
Impaired exercise capacity in individuals with non-obstructive small airway dysfunction
Highlight box
Key findings
• Although individuals with non-obstructive small airway dysfunction (SAD) have relatively preserved spirometry (post-bronchodilator FEV1/FVC ≥0.70), they have impaired exercise capacity regardless of smoking status that may be associated with ventilatory inefficiency.
What is known and what is new?
• Non-obstructive SAD has worse overall health and consequently a greater decline in pulmonary function than controls.
• This study clarifies the degree of impaired exercise capacity and its potential cause in individuals with non-obstructive SAD.
What is the implication, and what should change now?
• These findings have implications for better understanding of the physiological and clinical abnormalities in individuals with non-obstructive SAD.
Introduction
A forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) ratio of less than 0.70 after use of a bronchodilator is currently required to diagnose chronic obstructive pulmonary disease (COPD). However, a substantial proportion of individuals who do not meet this definition have small airway dysfunction (SAD). In China, the prevalence of spirometry-defined SAD without COPD in adults aged 20 years or older has been reported to be 11.3% [95% confidence interval (CI): 10.3–12.5%] (1). When compared with controls, this population has greater decline in pulmonary function (2,3). Therefore, non-obstructive SAD is considered to be a precursor of COPD and warrants attention (4-6).
Abnormalities of the small airways include premature airway closure and air trapping (7). Small airway impairment is associated with delayed lung emptying that can be amplified by an increased ventilatory requirement during exercise, and is one of the main reasons for exercise intolerance in symptomatic patients with mild COPD (8). However, it remains unclear whether individuals with SAD who do not have airway obstruction have impaired exercise capacity.
Furthermore, unlike in smokers, there have been few studies of the relationship between abnormalities of the small airways and cardiorespiratory fitness in never-smokers (9-11), and whether non-smokers have similarly abnormal physiologic responses to cardiopulmonary exercise testing (CPET) is still unclear. This issue is particularly pressing in developing countries, where other risk factors for developing SAD, namely outdoor air pollution and exposure to tobacco smoke, are increasing in prevalence (1).
There are many approaches available for evaluation of small airway function, including spirometry, forced oscillation, nitrogen washout, peripheral wedged catheters, and high-resolution computed tomography (12). Spirometry is the most widely used method in clinical practice, and we have used post-bronchodilator spirometry to assess SAD because of the need to obtain data comparable with those reported previously, especially in the Chinese population (1,13).
Accordingly, the main purpose of this study was to better understand exercise capacity in spirometry-defined SAD without airway obstruction, including in never-smokers and ever-smokers. Exercise tolerance was compared among participants with SAD but no COPD, a group of controls with normal spirometry, and a group of patients with Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage I. We presented the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1328/rc).
Methods
Study design and participants
In this study, we used data collected from participants enrolled consecutively in part of the National Science and Technology Support Plan Program for the 12th and 13th Five-Year Plans, which were community-based, observational surveys of COPD conducted in Guangdong, China in 2012–2019 (14,15). Questionnaire and spirometry data were collected from all participants. A subset of participants had CPET available. The present study includes participants with eligible questionnaires, spirometry, and CPET data who were enrolled from July 2012 to August 2019. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Scientific Research Project Review of the First Affiliated Hospital of Guangzhou Medical University (No. 2013-37). All study participants provided written informed consent.
The main inclusion criteria were as follows: aged 40–80 years; acceptable CPET data; eligible spirometry before and after a bronchodilator test; and a completed standard epidemiological respiratory questionnaire. The following exclusion criteria were applied: GOLD stage II–IV [ratio of FEV1 to FVC <0.70 and FEV1 <80% of the predicted value]; respiratory tract infection or exacerbation in the 4 weeks before screening; previous lobectomy; malignant tumor newly discovered and being treated; history of other lung disease (e.g., asthma, lung cancer, active pulmonary tuberculosis, pneumoconiosis, extensive bronchiectasis, pulmonary aspergillus); and severe cardiovascular disease or other contraindications to CPET.
We used three indicators of post-bronchodilator spirometry to assess SAD, namely, maximal mid-expiratory flow, forced expiratory flow at 50% of vital capacity, and forced expiratory flow at 75% of vital capacity. When at least two of these three indicators were below 65% of predicted values, we considered the participants to have SAD (1,13). Non-COPD was defined as a post-bronchodilator spirometry FEV1/FVC value of ≥0.70, mild COPD as a post-bronchodilator FEV1/FVC <0.70, and FEV1 ≥80% of the predicted value.
The participants were divided into a control group (without COPD or SAD), a SAD group (SAD without COPD), and a GOLD I group (mild COPD).
Questionnaire
A revised version of the standardized questionnaire used in the International Burden of Obstructive Lung Diseases study was administered during an in-person interview (16). Demographic information, chronic respiratory symptoms, smoking status, and smoking index were included (14). Chronic respiratory symptoms included chronic sputum, cough, dyspnea, and/or wheezing. Study participants who had smoked fewer than 100 cigarettes in their lifetime were defined as never-smokers and otherwise as ever-smokers. Ever-smokers included current smokers (smoking at baseline) and former smokers (had quit smoking before baseline). The smoking index was defined as the number of packs of cigarettes smoked daily multiplied by years of smoking.
Spirometry
All patients underwent spirometry before and after administration of a bronchodilator (salbutamol sulfate aerosol, 400 µg). Portable spirometers (CareFusion, Yorba Linda, CA, USA) and quality control software (SentrySuite version 2.3, CareFusion) were used to obtain the spirometric data and calibrated daily. Spirometry was performed using the standard methods recommended by the European Respiratory Society and American Thoracic Society (17), and quality grades A, B, and C were considered acceptable for analysis. Predicted values for spirometric variables were derived using the 1993 European Community for Steel and Coal reference values (18).
CPET
A maximal incremental CPET (COSMED, Rome, Italy) was performed on a calibrated cycle ergometer (Quark PFT Ergo Bp900, Rome, Italy) and supervised by a physician. The protocol was as follows: 2 min of rest, 2 min of unloaded cycling at 55–65 rpm, stepwise increases in workload of 5–30 W/min at 55–65 rpm until limited by symptoms or the test was terminated by the physician because of electrocardiographic abnormalities or chest pain (for a total of approximately 8–12 min), 10 min of recovery, and 3 min of rest. During the entire test period, oxygen consumption, carbon dioxide production (VCO2), changes in airflow, and heart rate were monitored by a mask sampling line and a 12-lead electrocardiogram.
Measurements of exercise tolerance [peak work rate and percentage of predicted peak oxygen uptake ()] and ventilatory efficiency [ventilatory equivalents for carbon dioxide at the ventilatory threshold ()] were obtained (19). The V-slope method was used to determine the anaerobic threshold, namely, the VO2 value at which the slope of the carbon dioxide production vs. VO2 changes from ≤1 to a slope steeper than 1 (20). The predicted values were determined according to the equations proposed by Wasserman and Hansen (21) and considered to indicate impaired exercise capacity when was below 84% of the predicted value (20,22).
Statistical analysis
A Kolmogorov–Smirnov test was used to explore whether the quantitative information accorded with normal distribution. A one-way analysis of variance or Kruskal–Wallis test were used to evaluate differences among control, SAD, and GOLD I groups, adjusted for multiple comparisons using Bonferroni correction method. Chi-squared tests or Fisher’s exact tests were used to compare the difference in categorical variables. The risk of impaired exercise capacity (<84% of the predicted value) was evaluated using multivariate logistic regression after adjustment for sex, age, body mass index (BMI), smoking status, and smoking index. Objective measurements of exercise tolerance (peak work rate and %pred) and ventilatory efficiency () were compared among groups by analysis of multivariate linear regression. Model 1 did not include any covariates, model 2 was adjusted for sex and age, and model 3 was adjusted for sex, age, BMI, smoking status, and smoking index to assess the robustness of the association. Correlations between exercise capacity and ventilatory efficiency were determined by Pearson’s correlation coefficients.
Subgroup analyses were performed according to smoking status (never-smokers and ever-smokers), given that smoking status may affect assessment of exercise capacity (23-25). We also performed three sensitivity analyses. First excluding participants with preserved ratio impaired spirometry (FEV1/FVC ≥0.70 and FEV1 <80%pred) to avoid confounding by restrictive processes. Second excluding participants with sub-maximal exercise defined as a peak respiratory exchange ratio lower than 1.10. Third, participants were grouped using the Global Lung Initiative 2012 reference equations for South East Asian populations. No imputation for missing values was performed; rather, observations with relevant missing values were excluded from the respective analyses. All statistical analyses were performed using SPSS 24.0 (IBM SPSS, Armonk, NY, USA). The statistical tests were two-sided and considered statistically significant at P<0.05.
Results
Demographic and clinical characteristics
A total of 865 participants were recruited from the community, 481 of whom had a completed questionnaire, acceptable CPET, and spirometry data and were included in the final analysis. There were 85 participants in the control group, 157 in the SAD group, and 239 in the GOLD I group (Figure 1).
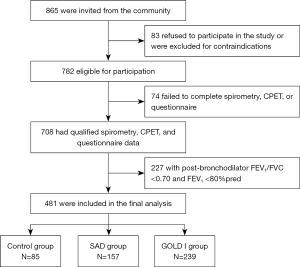
Table 1 lists the demographic and clinical characteristics of the participants by study group. The SAD group were older than the control group (58±8 vs. 55±7 years), had more smokers (former smokers, 21% vs. 8%; current smokers, 38% vs. 35%), and more participants with chronic respiratory symptoms (35% vs. 20%). There was no significant difference in sex, BMI, or smoking index between the SAD group and the control group. All post-bronchodilator spirometric parameters, except for FVC, were significantly lower in the SAD group than in the control group. There were no significant differences in chronic respiratory symptoms or FEV1 or predicted FEV1 values between the SAD and GOLD I groups. Comparisons of demographic and clinical characteristics among the groups stratified by smoking status are demonstrated in Table S1.
Table 1
| Variables | Control group (n=85) | SAD group (n=157) | GOLD I group (n=239) |
|---|---|---|---|
| Demographic data | |||
| Age, years | 55±7 | 58±8* | 62±8*† |
| Male sex | 49 [58] | 109 [69] | 200 [84]*† |
| BMI, kg/m2 | 23.9±3.2 | 23.2±3.4 | 22.3±3.1*† |
| Smoking status | |||
| Never | 48 [57] | 65 [41]* | 54 [23]*† |
| Former | 7 [8] | 32 [21]* | 41 [17]*† |
| Current | 30 [35] | 60 [38]* | 144 [60]*† |
| Smoking index, pack×years | 17±24 | 26±32 | 38±33*† |
| Symptoms‡ | |||
| Chronic cough | 15 [18] | 38 [25] | 64 [27] |
| Chronic sputum | 13 [16] | 39 [25] | 73 [31]* |
| Dyspnea | 1 [1] | 4 [3] | 5 [2] |
| Wheezing | 3 [4] | 17 [11] | 21 [9] |
| Any symptom | 17 [20] | 55 [35]* | 93 [39]* |
| Post-BD spirometry measures | |||
| FEV1, L | 2.70±0.54 | 2.33±0.47* | 2.34±0.47* |
| FVC, L | 3.33±0.73 | 3.13±0.70 | 3.62±0.72*† |
| FEV1/FVC, % | 81.7±4.6 | 74.9±4.1* | 64.8±4.0*† |
| FEV1%pred, % | 107±12 | 94±14* | 95±10* |
| MMEF, L | 2.69±0.58 | 1.63±0.35* | 1.14±0.37*† |
| FEF50, L | 3.54±0.73 | 2.31±0.50* | 1.67±0.51*† |
| FEF75, L | 0.98±0.38 | 0.54±0.18* | 0.37±0.17*† |
| MMEF %pred, % | 83±15 | 51±9* | 37±10*† |
| FEF50 %pred, % | 92±16 | 61±12* | 44±11*† |
| FEF75 %pred, % | 70±23 | 41±12* | 31±13*† |
Data are shown as the mean ± standard deviation or n [%]. The control group was defined as having normal lung function (non-COPD without SAD), the SAD group as having SAD but no COPD, and the GOLD I group as having mild COPD. Baseline characteristics were compared between the groups using one-way analysis of variance or Kruskal–Wallis test for continuous variables and chi-squared tests or Fisher’s exact tests for categorical variables. Pairwise comparisons between groups were performed using the Bonferroni method. *P<0.05 vs. the control group. †P<0.05 vs. the SAD group. ‡, numbers of participants with symptoms available: chronic cough =472, chronic sputum =478, wheezing =467. SAD, small airway dysfunction; GOLD, Global Initiative for Chronic Obstructive Lung Disease; BMI, body mass index; post-BD, post-bronchodilator; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; MMEF, maximal mid-expiratory flow; FEF50, forced expiratory flow at 50% of vital capacity; FEF75, forced expiratory flow at 75% of vital capacity.
Exercise capacity in non-obstructive SAD
Peak work rate was 8.7% lower in the SAD group than in the control group but not different from that in the GOLD I group (Table 2). The proportion of participants with a reported abnormality in was higher in the SAD group than in the control group (52.9% vs. 32.9%), as was the risk of impaired exercise capacity [adjusted odds ratio 2.53; 95% confidence interval (CI): 1.42–4.52; P=0.002], but neither was significantly different from the GOLD I group (Table 3). Using the control group as the referent group, multiple linear regression analysis showed that the peak work rate was lower in the SAD group (β=−10.5; 95% CI: −16.3 to −4.7; P<0.001) as was the %pred (β=−4.0; 95% CI: −7.7 to −0.2; P=0.039, Table 4). However, %pred was similar in the control and GOLD I groups (β=−2.4; 95% CI: −6.2 to 1.4; P=0.215).
Table 2
| Variables | Anaerobic threshold ‡ | Peak exercise | |||||
|---|---|---|---|---|---|---|---|
| Control | SAD | GOLD I | Control | SAD | GOLD I | ||
| Work rate, Watt | 81±19 | 71±20* | 68±22* | 138±28 | 126±28* | 121±27* | |
| , L/min | 1.03±0.22 | 0.97±0.24 | 0.97±0.23 | 1.49±0.32 | 1.42±0.31 | 1.44±0.33 | |
| % predicted maximum | 62±13 | 58±14 | 58±14 | 88±14 | 85±14 | 86±15 | |
| <84%pred at peak exercise, n (%) | 28 (32.9) | 83 (52.9)* | 108 (45.2)* | ||||
| HR, beats/min | 112±18 | 105±17* | 99±20*† | 144±17 | 142±19 | 139±17 | |
| % predicted maximum | 68±11 | 65±11 | 63±13* | 87±9 | 88±11 | 88±11 | |
| O2 pulse, mL O2/beat | 9.0±2.0 | 8.9±2.2 | 9.1±2.0 | 10.4±2.1 | 10.1±2.2 | 10.3±2.2 | |
| E, L/min | 29.9±7.0 | 28.5±6.7 | 30.2±8.3 | 52.9±15.4 | 51.8±14.3 | 52.7±13.5 | |
| % estimated MVV | 32±7 | 36±10* | 38±11* | 56±13 | 64±15* | 65±14* | |
| 31.1±3.8 | 32.9±4.3* | 34.7±5.2*† | 30.5±4.3 | 32.1±4.5 | 34.1±5.5*† | ||
| >34 at the anaerobic threshold | 17 (21.8) | 53 (34.2) | 112 (48.1)*† | ||||
| 28.9±4.0 | 29.3±4.0 | 30.7±5.0*† | 35.2±4.9 | 35.9±5.9 | 37.0±6.5 | ||
| PETCO2, mmHg | 40.3±4.4 | 38.6±4.3* | 37.1±4.7*† | 40.5±5.2 | 39.1±5.1 | 37.4±5.4*† | |
| fR, breaths/min | 25±5 | 23±5 | 22±5* | 33±7 | 33±6 | 34±7 | |
| R | 0.93±0.08 | 0.89±0.08* | 0.89±0.08* | 1.16±0.10 | 1.12±0.10* | 1.09±0.10*† | |
| VT, L | 1.21±0.30 | 1.20±0.31 | 1.28±0.32† | 1.62±0.41 | 1.57±0.36 | 1.59±0.39 | |
Data are shown as the mean ± standard deviation or n (%). The control group was defined as having normal lung function (non-COPD without SAD), the SAD group as having SAD but no COPD, and the GOLD I group as having mild COPD. Measurements were compared between groups using one-way analysis of variance or Kruskal–Wallis test for continuous variables and chi-squared tests or Fisher’s exact tests for categorical variables. Pairwise comparisons between groups were performed using the Bonferroni method. *P<0.05 vs. the control group. †P<0.05 vs. the SAD group. ‡, anaerobic threshold could not be identified in 15 participants. SAD, small airway dysfunction; GOLD, Global Initiative for Chronic Obstructive Lung Disease; , oxygen uptake; E, minute ventilation volume; HR, heart rate; MVV, maximum ventilatory volume; , ventilatory equivalents for carbon dioxide; , ventilatory equivalents for oxygen; PETCO2, end-tidal carbon dioxide partial pressure; fR, respiratory frequency; R, respiratory exchange ratio; VT, tidal volume; COPD, chronic obstructive pulmonary disease.
Table 3
| Variables | Participants (n) | <84%pred, n (%) | Univariate | Adjusted* | |||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||||
| All participants | |||||||
| Control group | 85 | 28 (32.9) | 1 (Ref.) | 1 (Ref.) | |||
| SAD group | 157 | 83 (52.9) | 2.28 (1.32–3.96) | 0.003 | 2.53 (1.42–4.52) | 0.002 | |
| GOLD I group | 239 | 108 (45.2) | 1.68 (1.00–2.82) | 0.051 | 1.79 (1.00–3.21) | 0.049 | |
| Ever-smokers | |||||||
| Control group | 37 | 16 (43.2) | 1 (Ref.) | 1 (Ref.) | |||
| SAD group | 92 | 56 (60.9) | 2.04 (0.94–4.43) | 0.071 | 2.44 (1.08–5.51) | 0.032 | |
| GOLD I group | 185 | 89 (48.1) | 1.22 (0.60–2.48) | 0.589 | 1.70 (0.79–3.66) | 0.171 | |
| Never-smokers | |||||||
| Control group | 48 | 12 (25.0) | 1 (Ref.) | 1 (Ref.) | |||
| SAD group | 65 | 27 (41.5) | 2.13 (0.94–4.83) | 0.070 | 2.38 (1.02–5.58) | 0.046 | |
| GOLD I group | 54 | 19 (35.2) | 1.63 (0.69–3.85) | 0.266 | 1.98 (0.77–5.05) | 0.155 | |
The control group was defined as having normal lung function (non-COPD without SAD), the SAD group as having SAD but no COPD, and the GOLD I group as having mild COPD. The risks of impaired exercise capacity (<84% of predicted values) were evaluated using dichotomous logistic regression. *, adjusted for sex, age, body mass index, smoking status, and smoking index (only adjusted for sex, age, and body mass index in never-smokers). , peak oxygen uptake; OR, odds ratio; CI, confidence interval; SAD, small airway dysfunction; COPD, chronic obstructive pulmonary disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease.
Table 4
| Variables | Control group | SAD group | GOLD I group | |||
|---|---|---|---|---|---|---|
| β (95% CI) | P | β (95% CI) | P | |||
| Exercise capacity | ||||||
| Peak work rate, Watt | ||||||
| Model 1 | Ref. | −11.9 (−19.2 to −4.6) | 0.002 | −16.7 (−23.5 to −9.8) | <0.001 | |
| Model 2 | Ref. | −11.9 (−17.8 to −5.9) | <0.001 | −14.2 (−20.1 to −8.3) | <0.001 | |
| Model 3 | Ref. | −10.5 (−16.3 to −4.7) | <0.001 | −11.1 (−16.9 to −5.2) | <0.001 | |
| %pred | ||||||
| Model 1 | Ref. | −3.7 (−7.6 to 0.1) | 0.058 | −1.9 (−5.5 to 1.8) | 0.313 | |
| Model 2 | Ref. | −4.0 (−7.7 to −0.2) | 0.039 | −3.0 (−6.7 to 0.8) | 0.119 | |
| Model 3 | Ref. | −4.0 (−7.7 to −0.2) | 0.039 | −2.4 (−6.2 to 1.4) | 0.215 | |
| Ventilation efficiency | ||||||
| Model 1 | Ref. | 1.8 (0.5 to 3.0) | 0.006 | 3.6 (2.4 to 4.7) | <0.001 | |
| Model 2 | Ref. | 1.2 (0.02 to 2.4) | 0.047 | 2.0 (0.8 to 3.2) | 0.001 | |
| Model 3 | Ref. | 1.1 (−0.1 to 2.3) | 0.072 | 1.5 (0.3 to 2.7) | 0.012 | |
The control group was defined as having normal lung function (non-COPD with no SAD), the SAD group as having SAD but no COPD, and the GOLD I group as having mild COPD. Exercise capacity and ventilation efficiency were compared between groups using multivariate linear analysis. Model 1 (unadjusted). Model 2 (adjusted for sex and age). Model 3 (adjusted for sex, age, body mass index, smoking status, and smoking index). SAD, small airway dysfunction; GOLD, Global Initiative for Chronic Obstructive Lung Disease; β, beta-coefficient; CI, confidence interval; %pred, peak oxygen uptake in percentage of predicted value; , ventilatory equivalents for carbon dioxide at ventilatory threshold; COPD, chronic obstructive pulmonary disease.
Associations between ventilatory efficiency and exercise capacity in non-obstructive SAD
Fifty-three of 155 participants (34.2%) in the SAD group had CPET evidence of ventilatory inefficiency (>34), which was slightly higher than that in the control group (P=0.069). The mean levels were higher in the SAD group than in the control group (32.9±4.3 vs. 31.1±3.8; P<0.05) but still within the normal range (Table 2). Using the control group as the referent group, multiple linear regression analysis adjusted for potential confounders showed that there was a trend towards higher in the SAD group that nearly reached statistical significance (β=1.1; 95% CI: −0.1 to 2.3; P=0.072, Table 4). In the SAD group, both peak work rate and %pred were negatively correlated with (r=−0.34, P<0.001, and r=−0.38, P<0.001, respectively, Table 5).
Table 5
| Variables | Correlation with peak work rate (Watt) | Correlation with %pred (%) | |||
|---|---|---|---|---|---|
| r | P value | r | P value | ||
| All participants | −0.35 | <0.001 | −0.30 | <0.001 | |
| SAD group | −0.34 | <0.001 | −0.38 | <0.001 | |
Correlation coefficients were tested among exercise capacity with in all participants and in the SAD group, respectively. %pred, peak oxygen uptake in percentage of predicted value; , ventilatory equivalents for carbon dioxide at ventilatory threshold; SAD, small airway dysfunction.
Subgroup and sensitivity analysis
When never-smokers and ever-smokers were considered separately, the risk of impaired exercise capacity was still higher in participants with SAD who did not meet the spirometric criteria for COPD than in controls (ever-smokers: adjusted odds ratio =2.44, 95% CI: 1.08–5.51, P=0.032; never-smokers: adjusted odds ratio =2.38, 95% CI: 1.02–5.58, P=0.046) but was not significantly different from that in the GOLD I group (Table 3). Ever-smokers in the SAD group had a 10.7% lower peak work rate and a 9.8% lower tidal volume than those in the control group (both P<0.05) but had a mildly elevated (Table S2). Compared with the controls, never-smokers in the SAD group tended to have a lower peak work rate (P=0.063), and a significantly higher (P=0.038); however, tidal volume was not significantly different between the two groups (Table S3).
Sensitivity analysis excluding participants with preserved ratio impaired spirometry (n=22, all in the SAD group) or participants with sub-maximal exercise, defined as a peak respiratory exchange ratio lower than 1.10 (n=207, of which 20 were in the control group, 61 were in the SAD group, and 126 were in the GOLD I group), yielded consistent results for the primary outcome variable (Table S4 and Table S5). These group differences in the risk of impaired exercise capacity remained present when we change the reference values (Table S6).
Discussion
This study had three novel findings. First, exercise capacity was poorer in participants with non-obstructive SAD who did not meet spirometric criteria for COPD than in controls but was comparable with that in patients with mild COPD. Second, in the SAD group, a higher risk of impaired exercise capacity was found not only in smokers but also in never-smokers. Third, impaired exercise capacity in patients with non-obstructive SAD appeared to be associated with ventilatory inefficiency.
Participants in the SAD group, even those with relatively preserved spirometry (post-bronchodilator FEV1/FVC ≥0.70), had lower FEV1 and were more likely to report chronic respiratory symptoms than those in the control group. Although still within the normal range, patients with non-obstructive SAD had worse exercise capacity and ventilatory efficiency during exercise relative to controls. In a study of 4,730 healthy middle-aged men, Hansen et al. found that lower levels of cardiorespiratory fitness (determined as maximal oxygen uptake) were associated with a higher long-term risk of incident COPD and death from COPD (26). Furthermore, spirometry-defined SAD itself is reportedly associated with an accelerated decline in lung function (2). Collectively, these results indicated that exclusive reliance on spirometry may result in underestimation of clinically important physiologic impairment and reinforce the pressing need for a better understanding of the physiological and clinical abnormalities in patients with non-obstructive SAD.
Previous studies have clearly shown that exercise tolerance is lower in smokers without spirometric COPD than in controls and that extensive SAD was one of the reasons for this finding (11). Our present study confirmed this finding and found that the risk of impaired exercise capacity was higher in smokers with SAD but no airflow obstruction than in controls. However, smoking exposure is not the only factor associated with SAD, and a number of other factors also play a role (1). For example, Petsonk et al. found that dust exposure can have an impact on many pathological processes in the small airways and considered it to be the main cause of the inefficient ventilation and exercise intolerance documented in miners (27). Our study extends these findings to never-smokers and found that patients with non-obstructive SAD who had never smoked also had impaired exercise capacity. This finding has implications for better recognition of chronic respiratory diseases in developing countries where outdoor air pollution and exposure to tobacco smoke are increasingly prevalent.
A substantial obstruction in the small airways could affect the distribution of ventilation and gas exchange before spirometric airway obstruction reaches a clinically detectable level (9,28,29). Individuals with peripheral airway dysfunction may experience pulmonary gas trapping and dynamic lung hyperinflation during exercise (8,30). All these factors can negatively affect ventilatory efficiency, which has been assessed by measurement of the quantity of ventilation needed to eliminate metabolically produced CO2 (i.e., ) (19,31-34). Our results supported those of previous studies. We found that marginally higher in patients with non-obstructive SAD than in controls, suggesting greater ventilation/perfusion abnormalities. We also found that both peak work rate and were negatively correlated with , which was similar to the findings of Devin et al. (31). Therefore, we speculate that impaired exercise capacity in patients with non-obstructive SAD may be associated with ventilatory inefficiency. As the difference of between non-obstructive SAD and controls did not reach statistical significance (P=0.072), further research is required to confirm this speculation.
Elbehairy et al. found no difference in , whether expressed as the slope, intercept, or nadir, between smokers without COPD and controls (11). In our study, increase slightly in both smoking and nonsmoking patients with non-obstructive SAD but was only statistically relevant in never-smokers. Moreover, the increase in peak tidal volume was significantly smaller in the smoking participants with non-obstructive SAD than in the control group, with the exception of never-smokers. This suggests that never-smokers with non-obstructive SAD are likely to have a different underlying pathology that can cause a different ventilatory response to exercise. It is also possible that an even larger sample size will be required for detection of a significant case-control difference in smokers. These ideas warrant further study.
We are satisfied that the reduced exercise performance in our participants was not the result of reduced motivational effort because (I) we encouraged the participants to continue exercise until limited by symptoms or an abnormal electrocardiogram and (II) sensitivity analysis excluding participants with sub-maximal exercise, defined as a peak respiratory exchange ratio lower than 1.10, yielded consistent results for the primary outcome variable. Significant cardiac impairment was also unlikely to have contributed to the impaired exercise capacity in non-obstructive SAD because patients with active cardiac comorbidity were excluded and heart rate responses and reserve at peak exercise were normal, as was the O2 pulse. We also performed a sensitivity analysis excluding participants with preserved ratio impaired spirometry (FEV1/FVC ≥0.70 and FEV1% <80%) to avoid the effect of restrictive lung disease. It is well known that sex, age, and BMI can also affect test performance (20,29). We adjusted for major confounding factors after univariable analysis to strengthen our findings.
This study has several limitations. First, the diagnosis of SAD was based entirely on post-bronchodilator spirometry, which is more variable than that based on impulse oscillometry, pathological examination, and high-resolution computed tomography. Therefore, our results pertain only to post-bronchodilator spirometry-defined SAD. The cutoff points selected require validation, but are routinely and widely used in clinical practice when diagnosing SAD. Second, operating lung volumes, dyspnea, and the ratio of dead space volume to tidal volume (invasive methods to obtain arterial blood gases are needed) were not measured during CPET, but would have greatly improved our ability to infer functional status from CPET. Finally, the data are derived from a cross-sectional study, which restricts causal interpretations.
Conclusions
Although individuals with non-obstructive SAD have relatively preserved spirometry (post-bronchodilator FEV1/FVC ≥0.70), they have impaired exercise capacity regardless of smoking status that may be associated with ventilatory inefficiency.
Acknowledgments
We thank all the study participants and personnel who assisted with the study. We are also grateful to the following individuals and institutions: Professor Rongchang Chen and Professor Jinping Zheng (The First Affiliated Hospital of Guangzhou Medical University) for providing the cardiopulmonary exercise testing equipment; the Lianping County People’s Hospital and Wengyuan County People's Hospital for providing us with a study follow-up site; Bijia Lin, Shaodan Wei, Xiaopeng Ling, Wenjun Lai, Qiaoyi He, and Yunsong Chen (Nanshan Medical Development Foundation of Guangdong Province, National Center for Respiratory Medicine, State Key Laboratory of Respiratory Disease, National Clinical Research Center for Respiratory Disease, Guangzhou Institute of Respiratory Health, The First Affiliated Hospital of Guangzhou Medical University) for the efforts in collecting and verifying the information analyzed in this study. Finally, we thank Liwen Bianji (Edanz) (www.liwenbianji.cn) for editing the English draft of this manuscript.
Funding: This work was supported by Local Innovative and Research Teams Project of Guangdong Pearl River Talents Program (No. 2017BT01S155), the 13th Five-Year National Key Research and Development Program (No. 2016YFC1304100), the National Science Foundation of China (Nos. 81970045, 81670040, and 81900044), Zhongnanshan Medical Foundation of Guangdong Province (Nos. ZNSA-2020003, ZNSA-2020012, and ZNSA-2020013), Guangdong Province Key Field R&D Program (No. 2020B1111330001), the Science and Technology Program of Guangzhou (No. 201904010071), the Guangdong Natural Science Foundation (No. 2018A0303130227), and the Construction of Formative Evaluation System for Medical Colleges Based on Wisdom Teaching Multimedia Big Data (No. 2021ALA02007).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1328/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1328/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1328/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1328/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Scientific Research Project Review of the First Affiliated Hospital of Guangzhou Medical University (No. 2013-37) and informed consent was taken from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Xiao D, Chen Z, Wu S, et al. Prevalence and risk factors of small airway dysfunction, and association with smoking, in China: findings from a national cross-sectional study. Lancet Respir Med 2020;8:1081-93. [Crossref] [PubMed]
- Stockley JA, Ismail AM, Hughes SM, et al. Maximal mid-expiratory flow detects early lung disease in α(1)-antitrypsin deficiency. Eur Respir J 2017;49:1602055. [Crossref] [PubMed]
- Bazzan E, Semenzato U, Turato G, et al. Symptomatic smokers without COPD have physiological changes heralding the development of COPD. ERJ Open Res 2022;8:00202-2022. [Crossref] [PubMed]
- Hogg JC, Chu F, Utokaparch S, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 2004;350:2645-53. [Crossref] [PubMed]
- McDonough JE, Yuan R, Suzuki M, et al. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med 2011;365:1567-75. [Crossref] [PubMed]
- Hogg JC, Paré PD, Hackett TL. The Contribution of Small Airway Obstruction to the Pathogenesis of Chronic Obstructive Pulmonary Disease. Physiol Rev 2017;97:529-52. [Crossref] [PubMed]
- Konstantinos Katsoulis K, Kostikas K, Kontakiotis T. Techniques for assessing small airways function: Possible applications in asthma and COPD. Respir Med 2016;119:e2-9. [Crossref] [PubMed]
- Ofir D, Laveneziana P, Webb KA, et al. Mechanisms of dyspnea during cycle exercise in symptomatic patients with GOLD stage I chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2008;177:622-9. [Crossref] [PubMed]
- Hogg JC, Macklem PT, Thurlbeck WM. Site and nature of airway obstruction in chronic obstructive lung disease. N Engl J Med 1968;278:1355-60. [Crossref] [PubMed]
- Gordon C, Gudi K, Krause A, et al. Circulating endothelial microparticles as a measure of early lung destruction in cigarette smokers. Am J Respir Crit Care Med 2011;184:224-32. [Crossref] [PubMed]
- Elbehairy AF, Guenette JA, Faisal A, et al. Mechanisms of exertional dyspnoea in symptomatic smokers without COPD. Eur Respir J 2016;48:694-705. [Crossref] [PubMed]
- McNulty W, Usmani OS. Techniques of assessing small airways dysfunction. Eur Clin Respir J 2014;1: [Crossref] [PubMed]
- Xing Z, Sun T, Janssens JP, et al. Airflow obstruction and small airway dysfunction following pulmonary tuberculosis: a cross-sectional survey. Thorax 2023;78:274-80. [Crossref] [PubMed]
- Liu S, Zhou Y, Liu S, et al. Association between exposure to ambient particulate matter and chronic obstructive pulmonary disease: results from a cross-sectional study in China. Thorax 2017;72:788-95. [Crossref] [PubMed]
- Liu S, Zhou Y, Liu S, et al. Clinical impact of the lower limit of normal of FEV(1)/FVC on detecting chronic obstructive pulmonary disease: A follow-up study based on cross-sectional data. Respir Med 2018;139:27-33. [Crossref] [PubMed]
- Buist AS, McBurnie MA, Vollmer WM, et al. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet 2007;370:741-50. [Crossref] [PubMed]
- Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005;26:319-38. [Crossref] [PubMed]
- Quanjer PH, Tammeling GJ, Cotes JE, et al. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J Suppl 1993;16:5-40.
- Neder JA, Berton DC, Arbex FF, et al. Physiological and clinical relevance of exercise ventilatory efficiency in COPD. Eur Respir J 2017;49:1602036. [Crossref] [PubMed]
- American Thoracic Society. ATS/ACCP Statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med 2003;167:211-77. Erratum in: Am J Respir Crit Care Med 2003;1451-2. [Crossref] [PubMed]
- Wasserman K, Hansen JE, Sue DY, et al. Principles of Exercise Testing and Interpretation: Including Pathophysiology and Clinical Applications. Philadelphia, PA: Lippincott Williams and Wilkins, 2012.
- Guazzi M, Adams V, Conraads V, et al. EACPR/AHA Joint Scientific Statement. Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Eur Heart J 2012;33:2917-27. [Crossref] [PubMed]
- Vozoris NT, O'donnell DE. Smoking, activity level and exercise test outcomes in a young population sample without cardiopulmonary disease. J Sports Med Phys Fitness 2015;55:787-96.
- Furlanetto KC, Mantoani LC, Bisca G, et al. Reduction of physical activity in daily life and its determinants in smokers without airflow obstruction. Respirology 2014;19:369-75. [Crossref] [PubMed]
- Regan EA, Lynch DA, Curran-Everett D, et al. Clinical and Radiologic Disease in Smokers With Normal Spirometry. JAMA Intern Med 2015;175:1539-49. [Crossref] [PubMed]
- Hansen GM, Marott JL, Holtermann A, et al. Midlife cardiorespiratory fitness and the long-term risk of chronic obstructive pulmonary disease. Thorax 2019;74:843-8. [Crossref] [PubMed]
- Petsonk EL, Stansbury RC, Beeckman-Wagner LA, et al. Small Airway Dysfunction and Abnormal Exercise Responses. A Study in Coal Miners. Ann Am Thorac Soc 2016;13:1076-80. [Crossref] [PubMed]
- Elbehairy AF, Faisal A, Guenette JA, et al. Resting Physiological Correlates of Reduced Exercise Capacity in Smokers with Mild Airway Obstruction. COPD 2017;14:267-75. [Crossref] [PubMed]
- Elbehairy AF, Ciavaglia CE, Webb KA, et al. Pulmonary Gas Exchange Abnormalities in Mild Chronic Obstructive Pulmonary Disease. Implications for Dyspnea and Exercise Intolerance. Am J Respir Crit Care Med 2015;191:1384-94. [Crossref] [PubMed]
- Di Marco F, Terraneo S, Job S, et al. Cardiopulmonary exercise testing and second-line pulmonary function tests to detect obstructive pattern in symptomatic smokers with borderline spirometry. Respir Med 2017;127:7-13. [Crossref] [PubMed]
- Phillips DB, Elbehairy AF, James MD, et al. Impaired Ventilatory Efficiency, Dyspnea, and Exercise Intolerance in Chronic Obstructive Pulmonary Disease: Results from the CanCOLD Study. Am J Respir Crit Care Med 2022;205:1391-402. [Crossref] [PubMed]
- Neder JA, Berton DC, Müller PT, et al. Ventilatory Inefficiency and Exertional Dyspnea in Early Chronic Obstructive Pulmonary Disease. Ann Am Thorac Soc 2017;14:S22-9. [Crossref] [PubMed]
- Forster HV, Pan LG. Breathing during exercise: demands, regulation, limitations. Adv Exp Med Biol 1988;227:257-76. [Crossref] [PubMed]
- Phillips DB, Collins SÉ, Stickland MK. Measurement and Interpretation of Exercise Ventilatory Efficiency. Front Physiol 2020;11:659. [Crossref] [PubMed]


