
Real-world experience of arbidol for Omicron variant of SARS-CoV-2
Highlight box
Key findings
• Arbidol significantly increased the negative conversion rate of Omicron variant of SARS-CoV-2 within the first week and accelerate the recovery time.
What is known and what is new?
• The new Omicron variant presents high transmissibility and infection rate, which brings a challenging situation worldwide. However, few efficacious medications are available currently.
• Arbidol shows an effective and safe treatment in asymptomatic-mild patients of Omicron variant during the pandemic of COVID-19.
What is the implication, and what should change now?
• Arbidol may be one of the sensible therapeutic regimens for outpatients suffering Omicrons. Randomized, multicenter, global clinical trials with larger sample size are still expected in the near future.
Introduction
It has been two years since the start of the coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), which is rapidly and continuously evolving and mutating, giving rise to various variants with variable degrees of infectivity and lethality. The most recent novel SARS-CoV-2 variant was first reported from a specimen collected on November 9th, 2021, named Omicron (B1.1.529) by World Health Organization (WHO) on November 26th, 2021 (1,2). In late February 2022, a wave of SARS-CoV-2 infection rapidly appeared in Shanghai, China. It is demonstrated that all of the new viral genomes in this pandemic were clustered into the SARS-CoV-2 BA.2.2 sub-lineage, while BA.2 is a sub-lineage of the Omicron (B1.1.529) (3). As of June 19th, 2022, about 65 thousand cases have been identified in Shanghai and 595 people have died with or from the Omicron variant (4).
The new Omicron variant of SARS-CoV-2 has created a highly challenging situation worldwide. This new variant underwent significant mutations when compared to its previous variants. It had a shorter incubation period and usually resulted in mild symptoms (5). However, it presented higher transmissibility and infection rate, as well as immune evasion against acquired immunity with breakthrough infections in vaccinated individuals (6). Thus, Omicron spread rapidly in a short period of time. So far, nirmatrelvir-ritonavir (Paxlovid) is the only recommended oral-antiviral drug in the updated guideline issued by the National Health Commission of the People’s Republic of China. Other intravenous therapies granted are not available for a great number of outpatients during the COVID-19 pandemic. Currently, Paxlovid is only used in mild to moderate COVID-19 patients who are at risk for progression (7). As a whole, it is urgent and critical for asymptomatic and mild outpatients to have access to other evidence-based Omicron treatments.
Arbidol, a small indole-derivative molecule, has been licensed in China for prophylaxis and treatment of influenza and other respiratory viral infections (8,9). So far, the antivirus effect of arbidol against SARS-CoV-2 has yet to be controversial. On the one hand, it has been found that arbidol has a good inhibitory effect against SARS-CoV-2 in vitro (10). Some clinical studies also suggested its beneficial effect either in monotherapy or combination therapy with other agents against COVID-19 (11-13). Our previous study on the original SARS-CoV-2 stain also demonstrated that arbidol could increase the negative conversion rate and accelerate the recovery time. On the other hand, there exist other studies which have found no benefit in using arbidol in COVID-19 patients (14). Arbidol, a broad-spectrum antiviral drug, is safe, convenient, and easily available as a medication for outpatients. However, its antiviral efficacy in the treatment of the new Omicron variant remains unknown.
This study describes a single-center, controlled, prospective, real-world study of the efficacy of arbidol in asymptomatic and mild Omicron infections, expecting our results could shed some light on the treatment of this new variant in this pandemic. We present the following article in accordance with the TREND reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-980/rc).
Methods
Ethics
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of Ruijin Hospital, Shanghai Jiaotong University School of Medicine (Shanghai, China) (No. 2020-28) and informed consent was taken from all the patients. The trial was registered on ClinicalTrial.gov with Trial Identifier NCT04260594.
Patients
Inclusion criteria: (I) aged 18 to 65 years old (including 18 and 65 years); (II) male and non-pregnant female; (III) respiratory tract specimens or hematology samples with positive results of SARS-CoV-2 detected by real-time transcriptase-polymerase chain reaction (RT-PCR); (IV) asymptomatic or mild clinical status, defined as having no or mild clinical symptoms, with no signs of pneumonia on imaging. Exclusion criteria: (I) the physician decision that involvement in the trial was not in the patient’s best interest; (II) known allergic reaction and/or severely allergic to arbidol; (III) hematologic dysfunction (platelet count <100×109/L, or hemoglobin level <90 g/L); (IV) severe hepatic dysfunction (total bilirubin level >2 times the normal upper limit, aspartic aminotransferase or alanine aminotransferase levels >3 times normal upper limit); (V) severe renal dysfunction (serum creatinine >1.5 times the upper limit of normal value, or calculated creatinine clearance rate <50 mL/min); (VI) treated with arbidol tablets before admission; (VII) history of severe heart disease or clinically significant arrhythmia considered unsafe for the trial.
Trial design and oversight
This was an investigator-initiated, prospective, open-label, controlled, and single-center trial conducted from Mar 26 to April 26, 2022. Patients meeting eligibility criteria were assigned in a 1:2 ratio to receive either standard-of-care (SOC) or SOC plus arbidol tablets (oral administration of 200 mg per time, three times a day for 5 days). SOC included traditional medicine, antibiotics, and other medications for patients’ comorbidities. All enrolled patients were isolated or treated in the inpatient unit of Ruijin Hospital. Abidor tablets were prescribed after the responsible physician was informed of the enrolment protocol and were dispensed by the pharmacy within 1 day and administered by the nurses.
The trial was conducted in accordance with the principles of the International Coordinating Conference on quality management of drug clinical trials. Clinical data were recorded by clinical research coordinators, followed by queries from clinical research associates.
Clinical and laboratory monitoring
Nasopharyngeal swab samples were obtained from patients the day before enrolment and every two days after enrolment until the patients were discharged. Positive or negative results for SARS-CoV-2 and cycle threshold (CT) values for open reading frame 1ab (ORF1ab) and nucleocapsid protein (N) in specific genomes were tested by RT-PCR. Laboratory tests for patients’ liver enzymes, blood cell counts, and immune-related indicators (percentage and absolute count of CD3+, CD4+, and CD8+ T cells) were performed on the day of pre-treatment and the day before discharge visits. Administration records of arbidol tablets and adverse events (AEs) were monitored daily by the responsible physician. In addition, patients’ demographic data, previous health status, pre-admission epidemiological characteristics, and treatment received after admission were thoroughly recorded.
Outcome measures
The primary endpoint was the negative conversion ratio of SARS-CoV-2 within the first week, defined as the percentage of negative viral changes detected in pathogen nucleic acid on day 7 after the first administration. Secondary endpoints included viral clearance ratio in the second week, overall negative conversion ratio, negative conversion time, and the duration of hospitalization. The patients were discharged after two consecutive negative nucleic acid tests (with an interval of >24 hours), and the patients’ admission and discharge times were recorded as the duration of hospitalization. Laboratory parameters such as the changes in lymphocyte count and the improvement in lymphocyte subsets (absolute CD3+, CD4+, CD8+ cells count) in peripheral blood were also included in the outcome analysis. Safety endpoints included AEs during treatment, severe AEs, and early discontinuation of therapy. The AEs were classified according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.
Statistical analysis
The trial was initially designed to enroll a total of 384 subjects, which would provide 80% power under a one-sided type I error of 2.5%. The sample size was based on the alternative hypothesis of a 15% increase in the virus nucleic acid negative rate. The allocation ratio between arbidol tablets and the control group was 2:1, and a 10% dropout rate has been considered in the original design.
The primary efficacy analysis was performed on a per-protocol (PP) basis for all patients who completed the trial. Subjects’ allocation, demographic data, and baseline characteristics were described in the statistical description part. Statistical analysis was conducted using SAS software, version 9.4 (SAS Institute Inc.). For quantitative variables, mean ± SD or median (IQR) were used for description, and a t-test or non-parametric test was used for hypothesis testing. Qualitative variables such as the number and proportions of cases were analyzed by Chi-square, adjusted Chi-square, or Fisher’s analysis for hypothesis testing. Efficacy analysis was based on a subset of the Full Analysis Set, including subjects with sufficient adherence to complete the trial protocol and with primary efficacy indicators. Binary outcomes were tested with the Chi-square test and Fisher’s analysis. Rates and 95% CI for those binary indicators were also reported. Virus clearance time was evaluated with survival analysis. Kaplan-Meier curves were plotted, and the Log-rank test was used between groups comparison. Cox regressions were used for hazard ratio (HR) and 95% CI estimation, with or without baseline variables adjusted. The safety analysis set was used for the overall analysis to summarize the AEs and serious AEs that occurred during the treatment of all patients. The number of cases and events of adverse reactions and serious adverse reactions was calculated.
Results
Patients
Between March 26, 2022, to April 26, 2022, 387 patients were screened, of whom 378 patients were eligible (Figure 1). In accordance with the 2:1 allocation ratio between arbidol and the control group, 253 patients were assigned to receive SOC plus arbidol and 125 patients to receive SOC. Due to centralized patient management of the medical appointment hospital, 7 patients in the arbidol group and 4 patients in the control group were transferred without completing the study. Finally, 246 patients in the arbidol group and 121 patients in the control group were included in the PP population for further analyses.
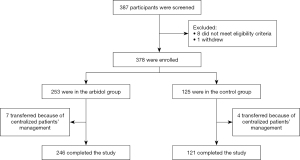
The median age of patients was 46.0 (IQR, 35.0–53.0); sex distribution was 128 (52.0%) men versus 118 (48.0%) women in the arbidol group and 38 (31.4%) versus 83 (68.6%) in the control group (Table 1). The most common comorbidity was hypertension, followed by diabetes and coronary heart disease, accounting for 10.6%, 4.9%, and 1.4%, respectively. Some imbalanced characteristics existed at enrollment between the groups, including more male and mild patients in the arbidol group (P<0.001). Patients with baseline C-reactive protein (CRP) values above 10 accounted for a higher proportion in the arbidol group compared to the control group (P<0.022). No other major differences in age, comorbidities and combined treatment with traditional medicine were observed between groups. Laboratory parameters such as the CT values of ORF1ab and N, lymphocyte counts and immune cell counts, including CD3+, CD4+, and CD8+ cells, did not differ significantly between groups at baseline.
Table 1
| Variables | Total (N=367) | Arbidol group (N=246) | Control group (N=121) | χ2 or t | P value |
|---|---|---|---|---|---|
| Age, year | 46.0 (35.0–53.0) | 44.5 (35.0–52.3) | 47.0 (35.5–54.0) | 1.398 | 0.163 |
| Sex | |||||
| Male | 166 (45.2%) | 128 (52.0%) | 38 (31.4%) | 13.930 | <0.001 |
| Female | 201 (54.8%) | 118 (48.0%) | 83 (68.6%) | ||
| Comorbidities | |||||
| Yes | 62 (18.8%) | 42 (20.2%) | 20 (16.5%) | 0.671 | 0.413 |
| No | 267 (81.2%) | 166 (79.8%) | 101 (83.5%) | ||
| Disease severity | |||||
| Asymptomatic | 124 (33.8%) | 68 (27.6%) | 56 (46.3%) | 12.595 | <0.001 |
| Mild | 243 (66.2%) | 178 (72.4%) | 65 (53.7%) | ||
| Combined treatment with traditional medicine | |||||
| Yes | 261 (88.8%) | 185 (88.9%) | 76 (88.4%) | 0.020 | 0.888 |
| No | 33 (11.2%) | 23 (11.1%) | 10 (11.6%) | ||
| Laboratory parameters | |||||
| CRP ≥10 | 29/290 (10.0%) | 26/207 (12.6%) | 3/83 (3.6%) | 5.268 | 0.022 |
| Lymphocyte count ×109 | 1.535±0.50 | 1.550±0.50 | 1.503±0.45 | 0.844 | 0.399 |
| CD3 count | 1,013.2±391.0 | 1,000.9±357.6 | 1,037.6±451.2 | 0.691 | 0.490 |
| CD4 count | 567.6±216.8 | 559.4±210.0 | 583.8±230.1 | 0.831 | 0.407 |
| CD8 count | 397.0±203.9 | 396.4±183.8 | 398.3±239.9 | 0.071 | 0.943 |
| Pre-treatment virological characteristics | |||||
| CT values of ORF1ab# | 23.5±5.3 | 23.1±5.2 | 24.3±5.6 | 1.636 | 0.196 |
| Ct values of N& | 22.2±5.5 | 21.6±5.3 | 23.6±5.6 | 2.807 | 0.402 |
Data are median (IQR), n (%) or mean ± SD. #, cycle threshold values for open reading frame 1ab; &, cycle threshold values for nucleocapsid. CRP, C-reactive protein.
Primary outcomes
The negative conversion rate of SARS-CoV-2 within the first week in the group of arbidol was 47.2% (116/246), which was significantly higher than that of the control group [35.5%, 43/121; odds ratio (OR): 1.619, 95% confidence interval (CI): 1.034–2.535; P=0.035; Table 2].
Table 2
| Variables | Total | Arbidol group | Control group | Differences | P value |
|---|---|---|---|---|---|
| Negative conversion rate | |||||
| First week | 159/367 (43.3%) | 116/246 (47.2%) | 43/121 (35.5%) | 1.619 (1.034–2.535)* | 0.035 |
| Second week | 181/208 (87.0%) | 118/130 (90.8%) | 63/78 (80.8%) | 2.341 (1.033–5.307)* | 0.038 |
| Overall | 340/367 (92.6%) | 234/246 (95.1%) | 106/121 (87.6%) | 2.759 (1.249–6.098)* | 0.009 |
| Negative conversion time, median day (IQR) | 8.9 (6.0–11.0) | 8.3 (5.0–11.0) | 10.0 (7.0–14.0) | 0.645 (0.516–0.808)# | <0.001 |
| Duration of hospitalization, median day (IQR) | 12.1 (10.0–16.0) | 11.4 (10.0–15.0) | 13.7 (10.0–18.0) | 1.214 (0.966–1.526)# | <0.001 |
| Post-treatment virological characteristics | |||||
| Ct values of ORF1ab& | 31.6±6.7 | 31.8±6.9 | 31.4±6.5 | – | 0.190 |
| Ct values of N$ | 30.9±6.9 | 31.1±7.0 | 30.6±6.7 | – | 0.491 |
Data are median (IQR), n (%) or mean ± SD. *, differences are expressed as odds ratio and 95% CI; #, differences are expressed as hazard ratio and 95% CI; &, cycle threshold values for open reading frame 1ab; $, cycle threshold values for nucleocapsid.
Secondary outcomes
The negative conversion rate within the second week was 90.8% (118/130) in the arbidol group, which was significantly higher than that in the control group (80.8%, 63/78; OR: 2.341, 95% CI: 1.033–5.307; P=0.038). Generally, Arbidol accelerated the clearance of SARS-CoV-2, with the overall negative conversion rate being 95.1% (234/246) in the arbidol group and 87.6% (106/121) in the control group (OR: 2.759, 95% CI: 1.249–6.098; P=0.009). The median negative conversion time was shorter in patients receiving arbidol than those in the control group (median 8.3 vs. 10.0 days; HR: 0.645, 95% CI: 0.516–0.808; P<0.001; Figure 2). In addition, the arbidol treatment shortened the median duration of hospitalization [median 11.4 days (Arbidol group) vs. 13.7 days (control group); HR: 1.214, 95% CI: 0.966–1.526; P<0.001].
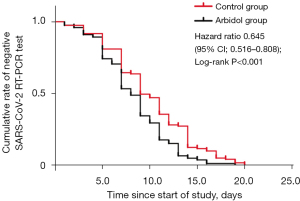
For post-treatment virological characteristics, CT values of ORF1ab and N were higher in the arbidol than in the control group, but there was no statistical difference [ORF1ab: P=0.190; average 31.8 (SD ±6.9) vs. 31.4 (SD ±6.5); N: P=0.491; average 31.1 (SD ±7.0) vs. 30.6 (SD±6.7)]. No cases in the arbidol or control group occurred with disease progression in the follow-up. No patients reported serious adverse reactions, and no one withdrew from the study due to untoward reactions. The most common AE was transaminase elevation in patients treated with arbidol tablets (3/246, 1.2%).
The evaluation of laboratory parameters
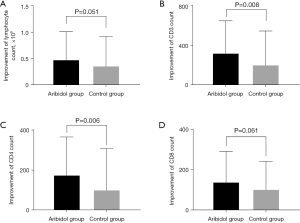
Considering that there were no differences in total lymphocyte counts and lymphocyte subsets counts, including CD3+, CD4+, and CD8+, between the arbidol group and the control group at baseline, differential changes in the above laboratory parameters were analyzed at the discharge compared to baseline. At the time of discharge, the up-regulation of lymphocyte counts in the arbidol group was numerically better than that in the control group [P=0.051, average 0.48 (SD ±0.53) vs. 0.36 (SD ±0.56) in arbidol group and the control group, Figure 3A]. Meanwhile, significances were observed in the improvement of CD3+ and CD4+ count between the abidol group and the control group [P=0.008, average 326.40 (SD ±326.34) vs. 203.16 (SD ±346.62) in differential changes of CD3+, Figure 3B; P=0.006, average 175.07 (SD ±189.70) vs. 100.33 (SD ±206.13) in differential changes of CD4+, Figure 3C]. The change of CD8+ count in the arbidol group was also better than that in the control group, but no statistical significance was reached [P=0.061, average 139.24 (SD ±151.27) vs. 100.68 (SD ±140.14) in arbidol group and the control group, Figure 3D]. As a whole, our results above indicated that arbidol was responsible for immunoregulation in virus infections.
Discussion
In the present study, we found the addition of arbidol tablets treatment was associated with a higher negative conversion rate and a shorter duration of negative conversion time as well as hospital discharge for patients infected by the Omicron variant of SARS-CoV-2. Besides, no serious side effects were found in arbidol tablet treatment.
Arbidol has been shown to display antiviral activity against a number of enveloped or non-enveloped RNA or DNA viruses, including influenza viruses A, B, and C, respiratory syncytial virus, SARS-CoV, adenovirus, parainfluenza type 5, poliovirus 1, rhinovirus 14, coxsackievirus B5, hantavirus, Chikungunya virus, hepatitis B virus (HBV) and hepatitis C virus (HCV) (15,16). Arbidol interferes with multiple stages of the virus life cycle by directly targeting viral proteins or virus-associated host factors. It can bind to hemagglutinin (HA), which enables the SARS-CoV-2 virus to attach to and enter the cells, and therefore reduce the virus’s infectivity and prevent the virus from entering the cells (8). A previously published study also indicated that arbidol may modulate the receptor-binding domain/angiotensin-converting enzyme 2 (RBD/ACE2) interaction in SARS-CoV-2 infection (17).
Currently, many clinical trials have been conducted with arbidol as a single agent or combination for COVID-19 treatment, but most of them were retrospective studies. Arbidol has been shown to be superior to the antiviral favipiravir, which did not improve the clinical recovery rate at day 7 compared to that in the arbidol group (13). Arbidol was also demonstrated superior to lopinavir/ritonavir in terms of treating COVID-19 by contributing to clinical and laboratory improvements (18). To our knowledge, this is the first prospective study evaluating the efficacy of arbidol in treating the Omicron variant of SARS-CoV-2. However, randomized, multicenter, global clinical trials with larger sample sizes are expected.
Lymphopenia is reported in many COVID-19 patients. The count of lymphocytes usually turned out to be an important indicator of the prognosis and clinical outcome (19). A previous study found that arbidol monotherapy led to a higher lymphocyte count than lopinavir/ritonavir in treating COVID-19 (20). Our study consistently found that the recovery of lymphocyte count in arbidol tablets group was significantly better than that in the control group, indicating this drug could promote the upregulation of lymphocytes. Moreover, the improvement of CD3+, CD4+, and CD8+ counts appears to be much greater in arbidol-treated patients than those in the control group. Both CD4+ and CD8+ T cells are responsible for the immunopathology and viral clearance of infection (21). Previous research confirmed that arbidol could reduce viral-induced inflammation by modulating the expression of pro-inflammatory cytokines in influenza-infected mice, indicating its immunomodulatory activity in anti-viral treatment (22).
A part of the patients enrolled in our study also received traditional Chinese medicine. Several Chinese herbal prescriptions were recommended and authorized by the Chinese government during Severe Acute Respiratory Syndromes (SARS), 2009 H1N1, and 2013 H7N9 pandemics (23-25). The purpose of traditional Chinese medicine treatment is to relieve symptoms and enhance physical fitness. Some herbs also exhibit beneficial immunomodulatory effects for the recovery of viral infection (26). Since there was no bias in the percentage of patients using traditional Chinese medicine between the arbidol group and the control group, we hypothesized the use of traditional Chinese medicine had no influence on our results.
Our study had some limitations. Given the large number of patients infected with the Omicron variant and the scarcity of healthcare resources at the time, the trial was not designed as a randomized controlled protocol with strict bias control. The use of simple randomization led to unevenness in the baseline characteristics of the two groups (e.g., patients in the arbidol group with higher CRP, symptomatic rather than asymptomatic). A previous study showed that asymptomatic patients had a longer duration of viral shedding, which might have contributed to the longer time to negative conversion in the control group (27). In this regard, we performed further subgroup analyses of the arbidol and control groups to analyze the difference in negative nucleic acid conversion in asymptomatic and mild patients (Table S1). For asymptomatic patients, the negative conversion rate within the first week was higher (41.2% vs. 19.6%; P=0.010) and the median negative conversion time was shorter (median 9.0 vs. 11.0 days; P<0.001) in patients receiving arbidol than those in the control group. For symptomatic patients, the treatment group tended to have a relatively higher negative conversion rate and shorter negative conversion time, but the imbalance in numbers between the two groups may be an explainable reason for the lack of statistical difference. In conclusion, arbidol tablets significantly increased the negative conversion rate of the Omicron variant of SARS-CoV-2 within the first week and accelerated the recovery of sufferers with COVID-19. Meanwhile, owing to its immunomodulatory activity, arbidol contributes to laboratory improvements, including lymphocytes as well as CD3+, CD4+, and CD8+ counts.
Conclusions
As a whole, arbidol may represent an effective and safe treatment in asymptomatic-mild patients suffering from Omicron variant during the pandemic of COVID-19. In addition, shorter negative conversion time in asymptomatic or mild patients treated with arbidol may also indirectly reduce the social transmission of Omicron carried by infected individuals, which is more valuable for countries and regions that have not adopted quarantine policies.
Acknowledgments
We thank the trial steering committee for their contributions in conducting the trial under very challenging field conditions (the chief, Yiping Xu); the chief of the clinical research center, Ruijin Hospital, Shanghai Jiaotong University School of Medicine, Jian Li, for his support of statistical analysis; other members of the clinical research center: Yun Qiu, Jianan Chen. We thank all the doctors and nurses in designated wards for their support of the trial and the patients for their bravery and altruism in participating in this trial. We thank the grant of Innovative research team of high-level local universities in Shanghai.
Funding: This work was supported by Shanghai Key Laboratory of Emergency Prevention, Diagnosis and Treatment of Respiratory Infectious Disease (No. 20dz2261100), Cultivation Project of Shanghai Major Infectious Disease Research Base (No. 20dz2210500), Shanghai Municipal Key Clinical Specialty (No. shslczdzk02202), and Shanghai Top-Priority Clinical Key Disciplines Construction Project (No. 2017ZZ02014).
Footnote
Reporting Checklist: The authors have completed the TREND reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-980/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-980/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-980/coif). All authors report funding from Shanghai Key Laboratory of Emergency Prevention, Diagnosis and Treatment of Respiratory Infectious Disease (No. 20dz2261100), Cultivation Project of Shanghai Major Infectious Disease Research Base (No. 20dz2210500), Shanghai Municipal Key Clinical Specialty (No. shslczdzk02202), and Shanghai Top-Priority Clinical Key Disciplines Construction Project (No. 2017ZZ02014). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Ruijin Hospital, Shanghai Jiaotong University School of Medicine (Shanghai, China) (No. 2020-28-3) and informed consent was taken from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Classification of Omicron (B.1.1.529): SARS-CoV-2 Variant of Concern. 2021. Available online: https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern
- Enhancing Readiness for Omicron (B.1.1.529): Technical Brief and Priority Actions for Member States. 2021. Available online: https://www.who.int/docs/default-source/coronaviruse/technical-brief-and-priority-action-on-omicron.pdf?sfvrsn=50732953_3
- Zhang X, Zhang W, Chen S. Shanghai's life-saving efforts against the current omicron wave of the COVID-19 pandemic. Lancet 2022;399:2011-2. [Crossref] [PubMed]
- Cumulative confirmed cases and deaths of COVID-19 in Shanghai on June 19, 2022. Available online: http://wsjkw.sh.gov.cn/xwfb/20220620/c65a10a571f24a9b90eebdd51a4bc17f.html
- Jansen L, Tegomoh B, Lange K, et al. Investigation of a SARS-CoV-2 B.1.1.529 (Omicron) Variant Cluster - Nebraska, November-December 2021. MMWR Morb Mortal Wkly Rep 2021;70:1782-4. [Crossref] [PubMed]
- Rahmani S, Rezaei N. Omicron (B.1.1.529) variant: Development, dissemination, and dominance. J Med Virol 2022;94:1787-8. [Crossref] [PubMed]
- Saravolatz LD, Depcinski S, Sharma M. Molnupiravir and Nirmatrelvir-Ritonavir: Oral Coronavirus Disease 2019 Antiviral Drugs. Clin Infect Dis 2023;76:165-71. [Crossref] [PubMed]
- Blaising J, Polyak SJ, Pécheur EI. Arbidol as a broad-spectrum antiviral: an update. Antiviral Res 2014;107:84-94. [Crossref] [PubMed]
- Sanders JM, Monogue ML, Jodlowski TZ, et al. Pharmacologic Treatments for Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020;323:1824-36. [Crossref] [PubMed]
- Wang X, Cao R, Zhang H, et al. The anti-influenza virus drug, arbidol is an efficient inhibitor of SARS-CoV-2 in vitro. Cell Discov 2020;6:28. [Crossref] [PubMed]
- Deng L, Li C, Zeng Q, et al. Arbidol combined with LPV/r versus LPV/r alone against Corona Virus Disease 2019: A retrospective cohort study. J Infect 2020;81:e1-5. [Crossref] [PubMed]
-
Liu Q Fang X Tian L The effect of Arbidol Hydrochloride on reducing mortality of Covid-19 patients: a retrospective study of real world date from three hospitals in Wuhan. 2020 . doi: - Chen C, Zhang Y, Huang J, et al. Favipiravir Versus Arbidol for Clinical Recovery Rate in Moderate and Severe Adult COVID-19 Patients: A Prospective, Multicenter, Open-Label, Randomized Controlled Clinical Trial. Front Pharmacol 2021;12:683296. [Crossref] [PubMed]
- Lian N, Xie H, Lin S, et al. Umifenovir treatment is not associated with improved outcomes in patients with coronavirus disease 2019: a retrospective study. Clin Microbiol Infect 2020;26:917-21. [Crossref] [PubMed]
- Boriskin YS, Leneva IA, Pécheur EI, et al. Arbidol: a broad-spectrum antiviral compound that blocks viral fusion. Curr Med Chem 2008;15:997-1005. [Crossref] [PubMed]
- Liu Q, Liu DY, Yang ZQ. Characteristics of human infection with avian influenza viruses and development of new antiviral agents. Acta Pharmacol Sin 2013;34:1257-69. [Crossref] [PubMed]
- Padhi AK, Seal A, Khan JM, et al. Unraveling the mechanism of arbidol binding and inhibition of SARS-CoV-2: Insights from atomistic simulations. Eur J Pharmacol 2021;894:173836. [Crossref] [PubMed]
- Zhu Z, Lu Z, Xu T, et al. Arbidol monotherapy is superior to lopinavir/ritonavir in treating COVID-19. J Infect 2020;81:e21-3. [Crossref] [PubMed]
- Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020;323:1061-9. [Crossref] [PubMed]
- Nojomi M, Yassin Z, Keyvani H, et al. Effect of Arbidol (Umifenovir) on COVID-19: a randomized controlled trial. BMC Infect Dis 2020;20:954. [Crossref] [PubMed]
- Liu Q, Zhou YH, Yang ZQ. The cytokine storm of severe influenza and development of immunomodulatory therapy. Cell Mol Immunol 2016;13:3-10. [Crossref] [PubMed]
- Liu Q, Xiong HR, Lu L, et al. Antiviral and anti-inflammatory activity of arbidol hydrochloride in influenza A (H1N1) virus infection. Acta Pharmacol Sin 2013;34:1075-83. [Crossref] [PubMed]
- Liu J, Manheimer E, Shi Y, et al. Chinese herbal medicine for severe acute respiratory syndrome: a systematic review and meta-analysis. J Altern Complement Med 2004;10:1041-51. [Crossref] [PubMed]
- Diagnostic and treatment protocol for human infections with avian influenza A (H7N9). Available online: https://www.chinacdc.cn/en/research_5311/Guidelines/201304/W020130425363145259626.pdf
- Zhong NS, Li YM, Yang ZF, et al. Chinese guidelines for diagnosis and treatment of influenza (2011). J Thorac Dis 2011;3:274-89. [Crossref] [PubMed]
- Poon PM, Wong CK, Fung KP, et al. Immunomodulatory effects of a traditional Chinese medicine with potential antiviral activity: a self-control study. Am J Chin Med 2006;34:13-21. [Crossref] [PubMed]
- Long QX, Tang XJ, Shi QL, et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med 2020;26:1200-4. [Crossref] [PubMed]


