined as significant in terms of diagnosis or treatment of chronic cough.
Thoracic CT scanning
Thoracic CT scan was performed in patients with at least one of the following criteria:
- any abnormalities found in CXR
- any abnormal respiratory signs found on physical examination except for diffused wheezing
- active smoking or ex-smoking with smoking cessation within the last 10 years
- any other risk factor for lung cancer
- CC persisting despite previous therapy, i.e., RCC
- inability to identify any trigger of CC despite diagnostics, i.e., UCC
Chest CT was not performed if:
- CXR was normal and no risk factors for lung cancer were present and cough disappeared or decreased significantly as a result of causal treatment (for asthma, non-asthmatic eosinophilic bronchitis, GER, UACS or discontinuation of ACEI)
- consent for CT was not granted by the patient
- the patient was pregnant
CT scans were performed with a 16-row CT scanner (LightSpeed 16 General Electric, USA) using: 1.25-mm collimation, 140 kV peak, 100–250 mA current and matrix size 512×512. CT scan with iodine contrast injection was planned only if mediastinal disorders were suspected.
In patients who had undergone chest CT within 6 months prior to enrollment, an analysis of the performed scanning was done and CT was not repeated for the purpose of the study.
The decision which CXR or CT findings should be defined as significant in terms of the CC causes was based on the consensus between the literature and expertise of the researchers (12,19). The chest CT findings construed as potentially related to the cause of CC are listed in Table 1. The response to cough therapy was assessed using cough related quality of life measured by Leicester Cough Questionnaire (LCQ). The significant response to cough treatment was defined as increase of LCQ >1.5 points (20).
Based on the results of CXR and chest CT, four groups of patients were distinguished:
- 1A: normal or nonsignificant CXR and normal or irrelevant abnormalities in thoracic CT (true negative CXR results),
- 1B: normal or nonsignificant CXR but relevant abnormalities in thoracic CT (false negative CXR results),
- 2A: significant abnormalities in CXR and thoracic CT (true positive results),
- 2B: significant abnormalities in CXR but not in thoracic CT (false positive results).
Statistical analysis
Differences between groups were compared using chi-square test for categorical variables and Mann Whitney U test for continuous variables. Received operating characteristic (ROC) curve was constructed to assess the diagnostic value of CXR with the use of the following parameters: diagnostic accuracy, sensitivity, specificity, NPV, positive predictive value (PPV), positive likelihood ratio (PLR) and negative likelihood ratio (NLR). The results of thoracic CT scans were used as the “gold standard”. P value less than 0.05 was considered statistically significant. The statistical analysis was performed using Statistica 13.1, StatSoft software package.
Based on our previous study (16) sample size calculation was estimated assuming that the diagnostic accuracy of CXR is ~60-70% and the estimated difference between AUC (area under the curve) of ROC for CXR and chest CT is at least 10%. Power analysis and sample size based on study by Haijan-Tilaki indicated that a sample size of 176 subjects would provide 90% statistical power to detect differences between the AUC of these two imaging tests (21). The number of enrolled subjects was increased by 25 to allow for a 15% drop-out rate. Thus, a total number of 201 subjects was required to provide an adequate power of the study.
Results
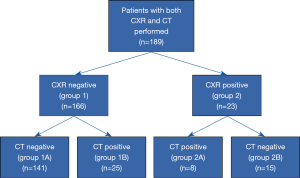
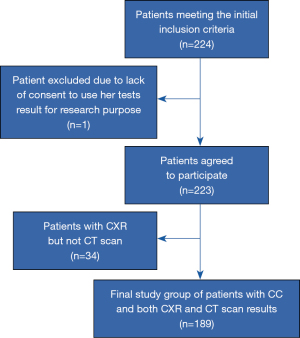
Only 1 of 224 patients meeting the initial inclusion criteria refused the results of her tests and treatment efficacy be analyzed for research purposes. Thirty four of the remaining 223 subjects (15.2%) were excluded because they had only CXR (but not CT) performed (all participants characteristics is given in Table S1). Thus, 189 patients were included in the final analysis (Figure 1).
The reasons of performing CT were: abnormal CXR (23/189 patients, 12%), presence of risk factor for lung cancer (32/189 patients, 17%), insufficient response to causal treatment of CC (134/189 patients, 71%). The median age of these patients was 58 years (IQR 44–67) and median duration of CC was 48 months (IQR 24–120). Majority of patients were women (136/189, 72.0%), only 6 (3.0%) were active smokers at enrollment and 129/189 (68.3%) were overweight or obese. Seventeen patients (9.0%) had a history of autoimmune disease, and 5 patients (2.6%) reported a history of malignancy. Twenty eight patients (28/189, 14.8%) had chest CT with intravenous iodine contrast medium injection. No adverse events related to performing CXR or chest CT were noted.
Among the 189 patients who underwent both CXR and chest CT scan, 166 subjects had normal or near normal (insignificant abnormalities) CXR. In 141 of them (141/166, 84.9%) this result was confirmed by thoracic CT which did not reveal any significant findings (group 1A). However, in 25 patients (25/166, 15.1%) with a normal CXR, CT presented various abnormalities that could have been related to CC (group 1B) (Figures 2,3).
The most common relevant abnormalities not seen in CXR but found in CT scans (group 1B) were as follows: hiatal hernia (8/25, 32.0%), tree-in-bud opacities (7/25, 28.0%), bronchiectasis (4/25, 16.0%), parenchymal opacities (both consolidations and ground glass opacities) (3/25, 12.0%). More than one abnormality was demonstrated in four patients. In all 25 patients with false negative CXR, the management was modified as a result of the findings revealed in thoracic CT scans. In 9 of these patients (9/166, 5.4%) modification of therapy due to result of thoracic CT led to improvement of cough related quality of life (increase of LCQ >1.5 points). The detailed data on the modification of diagnostic or therapeutic approach are given in Table 2.
Table 2
| No. of patients (N=25) | Type of abnormal finding in thoracic CT scan | Modification of the diagnostic protocol | Final diagnosis | Modification of treatment | No. of patients with decrease of CC |
|---|---|---|---|---|---|
| 8 | Hiatal hernia | 24 hours multichannel impedance-pH monitoring | GERD | Prokinetic drug added to previous PPI therapy, considered as candidates for fundoplication surgery | 3/8 |
| 1 | Bronchial wall thickening, parenchymal opacities | Patient did not give consent to bronchoscopy | Chronic bronchitis | Antibiotic therapy with amoxicillin with clavulanic acid | 1/1 |
| 1 | Deformation of the trachea and bronchial wall | Bronchoscopy | Tracheobronchopathia osteochondroplastica | No change in treatment | |
| 1 | Multiple pulmonary nodules | Bronchoscopy | Breast cancer metastases to the lungs | Chemotherapy | |
| 1 | GGO | Bronchoscopy | GGO pulmonary nodules requiring further surveillance | Follow-up with CT No change in treatment | |
| 5 | Tree-in-bud opacity | Bronchoscopy | Exclusion of mycobacterial infection; chronic bronchitis associated with chronic rhinosinusitis | Intensification of treatment of chronic bronchitis and rhinosinusitis | 1/5 |
| 1 | Tree-in-bud opacity in patient diagnosed with asthma | Bronchoscopy | Exclusion of mycobacterial infection, patient with asthma, BALF eosinophilia (2%) | Intensification of asthma treatment | 1/1 |
| 1 | Tree-in-bud pattern bronchiectasis | Bronchoscopy | Non-tuberculous mycobacteria related pulmonary disease (M. intracellulare) | Pulmonary rehabilitation, treatment with mucolytics; patient refused antimycobacterial treatment | 1/1 |
| 1 | Multiple pulmonary nodules, parenchymal opacities, mediastinal lymphadenopathy | Bronchoscopy | Sarcoidosis | No specific treatment, further surveillance | |
| 2 | Bronchiectasis | Bronchiectasis | Pulmonary rehabilitation, treatment with mucolytics and antibiotics on exacerbations | 2/2 | |
| 1 | Reticular opacities, honeycombing, traction bronchiectasis | Bronchoscopy | ILD related to rheumatoid arthritis | Methotrexate | |
| 1 | Nodule with spiculated margins | Bronchoscopy | Indeterminate SPN | Follow-up with CT | |
| 1 | Injury of the diaphragm with secondary hernia | Posttraumatic injury of the diaphragm with secondary hernia | Thoracic surgery |
CXR, chest radiograph; CT, computed tomography; CC, chronic cough; GERD, gastroesophageal reflux disease; PPI, proton pump inhibitor; GGO, ground glass opacities; BALF, bronchoalveolar lavage fluid; ILD, interstitial lung disease; SPN, solitary pulmonary nodule.
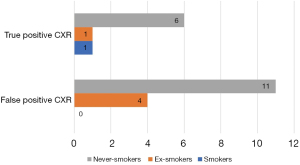
Plain CXR performed as one of the initial diagnostic tests revealed significant radiographic abnormalities in 23/189 patients (12.2%) (group 2). These included hilar lymphadenopathy (5/23, 21.7%), heart silhouette enlargement (5/23, 21.7%), single pulmonary nodules (SPN) suspected of being malignant (4/23, 17.4 %), pulmonary consolidation (3/23, 13%), peribronchial thickening (3/23, 13%), bronchiectasis (2/23, 8.7%), multiple pulmonary nodules (2/23, 8.7%), small volume pleural effusion (2/23, 13%), mediastinal cyst (probably bronchogenic) (1/23, 4.4%), tracheal stenosis due to external compression (1/23, 4.4%), unilateral elevation of the hemidiaphragm (1/23, 4.4%).
In 8 (8/23, 34.8 %) of these patients, the presence of significant changes was confirmed with CT scan (group 2A) (Figure 4). Chest CT scan revealed multiple pulmonary nodules with ground glass opacities and mediastinal lymphadenopathy (n=1), bronchiectasis with bronchial wall thickening and mucoid impaction (n=1), mediastinal lymphadenopathy (n=1), bronchiectasis (n=1), lung tumor (n=1), tracheal compression caused by goiter (n=1), coelomic cyst localized in the costocardiac angle (n=1) and pulmonary trunk dilatation (n=1). In the remaining 15 patients with abnormal CXR, CT scans showed a normal appearance of the thoracic structures or only the presence of irrelevant changes (group 2B). Thus, the percentage of false positive CXR among our patients with CC amounted 65.2% (15/23).
The sensitivity, specificity, PPV, NPV and diagnostic accuracy of CXR in the detection of abnormalities with a potential causal relationship with CC was 24.2% (95% CI: 11.1–42.3), 90.4% (95% CI: 84.6–94.5), 34.8% (95% CI: 19.8–53.6), 84.9% (95% CI: 82.2–87.3) and 78.8% (95% CI: 72.3–84.4), respectively. PLR was calculated as 2.52 and NLR as 0.84. The ROC analysis in the subgroup of smokers and ex-smokers (40/189, 21.0%) showed a slightly higher sensitivity and NPV: sensitivity 40.0% (95% CI: 5.3–85.3), specificity 88.6% (95% CI: 73.3–96.8), PPV 33.3% (95% CI: 10.8–67.3), NPV 91.2% (95% CI: 83.3–95.5) and diagnostic accuracy 82.5% (95% CI: 67.2–92.7).
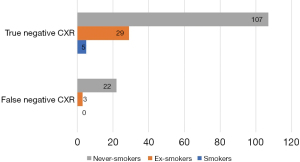
Patients with false negative CXR were older than those with true negative CXR (63 vs. 56 years, P=0.030). The analysis of false negative result of CXR in relation to age of patients with CC measured as AUC of ROC was 0.64 (95% CI: 0.51–0.76, P=0.029) and optimal cut off was 62 years (Figure 5). No differences were found in terms of other characteristics such as gender, duration of CC, smoking history, comorbidities or body mass index (BMI) (Table 3).
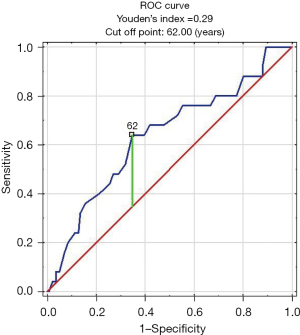
Table 3
| Patients’ characteristics | Patients with true negative CXR (n=141) | Patients with false negative CXR (n=25) | P value |
|---|---|---|---|
| Age (years) | 56 (42–67) | 63 (48–70) | 0.030 |
| Gender (F/M) | 98 (69%)/43 (31%) | 18 (72%)/7 (28%) | 0.840 |
| CC duration (months) | 48 (24–96) | 48 (36–137) | 0.771 |
| NS/ES/S | 107 (76%)/29 (21%)/5 (4%) | 25 (100%)/0/0 | 0.139 |
| Diagnosis of A or NAEB/GERD or LPR/UACS | 77 (55%)/112 (79%)/93 (66%) | 11 (44%)/14 (56%)/17 (68%) | 0.605 |
| BMI (kg/m2) | 26.8 (24.2–30.3) | 28.4 (25.0–33.0) | 0.350 |
Data are presented as median and interquartile range or numbers and percentages. F, female; M, male; CC, chronic cough; NS, non-smokers; ES, ex-smokers; S, smokers; A, asthma; NAEB, nonasthmatic eosinophilic bronchitis; GERD, gastroesophageal reflux disease; LPR, laryngopharyngeal reflux; UACS, upper airway cough syndrome; BMI, body mass index; CXR, chest radiograph; CT, computed tomography.
Discussion
Low diagnostic yield of CXR
Our study demonstrated a limited diagnostic yield of CXR in adults with CC referred to our cough clinic, with both significant percentage (15%) of false negative and high percentage (65%) of false positive results. Importantly, in patients with false negative CXR, thoracic CT scans revealed potentially relevant findings which affected either further diagnostic or therapeutic approach. In 5.4% of patients change in cough management driven by thoracic CT led to increase of cough-related quality of life. No less important is the fact that due to a high percentage of false positive CXR results, the probability of diagnosing significant changes in the thorax with the use of CT scanning was only 2.3-fold higher in patients with abnormal vs. normal CXR (35% vs. 15%). The results of our study suggest that CXR as the only imaging test might be insufficient to diagnose all relevant causes of CC in the population of adults with difficult-to- treat CC, who are referred to the reference cough clinics. The general indication for thoracic CT in patients with CC in our study are coherent with the ERS recommendations, but they widen the indications and include patients with any smoking history or other risk factors for lung cancer and patients with CC persisting despite previous therapy or idiopathic CC.
To the best of our knowledge, this study was one of a few studies published to date which compared the diagnostic yield of CXR and CT in patients with CC. The only prospective study by Kastelik et al. (14) was not specifically designed to compare the diagnostic performance of these two imaging techniques. Therefore, although all patients had undergone CXR, only approximately one third of them had a CT scan performed. Furthermore, neither the data on the significance of CXR findings nor the comparison of CXR and CT results were presented in that study.
There were also several retrospective studies which demonstrated a limited diagnostic sensitivity of CXR in patients with CC. Barnes et al. reported that thoracic CT revealed abnormalities in 9 of 21 subjects (43.0%) presenting with normal CXR (13). Slightly higher and lower percentages of false negative results of CXR in patients with CC were found in the study by McGarvey (20/34, i.e., 58.0%) (15) and in our previous study by Truba et al. (21/59, i.e., 36.0%) (16). However, it must be emphasized, that none of the previous studies analyzed the significance of new thoracic CT findings and their impact on further diagnostic work-up and CC management. On the contrary, the recent large retrospective study by Descazeaux et al. documented that chest CT may have an impact on the diagnosis only in 5.0% of patients with CC, while in 1.5% may lead to cough reduction (22). However, Descazeaux et al. concluded that although one quarter of CC patients had abnormal chest CT-scan, its impact on overall management or CC management was limited and chest CT should not be routinely performed particularly in patients with dry cough (22). The results of our study showed higher impact of thoracic CT on management of CC, as it revealed significant findings in 15% of adults with CC referred to our cough clinic, while in 5.4% of them chest CT contributed to modification of therapy and decrease of cough. Higher percentage of relevant findings in thoracic CT might result from selected cohort of our patients—those who are referred to cough clinic as they did not respond to antitussive treatment earlier. Thus we believe that thoracic CT might be useful in certain subgroups of adults with CC—if abnormalities are found on physical examination or in CXR, but also in active of ex-smokers and in patients with RCC or UCC. A few other authors share similar opinion. Li et al., states that as the role of chest CT in the identification of causes of CC is increasing CT may be recommended even as the first-line examination (23). Taking into consideration scarcity of these studies, we believe our findings add to the existing debate on the diagnostic role of imaging studies in adults with CC.
The limited diagnostic sensitivity of CXR in patients with CC is consistent with the overall view on the diagnostic yield of this imaging technique. Although CXR plays a pivotal role in the diagnosis of many lung diseases, it has an insufficient accuracy to detect pulmonary nodules, interstitial abnormalities, tree-in-bud opacities or bronchiectasis which can be related to CC. SPNs that are mostly benign, but can also be an early stage of lung cancer, are often missed in CXR due to superimposition of larger thoracic structures, poor viewing conditions and quality of images (24). Significant superiority of low dose spiral CT (LDCT) over CXR in diagnosing of early lung cancer was demonstrated by Kaneko et al. who reported detection rates of 0.43 and 0.12 for LDCT and CXR, respectively (25). Low diagnostic accuracy of CXR compared to chest CT has also been shown in diffuse infiltrative lung disease (DILD). DILD was diagnosed by chest CT in 28–42% of patients with normal CXR (26,27). Chronic cough is a leading symptom in patients with bronchiectasis. The role of CXR in this condition is also limited, while high resolution CT is a very accurate diagnostic tool with sensitivity and specificity amounting to 90% (28-30).
Our current study showed that hiatal hernia and tree-in-bud pattern were the most common thoracic CT findings in patients with CC and false negative CXR. As hiatal hernia is strongly associated with GERD (31), its diagnosis usually suggest the need for implementation or intensification of anti-reflux treatment (32). Tree-in-bud pattern was detected in 28% of patients with negative CXR results and in the majority of patients, it was related to chronic bronchitis and rhinosinusitis, leading to its more vigorous treatment. Of note, in our patients ILD or bronchiectasis were diagnosed rarely.
We believe the high percentage of false positive CXR results, later verified by negative chest CT may be viewed as an additional argument for the broader implementation of thoracic CT scanning into the diagnostic algorithm in adults with CC. As CT can exclude a number of apparent lesions diagnosed or suspected by CXR, it allows to accelerate the diagnostic process.
Radiation exposure related with CT scanning
In the context of the high prevalence of CC, a substantial exposure to radiation associated with chest CT imaging might be a concern (33-37). However, due to technological improvement of CT scanners, there are methods which enable reducing radiation exposure even by 30 to 90% with no quality compromise of CT images, i.e., automatic tube current modulation, tube potential reduction, filtering techniques or iterative reconstruction methods (IRIS, ASIR, iDose, SAFIRE) (38,39). The use of these methods allows to reduce the average dose of chest CT to 2–3 mSv (36). Considering role of chest CT in the diagnostics of adults with CC, we should balance profit and loss. Although there is a dispute about the cancerogenic effect of low doses of ionizing radiation (35,40,41), we believe it should not be a final argument against chest CT in diagnosing adults with CC (23,42,43). However, considering the low cost-effectiveness and radiation exposure of thoracic CT scanning in diagnosis of CC (5,14), there is certainly a need to define a group of patients which can particularly benefit from referral to CT. As in our study false negative CXR results were more common in patients older than 62 years, it seems that chest CT should be considered particularly in this group of patients. Surprisingly, we did not observe the same relationship in patients with relevant smoking history (smokers and ex-smokers). This can be explained by a low number of such patients (only 40) in our study and the fact that smokers rarely seek medical help attention because of CC despite the fact that smoking-associated chronic bronchitis is the most common cause of CC.
Limitations of the study
Our study has some limitations. Firstly, it was a single center study performed in the “cough clinic” setting and included mostly patients with difficult-to- treat CC. Therefore, the results refer mainly to a specific adult population treated in the “cough clinic”. Secondly, it was a retrospective analysis, but all patients were diagnosed according to worked out algorithm (see Figure S1). Thirdly, in majority of patients, CXR was performed only in postero-anterior view. Lateral CXR could show chest regions that are hidden behind mediastinum and perhaps reveal some additional abnormalities in these locations (43). Fourthly, CXR and CT were analyzed by only one radiology specialist, but then analyzed once more by respiratory physician. Next, as sometimes causal treatment of CC triggers might not be possible or its effect might be limited, we extended our definition of potential causal relationship to those situations when CT findings changed further diagnostic approach or management. Finally, as active smokers constituted only 3% of our population, the results of the study should not be extrapolated to active smokers with CC. Despite all these limitations, we believe that our results give arguments for considering thoracic CT as the additional imaging test in adults with CC, especially in patients older than 62 years old with either unsatisfactory response to treatment or risk factors for lung cancer.
Conclusions
Plain CXR shows a limited diagnostic yield in adults with difficult-to-treat CC referred to cough clinic. In these patients chest CT scans may add significant data and impact the diagnostic and therapeutic approach.
Acknowledgments
Part of this work was presented at the 11th International Cough Symposium, 21-22 January 2021.
The authors thank Dr. Marta Maskey-Warzechowska for language editing and proofreading of the final version of the manuscript.
Funding: None.
Footnote
Provenance and Peer Review: This article was a standard submission to the series “Cough Section” published in Journal of Thoracic Disease. The article has undergone external peer review.
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-111/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-111/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-111/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-111/coif). MD has received fees from Merck for consultations and lectures on chronic cough, outside the submitted work. EMG has received honorary for lectures on chronic cough from Merck and Polpharma, outside the submitted work. ARF has received fee from Polpharma for attendance at ERS International Congress (2019), outside the submitted work. RK reports research grant from the National Science Centre, Poland, honoraria for lectures from Chiesi, AstraZeneca, Polpharma and MSD, fees for Advisory Board participation from MSD and AstraZeneca; all the above outside the submitted work. Boehringer Ingelheim, Chiesi, AstraZeneca and MSD have covered his fee and travel expenses for international conferences, outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of the Medical University of Warsaw (No. KB/101/2009). Informed consent was obtained from all subjects involved in the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Morice AH, Fontana GA, Sovijarvi AR, et al. The diagnosis and management of chronic cough. Eur Respir J 2004;24:481-92. [Crossref] [PubMed]
- Morice AH, McGarvey L, Pavord I, et al. Recommendations for the management of cough in adults. Thorax 2006;61:i1-24. [Crossref] [PubMed]
- Irwin RS, Baumann MH, Bolser DC, et al. Diagnosis and management of cough executive summary: ACCP evidence-based clinical practice guidelines. Chest 2006;129:1S-23S. [Crossref] [PubMed]
- Chamberlain SA, Garrod R, Douiri A, et al. The impact of chronic cough: a cross-sectional European survey. Lung 2015;193:401-8. [Crossref] [PubMed]
- Morice AH, Millqvist E, Bieksiene K, et al. ERS guidelines on the diagnosis and treatment of chronic cough in adults and children. Eur Respir J 2020;55:1901136. [Crossref] [PubMed]
- Morice AH, Millqvist E, Belvisi MG, et al. Expert opinion on the cough hypersensitivity syndrome in respiratory medicine. Eur Respir J 2014;44:1132-48. [Crossref] [PubMed]
- Chung KF, McGarvey L, Mazzone S. Chronic cough and cough hypersensitivity syndrome. Lancet Respir Med 2016;4:934-5. [Crossref] [PubMed]
- Gibson P, Wang G, McGarvey L, et al. Treatment of Unexplained Chronic Cough: CHEST Guideline and Expert Panel Report. Chest 2016;149:27-44. [Crossref] [PubMed]
- McGarvey L, Gibson PG. What Is Chronic Cough? Terminology. J Allergy Clin Immunol Pract 2019;7:1711-4. [Crossref] [PubMed]
- Irwin RS, French CL, Chang AB, et al. Classification of Cough as a Symptom in Adults and Management Algorithms: CHEST Guideline and Expert Panel Report. Chest 2018;153:196-209. [Crossref] [PubMed]
- Irwin RS. Diagnosis and Management of Cough. Chest 2006;129:24S. [Crossref] [PubMed]
- Expert Panel on Thoracic Imaging. ACR Appropriateness Criteria® Chronic Cough. J Am Coll Radiol 2021;18:S305-19. [Crossref] [PubMed]
- Barnes TW, Afessa B, Swanson KL, et al. The clinical utility of flexible bronchoscopy in the evaluation of chronic cough. Chest 2004;126:268-72. [Crossref] [PubMed]
- Kastelik JA, Aziz I, Ojoo JC, et al. Investigation and management of chronic cough using a probability-based algorithm. Eur Respir J 2005;25:235-43. [Crossref] [PubMed]
- McGarvey LP, Heaney LG, Lawson JT, et al. Evaluation and outcome of patients with chronic non-productive cough using a comprehensive diagnostic protocol. Thorax 1998;53:738-43. [Crossref] [PubMed]
- Truba O, Rybka A, Klimowicz K, et al. Is a normal chest radiograph sufficient to exclude pulmonary abnormalities potentially associated with chronic cough?. Adv Respir Med. 2018;86: [Crossref] [PubMed]
- van der Bruggen-Bogaarts BA, van der Bruggen HM, van Waes PF, et al. Screening for bronchiectasis. A comparative study between chest radiography and high-resolution CT. Chest 1996;109:608-11. [Crossref] [PubMed]
- Zompatori M, Bnà C, Poletti V, et al. Diagnostic imaging of diffuse infiltrative disease of the lung. Respiration 2004;71:4-19. [Crossref] [PubMed]
- Collins J, Stern EJ. Chest Radiology, The Essentials. II-ed. Philadelphia: Lippincott Williams & Wilkins; 2008.
- Birring SS, Prudon B, Carr AJ, et al. Development of a symptom specific health status measure for patients with chronic cough: Leicester Cough Questionnaire (LCQ). Thorax 2003;58:339-43. [Crossref] [PubMed]
- Hajian-Tilaki K. Sample size estimation in diagnostic test studies of biomedical informatics. J Biomed Inform 2014;48:193-204. [Crossref] [PubMed]
- Descazeaux M, Brouquières D, Didier A, et al. Impact of chest computed tomography scan on the management of patients with chronic cough. ERJ Open Res 2021;7:00222-2021. [Crossref] [PubMed]
- Yu L, Xu X, Niu S. Should computed tomography and bronchoscopy be routine examinations for chronic cough? J Thorac Dis 2020;12:5238-42. [Crossref] [PubMed]
- Quekel LG, Kessels AG, Goei R, et al. Miss rate of lung cancer on the chest radiograph in clinical practice. Chest 1999;115:720-4. [Crossref] [PubMed]
- Kaneko M, Eguchi K, Ohmatsu H, et al. Peripheral lung cancer: screening and detection with low-dose spiral CT versus radiography. Radiology 1996;201:798-802. [Crossref] [PubMed]
- Padley SP, Hansell DM, Flower CD, et al. Comparative accuracy of high resolution computed tomography and chest radiography in the diagnosis of chronic diffuse infiltrative lung disease. Clin Radiol 1991;44:222-6. [Crossref] [PubMed]
- Volpe J, Storto ML, Lee K, et al. High-resolution CT of the lung: determination of the usefulness of CT scans obtained with the patient prone based on plain radiographic findings. AJR Am J Roentgenol 1997;169:369-74. [Crossref] [PubMed]
- Bonavita J, Naidich DP. Imaging of bronchiectasis. Clin Chest Med 2012;33:233-48. [Crossref] [PubMed]
- Singh A, Bhalla AS, Jana M. Bronchiectasis Revisited: Imaging-Based Pattern Approach to Diagnosis. Curr Probl Diagn Radiol 2019;48:53-60. [Crossref] [PubMed]
- Rosen MJ. Chronic cough due to bronchiectasis: ACCP evidence-based clinical practice guidelines. Chest 2006;129:122S-131S. [Crossref] [PubMed]
- Katzka DA, Kahrilas PJ. Advances in the diagnosis and management of gastroesophageal reflux disease. BMJ 2020;371:m3786. [Crossref] [PubMed]
- Sarma A, Heilbrun ME, Conner KE, et al. Radiation and chest CT scan examinations: what do we know? Chest 2012;142:750-60. [Crossref] [PubMed]
- Fazel R, Krumholz HM, Wang Y, et al. Exposure to low-dose ionizing radiation from medical imaging procedures. N Engl J Med 2009;361:849-57. [Crossref] [PubMed]
- McCollough CH, Bushberg JT, Fletcher JG, et al. Answers to Common Questions About the Use and Safety of CT Scans. Mayo Clin Proc 2015;90:1380-92. [Crossref] [PubMed]
- Mettler FA Jr, Huda W, Yoshizumi TT, et al. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology 2008;248:254-63. [Crossref] [PubMed]
- National Research Council. Health Risks from Exposure to low levels of ionizing radiation: BEIR VII Phase 2 NRC (US). Health risks from exposure to low levels of ionizing radiation: BEIR VII Phase 2. Washingt DC Natl Acad Press. 2006.
- Kubo T, Ohno Y, Kauczor HU, et al. Radiation dose reduction in chest CT--review of available options. Eur J Radiol 2014;83:1953-61. [Crossref] [PubMed]
- Ohno Y, Koyama H, Seki S, et al. Radiation dose reduction techniques for chest CT: Principles and clinical results. Eur J Radiol 2019;111:93-103. [Crossref] [PubMed]
- Doss M, Little MP, Orton CG. Point/Counterpoint: low-dose radiation is beneficial, not harmful. Med Phys 2014;41:070601. [Crossref] [PubMed]
- Hendee WR, O'Connor MK. Radiation risks of medical imaging: separating fact from fantasy. Radiology 2012;264:312-21. [Crossref] [PubMed]
- McCunney RJ. POINT: should radiation dose from CT scans be a factor in patient care? Yes. Chest 2015;147:872-4. [Crossref] [PubMed]
- Doss M. COUNTERPOINT: should radiation dose from CT scans be a factor in patient care? No. Chest 2015;147:874-7. [Crossref] [PubMed]
- Raoof S, Feigin D, Sung A, et al. Interpretation of plain chest roentgenogram. Chest 2012;141:545-58. [Crossref] [PubMed]