
Nocturnal continuous positive airway pressure offers symptomatic benefit in excessive dynamic airway collapse despite normal sleep study
Introduction
Excessive dynamic airway collapse (EDAC) and tracheobronchomalacia (TBM) are less well-known causes of obstructive airway disease and debilitating respiratory symptoms such as severe chronic cough, sputum production, wheeze and haemoptysis. Although EDAC and TBM have been described in the literature as different pathophysiological entities, current evidence so far shows no practical benefit in making distinction between the two as the symptoms, diagnostics and treatment goals are the same. They are commonly misdiagnosed as asthma or chronic obstructive pulmonary disease (COPD) or can occur concurrently. Symptoms can be of such severity to cause significant sleep disturbance, sleep disordered breathing, episodes of vomiting and collapse during coughing fits and in severe cases, near total airway obstruction. We present and discuss the case of a 63-year-old female referred to our respiratory unit for a trial of nocturnal continuous positive airway pressure (CPAP) due to EDAC diagnosed on flexible bronchoscopy.
Case presentation
A 63-year-old female was electively admitted to a specialist respiratory unit for respiratory assessment and a trial of CPAP therapy. She was referred following a fibreoptic bronchoscopy under conscious sedation which demonstrated possible EDAC of the right main bronchus. For months prior she had presented to hospital frequently with suspected asthma exacerbations which were refractory to treatment, as is often the case in undiagnosed EDAC/TBM (1).
The patient’s past medical history included late onset asthma (diagnosed 1995), recurrent lower respiratory tract infections, chronic rhinosinusitis with functional endoscopic sinus surgery (FESS) and septoplasty, previous primary right breast cancer (2005, stage 3, grade 3, 8/12 positive lymphadenopathy) successfully treated with surgery, radiotherapy and chemotherapy, previous primary lung cancer (2005, staging T1N1) successfully treated with left upper lobectomy, right total knee replacement and cervical spinal fusion (2021). Regular medications included a formoterol and beclomethasone diproprionate inhaler, tiotropium inhaler, cetirizine, omeprazole, vitamin D and calcium supplementation, tramadol, salbutamol nebulisers and salbutamol 100 mcg/dose inhaler when needed. She reported no allergies, had never smoked, only occasionally consumed alcohol in small amounts and used to work as an orthopaedic theatre nurse. Her father had a diagnosis of polymyalgia rheumatica, otherwise there was no family history of note.
Her main symptom was a chronic ‘barking’ dry cough of four years duration which significantly impacted on her quality of life and activities of daily living. Maximum duration of uninterrupted sleep was 40 minutes due to waking to cough, resulting in significant sleep disturbance, fatigue, and impairment of cognitive functioning. The patient typically had to get out of bed and shift posture, leaving her to sleep upright in an armchair frequently. She denied early morning headaches. Her most severe episodes of coughing could lead to haemoptysis, vomiting or episodes of loss of consciousness. She reported dyspnoea at rest as well as on minimal exertion, and orthopnoea requiring five pillows if lying supine. Lying on her right side was intolerable due to dyspnoea. She also described intermittent dysphagia to solid food of one-year duration which had not progressed but had led her to instigate a soft and bite-sized diet herself. Her weight had remained stable. Her height was 157 cm and her BMI was 35.3 kg/m2. On physical examination she looked fatigued, had a hoarse voice, audible wheeze and dry cough. Auscultation of the chest demonstrated widespread wheeze of the right side of the chest with radiation to the left upper zone.
Routine bloods showed a mildly elevated c-reactive protein (CRP) (28 mg/L) and neutrophil count (8.1×109/L), normal white cell and eosinophil count, normal liver and renal function and normal electrolytes. Electrocardiogram (ECG) showed normal sinus rhythm. Chest X-ray demonstrated ongoing blunting of the left costophrenic angle with some overlying atelectasis (previous left upper lobectomy), stable cardiomediastinal contours and clear right lung and pleural spaces. Venous blood gas showed no evidence of chronic respiratory failure (pH: 7.42, PaCO2: 5.7 kPa, HCO3: 26 mmol/L) and daytime oxygen saturations (SpO2) were 92-98% on room air. The spirometry indicated highly variable results due to interruption by coughing. However, the best effort revealed severe airway obstruction [forced expiratory volume in one second (FEV1) 1.02 L (44.9% predicted value), forced vital capacity (FVC) 1.56 L (52.7% predicted), FEV1/FVC ratio 65% (84.5% predicted)].
Given the patients symptomatology, a baseline sleep study was performed to determine whether sleep disordered breathing was present and therefore whether nocturnal CPAP therapy was indicated. The case was also discussed with rheumatology given the presentation and history, of note previous nasal septoplasty, regarding appropriate investigations for an underlying systemic diagnosis such as relapsing polychondritis, in which respiratory tract involvement is seen in up to 56% of cases (2).
On the first night, the nocturnal study using transcutaneous capnometry and oximetry while self-ventilating showed frequent waking during coughing episodes but no evidence of significant sleep-disordered breathing. The 4% oxygen desaturation index (4%ODI) was 4 events/hr, mean SpO2 was 96%, mean transcutaneous carbon dioxide (TcCO2) was 5.5 kPa, and the total time with SpO2 <90% was 0% of the night (Figure 1). Effectively, an overnight study in normal range. The following morning, the patient was reviewed. Given her symptom severity, it was agreed that an auto-set CPAP device would be trialled overnight to assess for symptomatic benefit, despite absence of data indicating respiratory failure or sleep disordered breathing based on the recordings and National Institute for Health and Care Excellence (NICE) guideline recommendations (2).
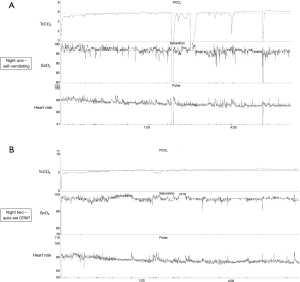
On the second night, auto-set CPAP (cycling at pressures of 4–20 cmH2O) was applied via a full face mask and it was tolerated well overnight. The 4%ODI was 1 event/hr, the mean SpO2 97%, the mean TcCO2 5.9 kPa, and the total analysis time with an SpO2 <90% was 0% (Figure 1). The following morning, the patient reported the “best night’s sleep” she had had in four years with reported “life changing” symptomatic improvement. She reported very good sleep quality with few interruptions (waking only once in 8 hours) and experienced less frequent coughing the following day, improved voice and having slept flat and supine all night in bed without the need to shift upright. The overnight auto-set CPAP had cycled in with pressures, most of the night, around 10–12 cmH2O. The patient was provided with an auto-set CPAP (range, 4–20 cmH2O) on discharge for use overnight.
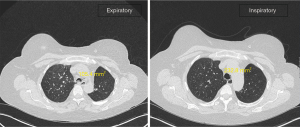
As an outpatient, a non-contrast CT thorax in expiratory phase was arranged to allow comparison with the inspiratory phase study and further characterise the extent of EDAC (Figure 2). This demonstrated significant (~55%) reduction in the luminal calibre of the distal trachea, proximal right main bronchus and bronchus intermedius on expiratory phase. There was mild generalised mosaicism, indicating associated air trapping, and some focal atelectasis within the inferior lingula. No pleural or lymph node abnormalities, and no aggressive osseous lesion were identified. A diagnostic criterion of 50% reduction of the cross-sectional area of the airways during expiration for EDAC/TBM is generally accepted in both bronchoscopy and CT scan studies (1,3).
Rheumatology followed her up within two weeks as an outpatient. Blood results showed negative anti-nuclear antibody (ANA), anti-neutrophil cytoplasmic antibody (ANCA), rheumatoid factor, and common connective tissue disease auto-antibodies. Complement components (C3, C4) and lupus anticoagulant were also normal. Erythrocyte sedimentation rate (ESR) was mildly elevated at 26 mm/hr. Due to her history of chronic joint stiffness, asthma, chronic sinusitis, haemoptysis, raised ESR and Immunoglobulin E (IgE), and evidence of nasal chondritis and costochondritis, the initial working diagnosis was relapsing polychondritis and she was referred for a positron emission tomography-computed tomography (PET-CT) scan. This showed no evidence of metabolically active polychondritis.
She was reviewed in her usual Asthma Clinic three months later where she reported that the nocturnal CPAP continued to make a significant difference to her quality of life by enabling her a good night sleep. She explained that she also found CPAP useful for when she had coughing fits by daytime and was interested in using an ambulatory CPAP machine during the day.
Discussion
TBM and EDAC have been described in the literature as two separate pathophysiological causes of central airway expiratory dynamic obstruction or otherwise collectively described as excessive central airway collapse (ECAC) (3-5). EDAC is characterised by inward bulging of muscular fibres in the posterior airway membrane during exhalation, as in this case, whilst TBM is characterised by weakness of the anterior tracheobronchial cartilage wall that may present with or without excessive dynamic invagination of the posterior membranous wall (3). As symptoms, diagnostics and treatment goals are the same, current evidence so far shows no practical benefit in making such distinction and EDAC/TBM will be used to refer to both in this discussion (6).
Under normal conditions, the trachea marginally dilates on inspiration and narrows on expiration due to the difference in intrathoracic and intraluminal pressures (1). Reduction and/or atrophy of longitudinal elastic fibers or impaired cartilage integrity of the airways predisposes to collapse, particularly under increased intrathoracic pressure during forced expiration, consequently causing airway obstruction (1). EDAC/TBM can be localized or occur throughout the airways and can be primary (congenital) or secondary (acquired) (1). The most common symptoms are dyspnoea, cough, sputum production, and hemoptysis (1).
Acquired causes include trauma (following intubation, tracheostomy, external chest trauma, lung transplantation), emphysema, chronic infection/bronchitis, chronic inflammation (such as relapsing polychondritis) and chronic external compression (malignant or benign tumors, cysts, abscesses, aortic aneurysms) (1).
In this case, the aetiology of EDAC/TBM appears multifactorial. The primarily right sided distribution of EDAC/TBM suggests that although chronic inflammation secondary to asthma requiring corticosteroid therapy (diagnosed 10 years prior to the malignancies) and recurrent infections may have contributed, previous lung surgery and radiotherapy (for right sided breast cancer) may be also significant. Furthermore, despite no diagnosis of rheumatological disease being made, the patients’ history of spinal surgery and a total knee replacement at a younger age than expected are suspicious for a connective tissue disorder.
It is unfortunately common for EDAC/TBM to be misdiagnosed and treated as asthma or COPD, as in this case, due to limited awareness of the condition (1). In one study of 4,283 patients with pulmonary disease, 542 (12.7%) had EDAC (>50% airway collapse) on bronchoscopy (4). Despite increasing awareness and research, true incidence remains unknown (3,5). Furthermore, there remains debate regarding the diagnostic criterion and classification of severity of EDAC/TBM. It should not be diagnosed on the >50% airway collapse criterion alone (6,7). One study revealed that 70–80% of healthy asymptomatic patients had >50% expiratory reduction in cross-sectional area on dynamic CT (6). Visualisation of dynamic tracheal or bronchial collapse by flexible bronchoscopy is the “gold standard” for diagnosing EDAC/TBM as the patient is able to follow commands to perform deep breathing, forced exhalation, and cough maneuvers to elicit the collapsibility of the airways (1,3-7). Since the 2000s, dynamic expiratory CT characterisation has been shown to be an alternative highly sensitive, non-invasive method for detecting EDAC/TBM, however further studies are required to compare specificity with flexible bronchoscopy (3). Despite the discrepancies with diagnostic criterion, with some studies defining >70% rather than >50% as the cut off, >50% remains generally accepted (7).
Only severe, refractory cases of EDAC/TBM (>90–100% collapse) are referred for surgical stenting (tracheobronchoplasty) whilst CPAP therapy has been identified as a ‘possible treatment’ for those requiring medical management (3,5). As described in this case, nocturnal CPAP therapy can result in life changing improvement in quality of life, and should be considered as a management option in severe symptomatology, regardless of the degree of EDAC/TBM on imaging.
Conclusions
EDAC/TBM should be considered in all cases of obstructive ventilatory defect refractory to medical management. Diagnosis is made with flexible bronchoscopy and/or inspiratory and expiratory phase CT scans. Rheumatological disorders, such as relapsing polychondritis, should be investigated for where EDAC/TBM has been diagnosed. Treatment should be individualised and “pneumatic stenting” with the use of CPAP may lead to symptomatic benefit, regardless of the degree of collapse (~55% in this case) or absence of sleep disordered breathing in overnight oximetry. Nocturnal CPAP therapy may improve quality of life and avoid further mechanical damage and associated complications due to chronic cough.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This brief report was commissioned by the editorial office, Journal of Thoracic Disease for the series “Clinical Update Sleep 2023”. The brief report has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1804/coif). The series “Clinical Update Sleep 2023” was commissioned by the editorial office without any funding or sponsorship. JS served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Journal of Thoracic Disease. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Carden KA, Boiselle PM, Waltz DA, et al. Tracheomalacia and tracheobronchomalacia in children and adults: an in-depth review. Chest 2005;127:984-1005. [Crossref] [PubMed]
- Obstructive sleep apnoea/hypopnoea syndrome and obesity hypoventilation syndrome in over 16s. NICE Guideline, No. 202. London: National Institute for Health and Care Excellence (NICE), 2021.
- Buitrago DH, Wilson JL, Parikh M, et al. Current concepts in severe adult tracheobronchomalacia: evaluation and treatment. J Thorac Dis 2017;9:E57-66. [Crossref] [PubMed]
- Ikeda S, Hanawa T, Konishi T, et al. Diagnosis, incidence, clinicopathology and surgical treatment of acquired tracheobronchomalacia. Nihon Kyobu Shikkan Gakkai Zasshi 1992;30:1028-35.
- López-Padilla D, García-Luján R, Puente Maestu L, et al. Tracheobronchomalacia treatment: how far have we come? J Thorac Dis 2016;8:3490-3. [Crossref] [PubMed]
- Kheir F, Majid A. Tracheobronchomalacia and excessive dynamic airway collapse: medical and surgical treatment. Semin Respir Crit Care Med 2018;39:667-73. [Crossref] [PubMed]
- McGinn J, Herbert B, Maloney A, et al. Quality of life outcomes in tracheobronchomalacia surgery. J Thorac Dis 2020;12:6925-30. [Crossref] [PubMed]


