
When to proceed to surgical rib fixation?—A single-institution clinical experience
Highlight box
Key findings
• In our ten years’ experience of surgical stabilization of rib fractures (SSRF), time to surgery was not associated to clinical outcome.
What is known and what is new?
• However, the impact of timing to SSRF on clinical outcomes has not been clearly defined, and remains a controversial topic. Some previous studies suggest that suggest that early surgery has a positive outcome. In this cohort of 159 subjects delay to surgery was not associated with either pneumonia or failure of extubating.
What is the implication, and what should change now?
• Our results suggest that SSRF could be delayed pending the stabilization of other clinical issues. SSRF in polytraumatized patients is feasible but should not interfere with the treatment of urgent visceral lesions and could be delayed with satisfactory post-operative results.
Introduction
Flail chest and multiple rib fractures are common injuries occurring after blunt chest trauma with an estimated frequency of 150 per 1,000,000 injuries (1). Overall mortality associated with multiple rib fractures is around 10%. Mortality increases in older patients (2). Identified predictors of mortality are age >65 years old (3), three or more rib fractures and the development of pneumonia post injury (4).
The most frequent evolution in chest wall injuries is the occurrence of pneumonia, which can lead to acute respiratory distress syndrome (ARDS) requiring prolonged ventilation (5). Surgical stabilization of rib fractures (SSRF) was described in the modern history of thoracic surgery, then largely replaced by internal stabilization using mechanical ventilation. Surgical rib fixation for chest wall trauma sparked an increased interest (6) in the 2000s following Tanaka et al. and Granetzny et al. (7,8) advocating the positive impact on outcomes of surgery vs. conservative management. These two publications showed the benefits of surgery (on length of mechanical ventilation, length of hospital stay, decrease of pneumonia rate, decrease of mortality and benefits on pulmonary function) for patients suffering from flail chest and re-opened an era of SSRF. Despite evidence of clinical benefits (9-11) less than 2% of flail chest and multiple rib fractures, patients undergo surgical intervention (12) and SSRF is still considered to be controversial by some surgeons (6). The tendency towards a non-surgical management attitude is mainly secondary to a lack of consensus regarding surgical indications (13). The Chest Wall Injury Society (CWIS) has edited a rib fractures taxonomy to indicate the need for potential surgery in displaced rib fractures (14).
However, the impact of timing to SSRF on clinical outcomes has not been clearly defined, and remains a controversial topic. We present our ten years retrospective evaluation of consecutive SSRF to evaluate the association between time to surgery and clinical outcome. Our secondary objective was to determine the factors associated with early pneumonia. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-735/rc) (15).
Methods
We conducted a retrospective review of real-world data from routine care of all the patients admitted in a Level I trauma center (Centre Hospitalo-Universitaire des Hospices Civiles de Lyon, France) with severe thoracic trauma who underwent SSRF from September 2010 to January 2020. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study received Institutional Review Board approval from the French Society of Thoracic and Cardio-Vascular Surgery (Société Française de Chirurgie Thoracique et Cardio-Vasculaire – IRB00012919 – 05/04/2022) and informed consent was taken from all the patients.
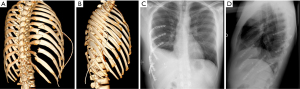
Severe thoracic trauma was defined by an Abbreviated Injury Scale (AIS) of 3 or more (16). All selected patients had 3 or more displaced rib fractures or flail chest [as defined per the taxonomy of Edwards et al. (17)], a respiratory rate >25 cycles/min or hypoxemia on pulse oximetry (<90% without oxygen) or a circulatory failure (systolic arterial pressure <110 mmHg, or more than 30% decrease in systolic arterial pressure). On admission, full body CT scan (Figure 1) screened all patients with severe thoracic trauma without severe hemodynamic instability or life-threatening injury. Patients were managed according to the guidelines of the French society of critical care and anesthesia (18). All life threatening or hemorrhagic lesions were treated before SSRF.
Standard management
All patients were directly admitted to Intensive Care Units (ICU). All patients were screened according to the current American College of Surgeon Committee on Trauma, Advanced Trauma Life Support (ATLS) guidelines (12,19). Optimized pain control was achieved via standard practices: non-narcotic (including non-steroidal anti-inflammatory drugs) and/or narcotic oral or IV medications; if feasible placement of a T4–T5 epidural catheter or serratus or paravertebral blocks. Aggressive airway drainage was achieved by means of frequent chest therapy and non-invasive ventilation with positive end expiratory pressure (PEEP). The attending physician who clinically monitored for pain control, oxygen saturation, functional respiratory status and temperature evaluated all the patients daily. A daily Chest X-ray was performed to screen for rib fracture displacement and pulmonary complications. When encountering fever, blood cultures were sampled.
Surgical treatment
Indication for SSRF were major rib fracture displacement (at least one bicortical displaced rib) with chest wall deformation and/or ongoing pain and/or mobile flail chest and/or hemothorax >200 mL/hour and/or suspicion of diaphragmatic laceration. Patient surgery positioning was adapted to injury localization as well as extra thoracic injuries such as spine trauma and pelvic or limb fractures. The posterolateral approach was preferably used for rib fractures of the lateral and posterior chest wall, whereas the anterior approach was used for anterior chest wall disruption or for patients with spine injuries or other injuries requiring strict supine positioning. SSRF was preferentially performed on fractures of the 3rd to the 9th ribs using the MatrixRibTM system (DePuy Synthes, Johnson & Johnson, USA) with plates and intra-medullary splints. The procedure did not evolve over this 10-years period and was standardized as follows: patients were under general anesthesia with double lumen endotracheal tube. Skin incisions were centered on the lesions; muscles were incised in the fiber axes or were mobilized without division whenever feasible. After exposure of rib fractures without opening the chest wall, an intercostal trocar was inserted to perform a video thoracoscopic exploration to manage clot removal and to identify any diaphragmatic laceration or rupture. Diaphragmatic repair was performed by a video thoracoscopic approach if possible or after a thoracotomy conversion using non-resorbable stitches with Teflon patches. Universal plates were preferably used to fix flail chest or multiple rib fractures with 3 screws on each side as recommended. The intramedullary splints were preferably used in patient requiring surgery in the supine position to fix lateral and posterior fractures by an anterior approach (trap door incisions). At the end of the procedure, the chest wall cavity was washed with 2–3 liters of 37 ℃ isotonic saline solution; a 24 Fr chest drain was inserted and placed at −20 mmHg suction. All patients were finally referred to an ICU for post-operative monitoring.
We defined time from hospital admission to surgery in three groups using cutoffs already chosen in the literature (20,21): early surgery (within 48 h after admission), mid (from 48 hours to 7 days) and late surgery (after 7 days). Complete patient main characteristic data and circumstances of injuries were collected using emergency unit reports. Numerous data were prospectively recorded such as chest wall fracture characterization with 3D CT scan reconstruction, length of mechanical ventilation, length of ICU and hospital stay; Home discharge or rehabilitation centers, pulmonary morbidity and overall mortality. The Chest Trauma Score (CTS) (22), the Injury Severity Score (ISS) and the new Simplified Acute Physiology Score (SAPS II) (23) were calculated to gather physiologic and clinical data.
Variables and definitions
Main clinical outcomes were defined as the development of pneumonia and extubation failure (need for reintubation within 48 h after endotracheal tube removal or inability for the patient to trigger and sustain spontaneous breathing after changing the setup of the mechanical ventilation to continuous spontaneous ventilation mode). Pneumonia was defined using Horan et al. pneumonia flow diagram (24). We defined early pneumonia (<5 post-operative days). and late pneumonia. Secondary outcomes/endpoints were numbers of ventilation days after surgery, length of stay in ICU and in hospital in days, tracheostomy and mortality rate at 30 days after trauma. Thoracic deformation was defined as the absence of symmetry of the chest wall after trauma on the X-ray.
Statistical analyses
A data monitoring and steering committee composed of two surgeons, an anesthesiologist, an epidemiologist and a statistician was assembled in order to analyze the independent association between time to surgery and clinical outcomes.
Data are presented with figures and percentages for categorical variables and median (interquartile range) for continuous variables. Normality was investigated using graphical methods for continuous variables. Non-parametric Kruskal-Wallis test was used to compare non-normal quantitative variables and ANOVA was used for normally distributed variables. Qualitative variables were compared with the Chi-squared test unless expected counts were less than 10, in which case Fischer’s exact test was used.
We faced an indication bias as the three groups were constituted retrospectively. In order to consider indication bias and potential confounders, covariates that might influence both timings of surgery and clinical outcomes were analyzed using a Directed Acyclic Graph (DAG). The first step consisted in the choice of the covariates to analyze (mechanism of trauma (motor vehicle crash, pedestrian/bicycle collision, fall from a height, other), uncontrolled pain as an indication to surgery, SAPS II, ISS, presence of hemothorax, presence and number of non-thoracic lesions, chest deformation as indication to surgery, CTS (less than 5 vs. 5 or more). The hypotheses of association were based on the literature and on pathophysiological knowledge. The second step was to identify covariates whose effect can be mediated by others and, then these new hypotheses were investigated by the monitoring and steering committee. Finally, all the direct associations were used to construct the DAG using the DAGitty software (25). A multivariable logistic regression model was used to calculate the odds ratio of main outcomes and covariates selected using the DAG. Sensitivity analyses were performed to analyze timing for surgery divided into two groups (within 48 vs. 48 h and more). Analyses were performed on complete data.
Then, an exploratory analysis was conducted to determine factors associated with early pneumonia. A first selection of the variables of interest was carried out with a univariable logistic regression model. Then, the variables of interest with a threshold of P<0.25 were implemented in a multivariable logistic regression model. Then, using a backward stepwise selection of covariates, the covariates were selected until the most appropriate model, defined by the lowest Akaike Information Criterion. A threshold of α= 0.05 was considered for significance for the final model. Analyses were performed with R software version 3.5.1.
Results
During the 10-year study period from September 2010 to January 2020, 159 patients were treated by SSRF: 57 in the early group, 83 in the mid group and 19 in the late group. The median age was 54 years (43–68 years). Gender was male in 117 cases (73.6%). The most frequent circumstances of admission were motor vehicle crash in 84 cases (52.8%) and fall from a height in 48 cases (30.2%); other trauma mechanisms occurred in 10.1% of cases (N=16) and ranged from skiing accidents to a “raging bull” charge. The three groups were quite similar in terms of demographic characteristics and mechanism of trauma (Table 1). All variables had less than 2% of missing data.
Table 1
| Early (N=57) | Mid (N=83) | Late (N=19) | P value | |
|---|---|---|---|---|
| Demographic features | ||||
| Male gender | 42 (73.7) | 60 (72.3) | 15 (78.9) | 0.838 |
| Age (years) | 55 [44–67] | 53 [43–70] | 52 [39–68] | 0.919 |
| Mechanism of the trauma | 0.825 | |||
| Motor vehicle crash | 28 (49.1) | 46 (55.4) | 10 (52.6) | |
| Pedestrian/Bicycle collision | 4 (7.0) | 5 (6.0) | 2 (10.5) | |
| Fall | 18 (31.6) | 26 (31.3) | 4 (21.1) | |
| Other | 7 (12.3) | 6 (7.2) | 3 (15.8) | |
| Clinical status at admission | ||||
| Glasgow Coma Scale | 15 [15–15] | 15 [14–15] | 15 [15–15] | 0.886 |
| Spontaneous ventilation | 45 (78.9) | 63 (75.9) | 16 (84.2) | 0.716 |
| ISS | 29 [16–41] | 33 [25–43] | 34 [25–42] | 0.231 |
| SAPS II | 24 [15–29] | 25 [16–32] | 29 [16–33] | 0.563 |
| Extra-thoracic lesions | 28 (49.1) | 35 (42.2) | 15 (78.9) | 0.015 |
| Spine fracture | 7 (12.3) | 14 (16.9) | 7 (36.8) | 0.050 |
| Including vertebral fracture | 0 (0.0) | 5 (6.0) | 3 (15.8) | 0.020 |
| Aortic isthmic lesion | 0 (0.0) | 1 (1.2) | 1 (5.3) | 0.204 |
| Abdominal lesion | 8 (14.0) | 20 (24.1) | 3 (15.8) | 0.306 |
| Orthopedic lesion | 17 (29.8) | 21 (25.3) | 10 (52.6) | 0.064 |
| Thoracic lesions | ||||
| Number of rib fractures | 7 [5–9] | 8 [6–10] | 9 [6–11] | 0.063 |
| Flail chest | 42 (73.7) | 64 (77.1) | 15 (78.9) | 0.855 |
| With more than 6 ribs | 7 (12.3) | 18 (21.7) | 4 (21.1) | 0.347 |
| Lung contusion | 46 (80.7) | 74 (89.2) | 19 (100.0) | 0.071 |
| Bilateral contusion | 9 (15.8) | 17 (20.5) | 4 (21.1) | 0.758 |
| Hemopneumothorax | 31 (54.4) | 49 (59.0) | 11 (57.9) | 0.860 |
| Hemothorax | 5 (8.8) | 9 (10.8) | 2 (10.5) | 0.921 |
| Pneumothorax | 14 (24.6) | 17 (20.5) | 5 (26.3) | 0.805 |
| Chest Trauma Score | 6 [5–7] | 7 [6–7] | 7 [6–8] | 0.082 |
| 5 or more | 51 (89.5) | 76 (91.6) | 17 (89.5) | 0.901 |
Data are presented as N (%) or median [interquartile range]. ISS, Injury Severity Score; SAPS II, Simplified Acute Physiology Score.
Pre-operative first 48 hours management
Thirty-five (22%) patients were intubated on the accident scene, and 124 (78%) patients were spontaneously breathing with or without oxygen supplementation with pulse oximetry >95%; however, 30% of initially non-ventilated patients required mechanical ventilation secondary to clinical deterioration or for emergency surgery. Noninvasive ventilation was required in 72 (45.3%) patients.
An epidural catheter was inserted in 52 (32.7%) and a paravertebral catheter was placed in 8. Two patients, with initially minor displacement, experienced transient cardiac arrest secondary to extreme hypotension following epidural catheter placement. A conservative treatment had originally been decided, but increased fracture displacement following cardio-pulmonary resuscitation required subsequent SSRF.
Lesions description
On admission to the ICU, median ISS and SAPS II were 32 [25–43] and 24 [15–32] respectively. After total body-CT-scan; the median number of rib fractures was 7 [6–10]. A flail chest was present in 121 (76.1%) patients, it was lateral in 90 (74.4%). Pulmonary lobe contusion was observed in 139 (87.4%) patients and involved more than 1 lobe in 67 (42.1%). A hemopneumothorax was described in 91 patients (57.2%). Two patients (1.3%) presented acute aortic isthmic rupture requiring urgent endovascular procedures using covered stent graft. Extra thoracic associated lesions were encountered in 49.1% of the patients. Among them, bony fractures including the upper and lower extremities were found in 48 (61.5%) patients. Abdominal injuries were found in 25 (32.1%) patients, liver contusion and splenic injuries were the most frequent (Table S1). Spine injuries were detected in 28 (17.6%) patients involving the transverse processes in 24 (85.7%).
Subjects from the late group presented a higher ISS and a higher SAPS II than the others. The early group had less spine fractures (and none vertebral fractures) when compared to the two other groups. There was no difference between groups in number of rib fractures, hemothorax, hemopneumothorax or CTS. The proportion of lung contusions increased with the delay to surgery.
Surgical rib fixation
The surgical indication was principally chest wall deformation in 126 (79%) and uncontrolled pain despite optimal analgesia in 97 (61%) patients. Additionally, 73 patients (45.9%) suffered from both chest wall deformation and uncontrolled pain (Table 2). SSRF was performed using 3 [2–4] plates and intramedullary splints in 22 cases (13.8%). During systematic associated video thoracoscopy, a diaphragmatic perforation requiring surgical stitches was encountered in 5 (3.1%). The median time to surgery was 3 days (2–5 days): the early group included 57 subjects, the mid group 83 patients and 19 made up the late group.
Table 2
| Variables | Overall population (N=159) | Early (N=57) | Mid (N=83) | Late (N=19) | P value |
|---|---|---|---|---|---|
| Indication of surgery | |||||
| Chest wall deformation | 126 (79.2) | 42 (73.7) | 68 (81.9) | 16 (84.2) | 0.423 |
| Mobile flail chest | 61(38.4) | 19 (33.3) | 36 (43.4) | 6 (31.6) | 0.393 |
| Uncontrolled pain | 97 (61.0) | 33 (57.9) | 53 (63.9) | 11 (57.9) | 0.744 |
| Time to surgery (days) | 3 [2–5] | 2 [1–2] | 4 [3–5] | 10 [9–11] | <0.001 |
| Number of fixed ribs | 4 [3–5] | 4 [3–4] | 4 [3–5] | 3 [3–5] | 0.379 |
| Ratio fixed/ non-fixed ribs | 0.50 [38–69] | 57 [44–78] | 50 [36–65] | 44 [26–58] | 0.010 |
| Systematic VATS results: associated phrenic perforation | 5 (3.1) | 1 (1.8) | 4 (4.8) | 0 (0.0) | 0.418 |
| Post-operative outcomes | |||||
| Pneumonia | 61 (38.4) | 21 (36.8) | 32 (38.6) | 8 (42.1) | 0.895 |
| Extubation failure after SSRF | 20 (12.6) | 9 (15.8) | 10 (12.0) | 1 (5.3) | 0.585 |
| Tracheostomy | 3 (1.9) | 0 (0.0) | 3 (3.6) | 0 (0.0) | 0.503 |
| LOV (days) | 0 [0–4] | 0 [0–3] | 0 [0–4] | 0 [0–8] | 0.789 |
| LOV after SSRF (days) | 0 [0–1] | 0 [0–1] | 0 [0–1] | 0 [0–1] | 0.924 |
| LOS Intensive care unit (days) | 8 [4–15] | 6 [4–11] | 9 [5–16] | 12 [6 –20] | 0.168 |
| LOS Hospital (days) | 18 [13–30] | 15 [11–25] | 18 [13–31] | 24 [18–32] | 0.034 |
| <30 days mortality | 4 (2.5) | 2 (3.5) | 2 (2.4) | 0 (0.0) | 0.999 |
Data are presented as N (%) or median [interquartile range]. VATS, video-assisted thoracoscopic surgery; SSRF, surgical stabilization of rib fracture; LOV, length of ventilation; LOS, length of stay.
Post-operative course and complications and outcomes
The median length of mechanical ventilation after SSRF was 0 days (0–1 day). Pneumonia was encountered in 61 (38.4%) patients and, most of the time, was diagnosed within <5 days after trauma (N=40; 65.6% of the pneumonia). Thirteen patients (8.2%) evolved towards an ARDS, two of them died at respectively day 14 and 10 (they belonged to the early group). Postoperative outcomes were not different between the three groups, the expected length of stay in hospital was significantly increased in the mid and late groups (Table 2).
Covariates selection with DAG found two sets of adjustment for the multivariable analysis (Figure S1) and results were similar: time to surgery was not independently associated with pneumonia or failure of extubation. Conclusions were similar in sensitivity analyses (Table 3). Multivariable analysis (P<0.05) identified early intubation on the trauma scene and multiple trauma as risk factors for early pneumonia (Table 4).
Table 3
| Variables | OR | 95% CI | P value |
|---|---|---|---|
| Main analyses | |||
| Pneumonia | |||
| Adjustment set 1: age, chest trauma scoring, ISS, mechanism of trauma, pain as indication to surgery, SAPS II | |||
| Early group | Ref | ||
| Mid group | 0.81 | 0.38–1.72 | 0.580 |
| Late group | 0.82 | 0.24–2.58 | 0.732 |
| Adjustment set 2: chest trauma scoring, hemothorax, ISS, mechanism of trauma, presence of non-thoracic lesions, pain as indication to surgery, SAPS II‡ | |||
| Early group | Ref | ||
| Mid group | 0.92 | 0.42–2.00 | 0.832 |
| Late group | 0.81 | 0.24–2.65 | 0.734 |
| Extubation failure | |||
| Adjustment set 1: age, chest trauma scoring, ISS, Mechanism of trauma, pain as indication to surgery, SAPS II | |||
| Early group | Ref | ||
| Mid group | 0.70 | 0.23–2.12 | 0.521 |
| Late group | 0.17 | 0.09–1.24 | 0.134 |
| Adjustment set 2: chest trauma scoring, hemothorax, ISS, mechanism of trauma, presence of non-thoracic lesions, pain as indication to surgery, SAPS II | |||
| Early group | Ref | ||
| Mid group | 0.82 | 0.25–2.65 | 0.737 |
| Late group | 0.18 | 0.08–1.65 | 0.182 |
| Sensitivity analyses | |||
| Pneumonia | |||
| Adjustment set 1: surgery after 48 h vs. surgery within 48 h (ref) | 0.81 | 0.39–1.68 | 0.568 |
| Adjustment set 2: surgery after 48 h vs. surgery within 48 h (ref) | 0.90 | 0.43–1.90 | 0.778 |
| Extubation failure | |||
| Adjustment set 1: surgery after 48 h vs. surgery within 48 h (ref) | 0.56 | 0.19–1.67 | 0.298 |
| Adjustment set 2: surgery after 48 h vs. surgery within 48 h (ref) | 0.66 | 0.21–2.09 | 0.474 |
OR, odds ratio; CI, confidence interval; ISS, Injury Severity Score; SAPS II, Simplified Acute Physiology Score.
Table 4
| Variables | Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value | Adjusted OR (95% CI) | P value | ||
| Gender: Male vs. Female (ref) | 1.38 (0.59–3.2) | 0.446 | |||
| Age (increase of 10 years) | 0.91 (0.74–1.12) | 0.374 | |||
| Glasgow Coma Scale | 0.88 (0.79–0.97) | 0.010 | 0.91 (0.80–1.03) | 0.144 | |
| Initial spontaneous ventilation (yes vs. no) | 0.18 (0.08–0.41) | <0.001 | |||
| Initial mechanical ventilation (yes vs. no) | 8.58 (3.84– 19.15) | <0.001 | 15.73 (4.66–53.11) | <0.001 | |
| Polytraumatized vs. isolated (ref) | 2.2 (1.06–4.57) | 0.032 | 2.84 (1.03 –7.86) | 0.041 | |
| ISS | 1.03 (1.00–1.05) | 0.024 | |||
| SAPS II | 1.01 (0.98–1.04) | 0.608 | |||
| Contusion | 0.149 | ||||
| No contusion (ref) | ref | – | |||
| Unilateral contusion | 3.69 (0.81–16.84) | 0.092 | |||
| Bilateral contusion | 2.86 (0.53–15.52) | 0.222 | |||
| Contusion–number of lobes | 0.032 | ||||
| None (ref) | ref | – | ref | – | |
| 1 lobe | 7.33 (0.92–58.1) | 0.063 | 10.50 (0.77–142.52) | 0.077 | |
| >1 lobes | 4.72 (0.5–44.67) | 0.046 | 1.43 (0.09–22.99) | 0.80 | |
| Number of ribs fractured | 1.11 (0.99–1.23) | 0.065 | |||
| Flail | 0.572 | ||||
| No flail | ref | – | |||
| Flail 3–6 ribs | 0.64 (0.28–1.48) | 0.299 | |||
| Flail >6 ribs | 0.83 (0.28–2.39) | 0.724 | |||
| Ratio fixed ribs/fractured ribs (increase of 10%) | 1.03 (0.89–1.18) | 0.726 | |||
| Delay to surgery (days) | 0.996 (0.91–1.009) | 0.934 | |||
OR, odds ratio; CI, confidence interval; ISS, Injury Severity Score; SAPS II, Simplified Acute Physiology Score.
A wound infection with septic material contamination was described in 4 (2.5%) cases during the first month and needed pleural decortication and antibiotic treatment for 6 weeks to treat germs identified by intraoperative samplings. Three of these required material removals without any complication or chest instability. Moreover, 2 patients (1.3%) were re-operated for hemothorax and a wound hematoma. The median length of ICU stay was 8 days (0–56 days) and the median length of in hospital stay was 18.5 days (5–109 days) (Table 2). Furthermore, 4 patients required surgical removal more than one year later due to complications induced by the prosthetic device (one patient suffered from chronic pain, two suffered from screw displacement and one from rib fracture three years later).
Discussion
Our results suggest that time to surgery is not associated with clinical outcomes (pneumonia and extubation failure). Early pneumonia was associated with initial intubation and polytraumas.
We referred to surgery patients with (I) need of urgent thoracotomy; (II) evident clinical or CT scan chest wall deformation; (III) presence of a paradoxical ventilation with respiratory insufficiency and (IV) failure of adequate analgesia management complicated with ineffective cough and bronchial congestion. Using these guidelines also reported by other authors, less than 10% of chest wall trauma would require surgery (14,26-28) with evident clinical benefits compared to conservative treatment.
Several studies (28-30) advocate the importance of an early SSRF within the first 72 hours. However, our findings suggest that surgery can be delayed without increasing negative clinical outcomes. Finally, the lack of significant impact of surgical delay prior to SSRF is a plea, especially in polytraumatized patients, to favor any other urgent procedure such as laparotomy or bone fracture reduction. The delay could also be used to correct metabolic disorders (hypothermia, bleeding and acidosis). Therefore, prolonged mechanical ventilation before surgery should not be considered as a contraindication of rib fixation. However, as demonstrated in a literature review (30), surgical rib fixation should be performed as soon as patients’ clinical status allows for safe surgery.
Our data suggest that the occurrence of early pneumonia is previously conditioned by the presence of a polytrauma and the need for mechanical ventilation set up on the “accident scene”. Early mechanical ventilation may be reflective of a worse initial clinical situation that is associated with early pneumonia. Intubation may not be itself associated with early pneumonia.
The systematic use of thoracic video-assisted thoracoscopic surgery (VATS) exploration allows for clot removal, pleural irrigation, lung extrication and repair of pulmonary lacerations (31-33). In this series, VATS lead to the discovery and repair of undiagnosed diaphragmatic perforations in 7% of cases (from 1–5 cm), especially in patients with lower rib fractures involving ribs 7 to 10. The early repair could prevent delayed diagnosis of intra thoracic abdominal herniation which could lead to respiratory complications or volvulus with bowel occlusion (34-36).
SSRF were initially realized using postero-lateral incisions (37) but could be improved and replaced using muscle-sparing dissection when feasible to avoid chest wall deformation and could reduce the risk of wound infection (2.5% in our current work). The future evolution would probably be in the use of minimally-invasive surgery (38-40) and in the development of 3D printed or resorbable devices. As we described a technique using plates and intra-medullary splints, we should be aware of the risk of rare and life-threatening organ injury (41) and consider other types of fixtures (42). Even though, Marasco et al. highlighted the risk of failure of SSRF of the posterior portion of the rib with one resorbable device (43) but should be used carefully (44). SSRF is difficult to perform in the posterior part of the rib and we may question the necessity to fix all fractures? Accessible rib fractures on the other hand can be fixed without parietal deformation (45,46). Surgical rib fixation would better be scheduled as part of a global process of clinical stabilization. Besides, as there is no significant difference in outcomes regarding to the proportion of fixed ribs, it seems reasonable to consider the necessity of fixation of all fractured ribs.
Even if our work did not show the negative impact of lung contusion on the risk of pneumonia development as significance was not reached, it is highly suspected that lung contusion is associated with pneumonia. Non-invasive ventilation can be useful to improve oxygenation and treat atelectasis (47). However, one of the strategic challenges to facilitating ventilation is the crucial role of analgesia to allow effective coughing efforts.
Multiple studies have demonstrated clinical benefit of SSRF. Indeed, many publications demonstrated a true benefit in the early post-traumatic period on length of ventilation and ICU stay (48). Granetzny et al. (8) demonstrated that SSRF statistically improves functional status on forced expiratory volume in the first second (FEV1) and vital capacity (VC). However, some studies suggested no effect of SSRF on long-term outcomes (49,50). A previous study showed (51) that the quality of life of patients after rib fixation with EQ-5D was quite satisfying although.
We believe that further studies are needed to evaluate the factors related to clinical issues for patients with extended trauma. Our results might suffer from a lack of power as the overall population included 159 subjects with only a small sample of subjects in the late group. This could explain a higher although non-significant pneumonia rate in the late group. Moreover, timing groups were constituted retrospectively as timing to SSRF was decided by surgical and medical teams regarding subjects’ clinical status. We analyzed possible confounders that might impact both group construction and outcomes. We focused on the association between the timing to surgery and clinical outcome. However, if our study shows some trends, it is difficult to evaluate why patients undergo early or late SSRF. A DAG was used with hypotheses on factors that might be associated with the delay to surgery (most of them were associated lesions and clinical status). Non-captured variables (as body mass index, tobacco consumption, chronic obstructive pulmonary disease or asthma) could be associated with the timing to surgery and influence our results. Our study underlines the need for prospective trials on this topic. These studies should combine prospective data from surgical and ICU teams such as mechanical ventilation parameters, lung compliance or metabolic disorders. As healthcare is multidisciplinary, analysis should also be multidisciplinary to offer a significant global evaluation.
Conclusions
In our 10 years’ experience of SSRF, time to surgery was not associated to clinical outcome. This suggests that SSRF could be delayed pending the stabilization of other clinical issues. SSRF in polytraumatized patients is feasible but should not interfere with the treatment of urgent visceral lesions and could be delayed with satisfactory post-operative results. The modern approach to multiple rib fractures should be multidisciplinary including intensivists and thoracic surgeons and should associate adequate pain control, physiotherapy, optimized ventilation or oxygen supplementation (from nasal O2 and non-invasive ventilation to mechanical ventilation) and surgical rib fixation.
Acknowledgments
The authors warmly thanks Dr. Guillaume William Taylor for the English revision.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-735/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-735/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-735/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-735/coif). GD reports personnal fees from Astra Zeneca for presentations. JSD reports honoraria for lectures and expertise from LFB Laboratory, les Ullis, France outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bastos R, Calhoon JH, Baisden CE. Flail chest and pulmonary contusion. Semin Thorac Cardiovasc Surg 2008;20:39-45. [Crossref] [PubMed]
- Flagel BT, Luchette FA, Reed RL, et al. Half-a-dozen ribs: the breakpoint for mortality. Surgery 2005;138:717-23; discussion 723-5. [Crossref] [PubMed]
- Bankhead-Kendall B, Radpour S, Luftman K, et al. Rib Fractures and Mortality: Breaking the Causal Relationship. Am Surg 2019;85:1224-7.
- Battle CE, Hutchings H, Evans PA. Risk factors that predict mortality in patients with blunt chest wall trauma: a systematic review and meta-analysis. Injury 2012;43:8-17. [Crossref] [PubMed]
- Sirmali M, Türüt H, Topçu S, et al. A comprehensive analysis of traumatic rib fractures: morbidity, mortality and management. Eur J Cardiothorac Surg 2003;24:133-8. [Crossref] [PubMed]
- Nirula R, Mayberry JC. Rib fracture fixation: controversies and technical challenges. Am Surg 2010;76:793-802.
- Tanaka H, Yukioka T, Yamaguti Y, et al. Surgical stabilization of internal pneumatic stabilization? A prospective randomized study of management of severe flail chest patients. J Trauma 2002;52:727-32; discussion 732. [Crossref] [PubMed]
- Granetzny A, Abd El-Aal M, Emam E, et al. Surgical versus conservative treatment of flail chest. Evaluation of the pulmonary status. Interact Cardiovasc Thorac Surg 2005;4:583-7. [Crossref] [PubMed]
- Leinicke JA, Elmore L, Freeman BD, et al. Operative management of rib fractures in the setting of flail chest: a systematic review and meta-analysis. Ann Surg 2013;258:914-21. [Crossref] [PubMed]
- Schuurmans J, Goslings JC, Schepers T. Operative management versus non-operative management of rib fractures in flail chest injuries: a systematic review. Eur J Trauma Emerg Surg 2017;43:163-8. [Crossref] [PubMed]
- Slobogean GP, MacPherson CA, Sun T, et al. Surgical fixation vs nonoperative management of flail chest: a meta-analysis. J Am Coll Surg 2013;216:302-11.e1. [Crossref] [PubMed]
- Ludwig C, Koryllos A. Management of chest trauma. J Thorac Dis 2017;9:S172-7. [Crossref] [PubMed]
- Mayberry JC, Ham LB, Schipper PH, et al. Surveyed opinion of American trauma, orthopedic, and thoracic surgeons on rib and sternal fracture repair. J Trauma 2009;66:875-9. [Crossref] [PubMed]
- Pieracci FM, Agarwal S, Doben A, et al. Indications for surgical stabilization of rib fractures in patients without flail chest: surveyed opinions of members of the Chest Wall Injury Society. Int Orthop 2018;42:401-8. [Crossref] [PubMed]
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007;370:1453-7. [Crossref] [PubMed]
- Rating the severity of tissue damage. I. The abbreviated scale. JAMA 1971;215:277-80. [Crossref] [PubMed]
- Edwards JG, Clarke P, Pieracci FM, et al. Taxonomy of multiple rib fractures: Results of the chest wall injury society international consensus survey. J Trauma Acute Care Surg 2020;88:e40-5. [Crossref] [PubMed]
- Bouzat P, Raux M, David JS, et al. Chest trauma: First 48hours management. Anaesth Crit Care Pain Med 2017;36:135-45. [Crossref] [PubMed]
- American College of Surgeon Committee on Trauma. Advanced Trauma Life Support (ATLS®), 10th edition. Chicago, Illinois; 2018.
- Pieracci FM, Coleman J, Ali-Osman F, et al. A multicenter evaluation of the optimal timing of surgical stabilization of rib fractures. J Trauma Acute Care Surg 2018;84:1-10. [Crossref] [PubMed]
- Sawyer E, Wullschleger M, Muller N, et al. Surgical Rib Fixation of Multiple Rib Fractures and Flail Chest: A Systematic Review and Meta-analysis. J Surg Res 2022;276:221-34. [Crossref] [PubMed]
- Chen J, Jeremitsky E, Philp F, et al. A chest trauma scoring system to predict outcomes. Surgery 2014;156:988-93. [Crossref] [PubMed]
- Bina WF 3rd. US hospital ships: more public health, less high tech. JAMA 1993;270:2927-8.
- Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 2008;36:309-32. [Crossref] [PubMed]
- Textor J, van der Zander B, Gilthorpe MS, et al. Robust causal inference using directed acyclic graphs: the R package 'dagitty'. Int J Epidemiol 2016;45:1887-94. [Crossref] [PubMed]
- Fokin AA, Wycech J, Weisz R, et al. Outcome Analysis of Surgical Stabilization of Rib Fractures in Trauma Patients. J Orthop Trauma 2019;33:3-8. [Crossref] [PubMed]
- Jayle CP, Allain G, Ingrand P, et al. Flail chest in polytraumatized patients: surgical fixation using Stracos reduces ventilator time and hospital stay. Biomed Res Int 2015;2015:624723. [Crossref] [PubMed]
- Pieracci FM, Leasia K, Bauman Z, et al. A multicenter, prospective, controlled clinical trial of surgical stabilization of rib fractures in patients with severe, nonflail fracture patterns (Chest Wall Injury Society NONFLAIL). J Trauma Acute Care Surg 2020;88:249-57. [Crossref] [PubMed]
- Su YH, Yang SM, Huang CH, et al. Early versus late surgical stabilization of severe rib fractures in patients with respiratory failure: A retrospective study. PLoS One 2019;14:e0216170. [Crossref] [PubMed]
- Prins JTH, Wijffels MME, Pieracci FM. What is the optimal timing to perform surgical stabilization of rib fractures? J Thorac Dis 2021;13:S13-25. [Crossref] [PubMed]
- Lin HL, Tarng YW, Wu TH, et al. The advantages of adding rib fixations during VATS for retained hemothorax in serious blunt chest trauma - A prospective cohort study. Int J Surg 2019;65:13-8. [Crossref] [PubMed]
- Schots JP, Vissers YL, Hulsewé KW, et al. Addition of Video-Assisted Thoracoscopic Surgery to the Treatment of Flail Chest. Ann Thorac Surg 2017;103:940-4. [Crossref] [PubMed]
- van Gool MH, van Roozendaal LM, Vissers YLJ, et al. VATS-assisted surgical stabilization of rib fractures in flail chest: 1-year follow-up of 105 cases. Gen Thorac Cardiovasc Surg 2022;70:985-92. [Crossref] [PubMed]
- Crandall M, Popowich D, Shapiro M, et al. Posttraumatic hernias: historical overview and review of the literature. Am Surg 2007;73:845-50.
- Kishore GS, Gupta V, Doley RP, et al. Traumatic diaphragmatic hernia: tertiary centre experience. Hernia 2010;14:159-64. [Crossref] [PubMed]
- Nayak HK, Maurya G, Kapoor N, et al. Delayed presentation of congenital diaphragmatic hernia presenting with intrathoracic gastric volvulus: a case report and review. BMJ Case Rep 2012;2012:bcr2012007332. [Crossref] [PubMed]
- Maury JM, Roquet G, Marcotte G, et al. Surgical fixation of rib fractures in chest wall trauma. Intensive Care Med 2015;41:1483-4. [Crossref] [PubMed]
- Fraser SF, Tan C, Kuppusamy MK, et al. The role of a video-assisted thoracic approach for rib fixation. Eur J Trauma Emerg Surg 2017;43:185-90. [Crossref] [PubMed]
- Hasenboehler EA, Bernard AC, Bottiggi AJ, et al. Treatment of traumatic flail chest with muscular sparing open reduction and internal fixation: description of a surgical technique. J Trauma 2011;71:494-501. [Crossref] [PubMed]
- Xia H, Zhu D, Li J, et al. Current status and research progress of minimally invasive surgery for flail chest. Exp Ther Med 2020;19:421-7. [Crossref] [PubMed]
- Ryomoto M, Sekiya N, Uemura H, et al. Titanium plate to fix crossed rib can cause cardiac tamponade: A cautionary note. Gen Thorac Cardiovasc Surg 2021;69:353-5. [Crossref] [PubMed]
- Uebayashi A, Takanashi Y, Oka M, et al. Placement of KANI® plate inside the chest wall for rib fixation: Prevention for organ injuries caused by crossed rib edges and plate claws. Respirol Case Rep 2022;10:e0914. [Crossref] [PubMed]
- Marasco SF, Sutalo ID, Bui AV. Mode of failure of rib fixation with absorbable plates: a clinical and numerical modeling study. J Trauma 2010;68:1225-33. [Crossref] [PubMed]
- Ashley DW, Christie DB 3rd, Long EL, et al. Prospective randomized trial of metal versus resorbable plates in surgical stabilization of rib fractures. J Trauma Acute Care Surg 2022;93:147-56. [Crossref] [PubMed]
- Marasco S, Liew S, Edwards E, et al. Analysis of bone healing in flail chest injury: do we need to fix both fractures per rib? J Trauma Acute Care Surg 2014;77:452-8. [Crossref] [PubMed]
- Nickerson TP, Thiels CA, Kim BD, et al. Outcomes of Complete Versus Partial Surgical Stabilization of Flail Chest. World J Surg 2016;40:236-41. [Crossref] [PubMed]
- Sutyak JP, Wohltmann CD, Larson J. Pulmonary contusions and critical care management in thoracic trauma. Thorac Surg Clin 2007;17:11-23. v. [Crossref] [PubMed]
- Olland A, Puyraveau M, Guinard S, et al. Surgical stabilization for multiple rib fractures: whom the benefit? -a prospective observational study. J Thorac Dis 2019;11:S130-40. [Crossref] [PubMed]
- Prins JTH, Van Lieshout EMM, Overtoom HCG, et al. Long-term pulmonary function, thoracic pain, and quality of life in patients with one or more rib fractures. J Trauma Acute Care Surg 2021;91:923-31. [Crossref] [PubMed]
- Marasco SF, Balogh ZJ, Wullschleger ME, et al. Rib fixation in non-ventilator-dependent chest wall injuries: A prospective randomized trial. J Trauma Acute Care Surg 2022;92:1047-53. [Crossref] [PubMed]
- Beks RB, de Jong MB, Houwert RM, et al. Long-term follow-up after rib fixation for flail chest and multiple rib fractures. Eur J Trauma Emerg Surg 2019;45:645-54. [Crossref] [PubMed]


