Immune-related thyroid dysfunction is associated with improved long-term prognosis in patients with non-small cell lung cancer treated with immunotherapy: a systematic review and meta-analysis
Highlight box
Key findings
• Thyroid dysfunction associated with immunotherapy is common and associated with a good prognosis.
What is known and what is new?
• Immunotherapy can cause thyroid dysfunction.
• Thyroid dysfunction associated with immunotherapy can affect the prognosis of patients with NSCLC.
What is the implication, and what should change now?
• Thyroid dysfunction associated with immunotherapy can be used as a biological indicator of good prognosis of immunotherapy.
Introduction
Overexpression of immune checkpoint molecules in the tumor microenvironment has been recognized to play a crucial role in anti-tumor immune evasion. This understanding has transformed the treatment of cancer (1-3). Their use is expected to increase in the coming years, given the continually increasing number of cancer types in which immune checkpoint inhibitors (ICIs) have shown clinical activity. Monoclonal antibodies targeting the CTLA-4 and programmed cell death 1/programmed cell death ligand-1 (PD-1/PD-L1) axes are the 2 main categories currently used in cancer immunotherapy. They all have immune-related adverse events (irAEs), which are unique side effects of ICIs similar to autoimmune reactions. Although irAEs can affect almost every organ in the body, they most commonly affect the skin, lung, endocrine, gastrointestinal tract, musculoskeletal, and other systems (4).
Since irAEs occur through an immune activation process, suggesting that depleted immune cells are reactivated to attack not only tumor cells but also normal tissues, the occurrence of irAEs could theoretically indicate a better response to ICIs treatment. However, whether irAE development can predict ICIs response remains controversial. Several recent studies have supported this hypothesis by showing favorable prognostic outcomes of various irAEs in response to immune checkpoint suppression in patients with non-small cell lung cancer (NSCLC) and melanoma (5-23).
However, because of conflicting results (24-36), no definitive conclusions can be drawn based on the results of each study. Different irAEs may lead to different prognostic differences. A systematic review of 16 studies reported that irAEs such as pneumonia, thyroid disease, myalgia, and mucosal toxicity were not significantly associated with overall survival (OS) (37). But immune-related thyroid dysfunction was found to be associated with prognosis in NSCLC, The mechanism is not well defined and may be related to reactivation of immune cells and disruption caused by severe adverse immunotherapy reactions, with moderate adverse immunotherapy reactions having a better response to ICIs treatment (10,12,13). Here, we conducted a systematic review of published studies for which survival data could be extracted to investigate the association between the development of thyroid dysfunction and the efficacy of ICIs in patients with irAE. We present the following article in accordance with the PRISMA reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-254/rc).
Methods
Literature search and data extraction
This study involved a one-arm meta-analysis of survival, with the aim of analyzing the relationship between thyroid dysfunction and survival prognosis in patients diagnosed with advanced lung cancer (LC) after immunotherapy. The objectives of the review were defined according to the following PECO criteria: P (population) = immune-related thyroid dysfunction diagnosed with LC; E (exposure): thyroid dysfunction after immunotherapy; C (comparison) = LC patients with normal and abnormal thyroid function after immunotherapy; O (outcome): progression-free survival (PFS) and OS. The literature search was conducted in the databases of MEDLINE and Embase on 31 December 2021 using the following search string: (thyroid dysfunction OR Immunotherapy OR ICI) AND (lung) AND (cancer OR carcinoma OR tumour OR malignancy) AND (survival OR prognosis OR outcome). No time, geographical, or language distinction limitation was applied, provided that English abstracts may be provided to determine eligibility for inclusion. After removing duplicate articles, first filter by title and abstract, and read those articles deemed likely to be included with full copies. Literature search and literature screening were performed independently by 3 researchers (Zhengjun Li, Ying Xia, and Mozhu Xia), and any differences of opinion are resolved by consensus or by seeking advice from the fourth senior researcher (Yi Ren). We finally included all the original full text containing basic data and survival analysis of patients with thyroid dysfunction after advanced LC patients immunotherapy into the review and meta-analysis. In order to be retained, an article must also report the value of the unadjusted hazard ratio (HR) obtained by fitting the survival analysis models, and the corresponding measure of statistical uncertainty [e.g., 95% confidence intervals (CIs), standard errors, or exact P value], or provide a Kaplan-Meier (KM) survival curve allowing to calculate an unadjusted HR using the method described by Parmar et al. (38). Editorials, commentaries, and letters that did not provide original data, as well as mere meeting abstracts, were excluded (the latter because they typically lacked much of the data information needed to properly interpret the results and assess the quality of the research). A list of references for all eligible papers, as well as previously published literature reviews and meta-analyses, were used to try to find other additional articles covering the same topic through a backreference chain approach.
Data extraction was carried out using a spreadsheet. The three authors (Zhengjun Li, Ying Xia, and Chang Liu) independently extracted and summarized the data, and questionable data were decided upon after joint discussion. We extracted the following information from each article eligible for inclusion: the country and year in which the study was conducted; study design; the number of patients with thyroid dysfunction after immunotherapy and the type of immunotherapy; classification of thyroid abnormalities, such as hyperthyroidism and hypothyroidism; follow-up survival data of patients with thyroid dysfunction after immunotherapy; The distribution of LC patients in terms of sex, age and tumor type [squamous cell carcinoma (SqCC) and non-SqCC)]; follow-up time (if any, median/mean and maximum); received therapeutic immune drugs; the statistical analysis methods and variables used for estimation adjustment are introduced in detail. The HR and 95% CI for the association between thyroid dysfunction after immunotherapy and the survival of LC patients were inverted when necessary to ensure that the reference group was always that of immunotherapy-associated thyroid dysfunction in LC patients, and then transformed into logHR and corresponding variance using Greenland’s formula (39). If two or more articles contain fully or partially overlapping study populations, we extract and enter the HR with the highest number of LC patients in the article, or the HR with the most adjustments when the study size is the same. The “rates” we extracted were adjusted in all the included literature to eliminate the influence of covariates, and there was no statistical difference in the underlying clinical variables.
Quality assessment
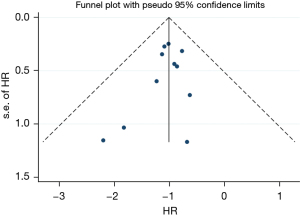
This study included 11 articles to analyze the survival of patients with thyroid dysfunction after immunotherapy. The included literature was evaluated by the Newcastle-Ottawa Scale (NOS). The quality of non-randomized controlled studies can be assessed using the NOS. Some appropriate modifications have been made to meet the needs of this study. The quality of the study was assessed by examining three items: patient selection, group comparability, and outcome evaluation. The study adopts the sequential star rating scale for scoring, and the higher the score, the higher the research quality. Each numbered item earns up to one star in the selection and exposure categories, and the comparability of two groups earns up to four stars. The quality of each study was rated as level 1 (0 to 5 stars) or level 2 (6 to 9 stars). The quality was 7–9 stars, is considered a low-risk bias. Funnel plots of the study results are shown in Figure 1. The funnel plots on overall survival rates following ICIs for the treatment of NSCLC showed symmetry, which suggested there was no publication bias.
Statistical analysis
For patients’ OS and PFS, study-specific log(HR) were pooled into a summary hazard risk (HR) through random effects models with maximum likelihood estimation, and corresponding 95% CI were calculated by assuming an underlying distribution (40). In all analyses, the extracted data is the adjusted data, patients with immune-related thyroid dysfunction were compared to those without abnormal thyroid function, the latter being the reference group: therefore, an HR of less than 1.00 indicated that patients with thyroid dysfunction after immunotherapy would have an increased survival rate, whereas an HR of more than 1.00 indicated the opposite. If there is no difference in the results between the 2 models, the random effects model is used in the report because it is used for indirect comparison. If the results of the 2 models differ, both results are reported. Heterogeneity was explored by χ2 and I2 testing. An I2<25% and an I2>50% reflected small and large inconsistency, respectively. If P>0.10, the studies were considered homogenous and a fixed-effects analysis model was used. When P<0.10 and I2<50%, these studies were considered to show heterogeneity, but heterogeneity was acceptable and fixed-effects analysis models were also used. When P<0.10 and I2>50%, the heterogeneity was too high to be accepted, and the random effects analysis model was used. Statistical analyses were conducted using Stata 15.0 software, version 15.0 (StataCorp., College Station, TX, USA). All tests were 2-sided and statistical significance was set at P values below 0.05.
Results
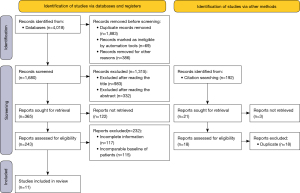
The MEDLINE and Embase literature searches yielded a total of 1,680 non-duplicate entries. The papers based on title (n=983) and abstract (n=332) were further excluded. A total of 243 full texts were retrieved and read. Among them, some did not meet the inclusion criteria: the main reasons for exclusion were that LC was not diagnosed during immunotherapy or thyroid dysfunction was not paid attention to after immunotherapy, and there was a lack of relevant survival follow-up data after thyroid dysfunction occurred during immunotherapy. One hundred and seventeen articles were deleted with incomplete data and 115 articles with unavailable patient data. When reading the full article, find 192 articles in the list of references. The literatures that could be evaluated after screening were all duplicated with those previously screened. Finally, a total of 11 studies (Figure 2) were included in the systematic review (11-13,15,41-47). The study was conducted by 3 people to extract data, statistical survival data, and perform statistical analysis.
The 11 articles that were included in the systematic review were published between 2015 and 2021 and included a total of 1,962 patients, these patients were diagnosed with advanced LC and had immune-related thyroid dysfunction after immunotherapy (Table 1). The patients came from 6 countries around the world, the average age at diagnosis of LC was about 58–72 years old, and the proportion of males was 41–78%. All LCs were in stage III-IV. Most studies had divided the pathological types into SqCC and other types, which may be related to PDL-1 expression in immunotherapy. All patients received anti-PD-1 or anti-PDL-1 therapy. The main drugs included were nivolumab, atezolizumab, and pembrolizumab, and some of them were in combination with CTLA-4. In some cases, immunotherapy was combined with chemotherapy.
Table 1
| Author, year | Country | No. of patients | Age, mean [range] or mean ± SD | Men, n (%) | Stage | Pathological subtype | Treatment | Study quality (NOS) |
|---|---|---|---|---|---|---|---|---|
| Kim 2017 (12) | Korea | 58 | 63.1 [49.0–68.0] | 43 (74.1) | IV | SqCC 20; non-SqCC 38 | Nivolumab/pembrolizumab | 8 stars |
| Peiró 2019 (41) | Spain | 55 | 60.5 [49.8–67] | 37 (78.7) | Advanced | NR | Nivolumab; nivolumab/ipilimumab | 9 stars |
| Osorio 2017 (13) | USA | 51 | 59 [39–80] | 21 (41.1) | IV | SqCC 9; non-SqCC 42 | Pembrolizumab | 8 stars |
| Luo 2021 (42) | USA | 744/551 | 63 [57–69]/67 [59–73] | MSKCC + VUMC: 379 (50.9); MSKCC: 260 (47%) | Advanced | MSKCC + VUMC: SqCC 197; non-SqCC 547; MSKCC: SqCC 124; non-SqCC 427 | Anti-PD-L1 ± CTLA-4 | 7 stars |
| Morimoto 2021 (43) | Japan | 70 | 69.5 [43–85] | 51 (72.9) | III/IV | SqCC 19; non-SqCC 51 | Immunotherapy (pembrolizumab/atezolizumab) plus chemotherapy | 8 stars |
| Haratani 2018 (11) | Japan | 134 | 68 [33–85] | 90 (67.0) | IIIB–IV | SqCC 33; non-SqCC 101 | Nivolumab | 8 stars |
| Grangeon 2019 (15) | France | 270 | 61 [32–84] | 177 (65.5) | IV | NR | Anti-PD-1/anti-PD-L1 | 9 stars |
| Campredon 2019 (44) | France | 105 | 61 [41–80] | 72 (68.6) | III/IV | NR | Nivolumab | 9 stars |
| Thuillier 2021 (45) | France | 134 | 62.5±8.9 | 94 (70.1) | IIIB–IV | SqCC 35; non-SqCC 96 | Nivolumab | 7 stars |
| Sakakida 2019 (46) | Japan | 150 | 72 [56–83] | 101 (67.3) | Advanced | NR | Nivolumab/pembrolizumab | 9 stars |
| Zhou 2021 (47) | China | 191 | 58 [32–85] | 138 (72.3) | IIIB–IV | SqCC 69; non-SqCC 105 | Nivolumab/pembrolizumab | 8 stars |
NOS, Newcastle-Ottawa scale; SqCC, squamous cell carcinoma; non-sqCC, non-squamous cell carcinoma; NR, not reported; MSKCC, Memorial Sloan Kettering Cancer Center; VUMC, Vanderbilt University Medical Center.
In the included literature, patients developed thyroid dysfunction at different times after immunotherapy, which was divided into hyperthyroidism and hypothyroidism. Thyroid function was evaluated by periodic hematologic tests at each cycle after immunotherapy. We found that among immunotherapy-related thyroid dysfunction cases, there were differences in the types of thyroid dysfunction with different immunotherapy modalities. In this study, the incidence of thyroid dysfunction was 13.2%, with some having a higher incidence of hypothyroidism and some having a higher incidence of hyperthyreosis. There was a total of 543 people in 5 articles, 38 patients with hyperthyroidism, accounting for 7% of the total, and 50 patients with hypothyroidism, accounting for 9.2% of the total. Thyroid dysfunction occurs in the early stage after immunotherapy and is one of the common adverse reactions (13,48-50). The cumulative incidence of thyroid dysfunction after immunotherapy is approximately 10%. Symptomatic treatment can be given to all patients with thyroid dysfunction, and no serious adverse reactions of grade 3-4 were found. Immunotherapy rarely induces a life-threatening thyroid storm (51). Most thyroid dysfunction can be controlled with treatment.
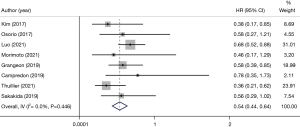
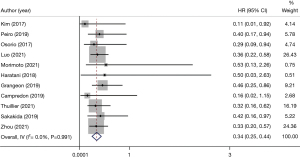
In all the included articles, patients with advanced NSCLC who underwent immunotherapy were followed-up. After data extraction and statistical analysis (Table 2), we found that patients with thyroid dysfunction after immunotherapy had significant improvement in PFS (HR 0.54, 95% CI: 0.44–0.64) (Figure 3) and OS (HR 0.34, 95% CI: 0.25–0.44) (Figure 4). This showed that patients with NSCLC who developed thyroid dysfunction after immunotherapy had a 66% lower risk of death and a 46% lower risk of disease progression.
Table 2
| Author, year | Thyroid dysfunction | No. of patients | PFS HR | 95% CI | OS HR | 95% CI |
|---|---|---|---|---|---|---|
| Kim 2017 (12) | Hyperthyroidism | 9 | 0.38 | 0.17–0.85 | 0.11 | 0.01–0.92 |
| Hypothyroidism | 10 | |||||
| Peiró 2019 (41) | Hyperthyroidism | 7 | – | – | 0.4 | 0.17–0.94 |
| Hypothyroidism | 10 | |||||
| Osorio 2017 (13) | Hyperthyroidism | 10 | 0.58 | 0.27–1.21 | 0.29 | 0.09–0.94 |
| Hypothyroidism | ||||||
| Luo 2021 (42) | Hyperthyroidism | 65 | 0.68 | 0.52–0.88 | 0.36 | 0.22–0.58 |
| Hypothyroidism | ||||||
| Morimoto 2021 (43) | Hyperthyroidism | 9 | 0.46 | 0.17–1.29 | 0.53 | 0.13–2.26 |
| Hypothyroidism | ||||||
| Haratani 2018 (11) | Hyperthyroidism | 10 | – | – | 0.504 | 0.027–2.629 |
| Hypothyroidism | ||||||
| Grangeon 2019 (15) | Hyperthyroidism | 53 | 0.58 | 0.39–0.85 | 0.46 | 0.25–0.86 |
| Hypothyroidism | ||||||
| Campredon 2019 (44) | Hyperthyroidism | 8 | 0.78 | 0.35–1.73 | 0.16 | 0.02–1.15 |
| Hypothyroidism | 6 | |||||
| Thuillier 2021 (45) | Hyperthyroidism | 5 | 0.36 | 0.21–0.62 | 0.32 | 0.16–0.62 |
| Hypothyroidism | 9 | |||||
| Sakakida 2019 (46) | Hyperthyroidism | 25 | 0.56 | 0.29–1.02 | 0.42 | 0.16–0.97 |
| Hypothyroidism | ||||||
| Zhou 2021 (47) | Hyperthyroidism | 9 | – | – | 0.334 | 0.196–0.571 |
| Hypothyroidism | 15 |
PFS, progression-free survival; HR, hazard ratio; CI, confidence interval; OS, overall survival.
Discussion
In the present study, thyroid dysfunction during immunotherapy was associated with longer PFS and OS, adjusting for other factors. Objective response rate and persistence control correlate with the treatment pattern of immunotherapy and are more common in patients with thyroid dysfunction. This is the first meta-analysis study to systematically demonstrate that thyroid dysfunction is associated with prognosis after immunotherapy for advanced NSCLC.
Immunotherapy is a new therapy for the treatment of metastatic malignancies by inhibiting the mechanism by which cancer cells evade host T cells (12). However, inhibitory checkpoint blocking may also lead to attacks on other tissues in the process of activating host T cells against malignant antigens. Therefore, PD-1 blocking can lead to irAEs, forming a series of adverse reactions related to autoimmune tissue destruction, and thyroid related irAEs are relatively common. Thyroid dysfunction associated with immunotherapy developed in approximately 13.2% of the patients in this study, with a median onset time of 40 days after drug initiation. Many immunodrugs were used in the included literature. Different immunotherapy drugs may have different effects on thyroid dysfunction, which may be different from the rate and occurrence time of side effects of a certain drug. The study included patients of different ethnic groups in 6 countries, with different ethnic groups having different rates of thyroid dysfunction.
Studies have found significant differences in the prognosis of irAEs when stratified according to severity. A recent meta-analysis reported that the occurrence of low-grade rather than high-grade irAEs may be a prognostic factor for clinical outcomes in patients with solid tumors (52). In our study, immunotherapy-related thyroid dysfunction in patients with advanced LC was found to have mild or moderate adverse effects, and no severe adverse effects were found. When the study stratified moderate and mild adverse events, the PFS and OS data of moderate adverse events were slightly improved compared with those of mild adverse events. As a result, severe irAEs often force ICIs treatment to be interrupted and may be associated with adverse clinical outcomes. In addition, severe irAEs can sometimes cause serious, life-threatening events that require immunosuppressive therapy or discontinuation of treatment. The inflammatory tumor microenvironment may be reactivated by immunosuppressive agents, ultimately promoting tumor progression. A previous study has also reported a negative impact of ICI discontinuation due to irAEs on clinical outcomes in NSCLC (53).
Some studies have found that thyroid dysfunction has no prognostic relevance in malignancies other than NSCLC, such as melanoma, when using immunotherapy for malignancies (10,12,13). There was no significant difference in prognosis between hyperthyroidism and hypothyroidism in immune-related thyroid dysfunction when viewed separately. These results suggest that thyroid dysfunction may be a biological indicator for evaluating the efficacy of immunotherapy in advanced NSCLC. In our study, 8 articles explained the relationship between immunotherapy-related thyroid dysfunction and PFS (HR 0.54, 95% CI: 0.44–0.64) (Figure 3).
A total of 11 studies showed the relationship between immunotherapy-related thyroid dysfunction and OS (HR 0.34, 95% CI: 0.25–0.44) (Figure 4).
However, we did not separate any differences in OS or PFS for other irAE types in NSCLC, possibly due to the lack of patients in each group of irAEs. To our knowledge, no association has been reported between ICIs outcomes and the occurrence of pneumonia or other less frequent irAEs such as colitis, hepatitis, and other endocrine dysfunctions.
We demonstrated a statistically significant association between thyroid dysfunction and prognosis after immunotherapy for NSCLC. The occurrence of thyroid dysfunction can be used to predict future treatment response. The association between the occurrence of thyroid dysfunction after immunotherapy and the efficacy of ICIs underscores the need for better diagnosis and management of thyroid dysfunction so that ICIs can be continued for as long as possible. Our study shows that the efficacy of ICIs is significantly associated with the incidence of thyroid dysfunction. Thyroid dysfunction may occur due to the strongest T cell activation. Further prospective studies are needed to understand the underlying mechanisms and to relate the duration of efficacy to the duration or severity of thyroid dysfunction, as well as the impact of discontinuation of ICIs on response and survival in severe irAE. Further prospective trials are needed to evaluate the association between ICIs efficacy and lower frequency of irAEs (i.e., pneumonia, hepatitis, colitis, and cutaneous adverse events). Longer patient follow-up after ICI therapy is discontinued is also needed to determine whether the duration of response is longer in patients with irAEs than in nonpatients, even when treatment is discontinued.
There are some limitations to our study. First, the number of included studies was small, and there were racial disparities in the efficacy of immunotherapy. This may have influenced the results of the analysis. Second, the study was retrospective, and the reporting of adverse events may have been biased. In fact, we only conducted a “rate” meta-analysis to assist in illustrating this clinical phenomenon and viewpoint. So more randomized controlled trial data is needed to confirm this idea. Third, immunotherapy may include multidrug regimens, and adverse events may not have been irAEs. For example, some patients may have abnormal thyroid function before immunotherapy. However, even if none of the reported irAEs were adverse events caused by ICIs, irAEs may still be associated with clinical efficacy of immunotherapy plus chemotherapy because they are associated with prolonged PFS and OS.
Conclusions
Our meta-analysis shows that immunotherapy-related thyroid dysfunction may have a favorable therapeutic effect on the outcome of immunotherapy in patients with advanced NSCLC. Further large, prospective, observational studies are needed to confirm our findings.
Acknowledgments
Funding: This work was supported by the Shenyang Science and Technology Planning Project (No. 22-321-33-70).
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-254/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-254/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science 1996;271:1734-6. [Crossref] [PubMed]
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252-64. [Crossref] [PubMed]
- Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 2000;192:1027-34. [Crossref] [PubMed]
- Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2018;36:1714-68. [Crossref] [PubMed]
- Indini A, Di Guardo L, Cimminiello C, et al. Immune-related adverse events correlate with improved survival in patients undergoing anti-PD1 immunotherapy for metastatic melanoma. J Cancer Res Clin Oncol 2019;145:511-21. [Crossref] [PubMed]
- Owen DH, Wei L, Bertino EM, et al. Incidence, Risk Factors, and Effect on Survival of Immune-related Adverse Events in Patients With Non-Small-cell Lung Cancer. Clin Lung Cancer 2018;19:e893-900. [Crossref] [PubMed]
- Okada N, Kawazoe H, Takechi K, et al. Association Between Immune-Related Adverse Events and Clinical Efficacy in Patients with Melanoma Treated With Nivolumab: A Multicenter Retrospective Study. Clin Ther 2019;41:59-67. [Crossref] [PubMed]
- Verzoni E, Cartenì G, Cortesi E, et al. Real-world efficacy and safety of nivolumab in previously-treated metastatic renal cell carcinoma, and association between immune-related adverse events and survival: the Italian expanded access program. J Immunother Cancer 2019;7:99. [Crossref] [PubMed]
- Faje AT, Lawrence D, Flaherty K, et al. High-dose glucocorticoids for the treatment of ipilimumab-induced hypophysitis is associated with reduced survival in patients with melanoma. Cancer 2018;124:3706-14. [Crossref] [PubMed]
- Freeman-Keller M, Kim Y, Cronin H, et al. Nivolumab in Resected and Unresectable Metastatic Melanoma: Characteristics of Immune-Related Adverse Events and Association with Outcomes. Clin Cancer Res 2016;22:886-94. [Crossref] [PubMed]
- Haratani K, Hayashi H, Chiba Y, et al. Association of Immune-Related Adverse Events With Nivolumab Efficacy in Non-Small-Cell Lung Cancer. JAMA Oncol 2018;4:374-8. [Crossref] [PubMed]
- Kim HI, Kim M, Lee SH, et al. Development of thyroid dysfunction is associated with clinical response to PD-1 blockade treatment in patients with advanced non-small cell lung cancer. Oncoimmunology 2017;7:e1375642. [Crossref] [PubMed]
- Osorio JC, Ni A, Chaft JE, et al. Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann Oncol 2017;28:583-9. [Crossref] [PubMed]
- Yamauchi I, Yasoda A, Matsumoto S, et al. Incidence, features, and prognosis of immune-related adverse events involving the thyroid gland induced by nivolumab. PLoS One 2019;14:e0216954. [Crossref] [PubMed]
- Grangeon M, Tomasini P, Chaleat S, et al. Association Between Immune-related Adverse Events and Efficacy of Immune Checkpoint Inhibitors in Non-small-cell Lung Cancer. Clin Lung Cancer 2019;20:201-7. [Crossref] [PubMed]
- Ricciuti B, Genova C, De Giglio A, et al. Impact of immune-related adverse events on survival in patients with advanced non-small cell lung cancer treated with nivolumab: long-term outcomes from a multi-institutional analysis. J Cancer Res Clin Oncol 2019;145:479-85. [Crossref] [PubMed]
- Lei M, Michael A, Patel S, et al. Evaluation of the impact of thyroiditis development in patients receiving immunotherapy with programmed cell death-1 inhibitors. J Oncol Pharm Pract 2019;25:1402-11. [Crossref] [PubMed]
- Ishihara H, Takagi T, Kondo T, et al. Association between immune-related adverse events and prognosis in patients with metastatic renal cell carcinoma treated with nivolumab. Urol Oncol 2019;37:355.e21-9. [Crossref] [PubMed]
- Toi Y, Sugawara S, Sugisaka J, et al. Profiling Preexisting Antibodies in Patients Treated With Anti-PD-1 Therapy for Advanced Non-Small Cell Lung Cancer. JAMA Oncol 2019;5:376-83. [Crossref] [PubMed]
- Cortellini A, Chiari R, Ricciuti B, et al. Correlations Between the Immune-related Adverse Events Spectrum and Efficacy of Anti-PD1 Immunotherapy in NSCLC Patients. Clin Lung Cancer 2019;20:237-247.e1. [Crossref] [PubMed]
- Berner F, Bomze D, Diem S, et al. Association of Checkpoint Inhibitor-Induced Toxic Effects With Shared Cancer and Tissue Antigens in Non-Small Cell Lung Cancer. JAMA Oncol 2019;5:1043-7. [Crossref] [PubMed]
- Ahn BC, Pyo KH, Xin CF, et al. Comprehensive analysis of the characteristics and treatment outcomes of patients with non-small cell lung cancer treated with anti-PD-1 therapy in real-world practice. J Cancer Res Clin Oncol 2019;145:1613-23. [Crossref] [PubMed]
- Nakamura Y, Tanaka R, Asami Y, et al. Correlation between vitiligo occurrence and clinical benefit in advanced melanoma patients treated with nivolumab: A multi-institutional retrospective study. J Dermatol 2017;44:117-22. [Crossref] [PubMed]
- Judd J, Zibelman M, Handorf E, et al. Immune-Related Adverse Events as a Biomarker in Non-Melanoma Patients Treated with Programmed Cell Death 1 Inhibitors. Oncologist 2017;22:1232-7. [Crossref] [PubMed]
- Ksienski D, Wai ES, Croteau N, et al. Efficacy of Nivolumab and Pembrolizumab in Patients With Advanced Non-Small-Cell Lung Cancer Needing Treatment Interruption Because of Adverse Events: A Retrospective Multicenter Analysis. Clin Lung Cancer 2019;20:e97-e106. [Crossref] [PubMed]
- Rogado J, Sánchez-Torres JM, Romero-Laorden N, et al. Immune-related adverse events predict the therapeutic efficacy of anti-PD-1 antibodies in cancer patients. Eur J Cancer 2019;109:21-7. [Crossref] [PubMed]
- Lesueur P, Escande A, Thariat J, et al. Safety of combined PD-1 pathway inhibition and radiation therapy for non-small-cell lung cancer: A multicentric retrospective study from the GFPC. Cancer Med 2018;7:5505-13. [Crossref] [PubMed]
- Lisberg A, Tucker DA, Goldman JW, et al. Treatment-Related Adverse Events Predict Improved Clinical Outcome in NSCLC Patients on KEYNOTE-001 at a Single Center. Cancer Immunol Res 2018;6:288-94. [Crossref] [PubMed]
- Bjørnhart B, Hansen KH, Jørgensen TL, et al. Efficacy and safety of immune checkpoint inhibitors in a Danish real life non-small cell lung cancer population: a retrospective cohort study. Acta Oncol 2019;58:953-61. [Crossref] [PubMed]
- Lang N, Dick J, Slynko A, et al. Clinical significance of signs of autoimmune colitis in 18F-fluorodeoxyglucose positron emission tomography-computed tomography of 100 stage-IV melanoma patients. Immunotherapy 2019;11:667-76. [Crossref] [PubMed]
- Fujimoto D, Yoshioka H, Kataoka Y, et al. Efficacy and safety of nivolumab in previously treated patients with non-small cell lung cancer: A multicenter retrospective cohort study. Lung Cancer 2018;119:14-20. [Crossref] [PubMed]
- Sato K, Akamatsu H, Murakami E, et al. Correlation between immune-related adverse events and efficacy in non-small cell lung cancer treated with nivolumab. Lung Cancer 2018;115:71-4. [Crossref] [PubMed]
- Sanlorenzo M, Vujic I, Daud A, et al. Pembrolizumab Cutaneous Adverse Events and Their Association With Disease Progression. JAMA Dermatol 2015;151:1206-12. [Crossref] [PubMed]
- de Moel EC, Rozeman EA, Kapiteijn EH, et al. Autoantibody Development under Treatment with Immune-Checkpoint Inhibitors. Cancer Immunol Res 2019;7:6-11. [Crossref] [PubMed]
- Weber JS, Hodi FS, Wolchok JD, et al. Safety Profile of Nivolumab Monotherapy: A Pooled Analysis of Patients With Advanced Melanoma. J Clin Oncol 2017;35:785-92. [Crossref] [PubMed]
- Horvat TZ, Adel NG, Dang TO, et al. Immune-Related Adverse Events, Need for Systemic Immunosuppression, and Effects on Survival and Time to Treatment Failure in Patients With Melanoma Treated With Ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol 2015;33:3193-8. [Crossref] [PubMed]
- Ouwerkerk W, van den Berg M, van der Niet S, et al. Biomarkers, measured during therapy, for response of melanoma patients to immune checkpoint inhibitors: a systematic review. Melanoma Res 2019;29:453-64. [Crossref] [PubMed]
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998;17:2815-34. [Crossref] [PubMed]
- Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev 1987;9:1-30. [Crossref] [PubMed]
- van Houwelingen HC, Arends LR, Stijnen T. Advanced methods in meta-analysis: multivariate approach and meta-regression. Stat Med 2002;21:589-624. [Crossref] [PubMed]
- Peiró I, Palmero R, Iglesias P, et al. Thyroid dysfunction induced by nivolumab: searching for disease patterns and outcomes. Endocrine 2019;64:605-13. [Crossref] [PubMed]
- Luo J, Martucci VL, Quandt Z, et al. Immunotherapy-Mediated Thyroid Dysfunction: Genetic Risk and Impact on Outcomes with PD-1 Blockade in Non-Small Cell Lung Cancer. Clin Cancer Res 2021;27:5131-40. [Crossref] [PubMed]
- Morimoto K, Yamada T, Takumi C, et al. Immune-Related Adverse Events Are Associated With Clinical Benefit in Patients With Non-Small-Cell Lung Cancer Treated With Immunotherapy Plus Chemotherapy: A Retrospective Study. Front Oncol 2021;11:630136. [Crossref] [PubMed]
- Campredon P, Mouly C, Lusque A, et al. Incidence of thyroid dysfunctions during treatment with nivolumab for non-small cell lung cancer: Retrospective study of 105 patients. Presse Med 2019;48:e199-207. [Crossref] [PubMed]
- Thuillier P, Joly C, Alavi Z, et al. Thyroid dysfunction induced by immune checkpoint inhibitors is associated with a better progression-free survival and overall survival in non-small cell lung cancer: an original cohort study. Cancer Immunol Immunother 2021;70:2023-33. [Crossref] [PubMed]
- Sakakida T, Ishikawa T, Uchino J, et al. Clinical features of immune-related thyroid dysfunction and its association with outcomes in patients with advanced malignancies treated by PD-1 blockade. Oncol Lett 2019;18:2140-7. [Crossref] [PubMed]
- Zhou Y, Xia R, Xiao H, et al. Thyroid function abnormality induced by PD-1 inhibitors have a positive impact on survival in patients with non-small cell lung cancer. Int Immunopharmacol 2021;91:107296. [Crossref] [PubMed]
- Lee H, Hodi FS, Giobbie-Hurder A, et al. Characterization of Thyroid Disorders in Patients Receiving Immune Checkpoint Inhibition Therapy. Cancer Immunol Res 2017;5:1133-40. [Crossref] [PubMed]
- Quandt Z, Young A, Perdigoto AL, et al. Autoimmune Endocrinopathies: An Emerging Complication of Immune Checkpoint Inhibitors. Annu Rev Med 2021;72:313-30. [Crossref] [PubMed]
- Robert C, Joshua AM, Kefford R, et al. Association of immune-related thyroid disorders with pembrolizumab (pembro, MK-3475) in patients (pts) with advanced melanoma treated in KEYNOTE-001. J Clin Orthod. American Society of Clinical Oncology 2015;33:9050-9050.
- Barroso-Sousa R, Barry WT, Garrido-Castro AC, et al. Incidence of Endocrine Dysfunction Following the Use of Different Immune Checkpoint Inhibitor Regimens: A Systematic Review and Meta-analysis. JAMA Oncol 2018;4:173-82. [Crossref] [PubMed]
- Zhou X, Yao Z, Yang H, et al. Are immune-related adverse events associated with the efficacy of immune checkpoint inhibitors in patients with cancer? A systematic review and meta-analysis. BMC Med 2020;18:87. [Crossref] [PubMed]
- Naqash AR, Ricciuti B, Owen DH, et al. Outcomes associated with immune-related adverse events in metastatic non-small cell lung cancer treated with nivolumab: a pooled exploratory analysis from a global cohort. Cancer Immunol Immunother 2020;69:1177-87. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)