Honeycomb lung is a major risk factor for preoperative radiological tumor size underestimation in patients with primary lung cancer
Highlight box
Key findings
• Honeycomb lung adjacent to lung cancer frequently causes radiological tumor size underestimation.
What is known and what is new?
• Risk of preoperative underestimation of tumor size in patients with lung cancer complicated with idiopathic interstitial pneumonias was reported to be high.
• This manuscript shows honeycomb lung adjacent to the tumor is a major risk factor for preoperative tumor size underestimation.
What is the implication, and what should change now?
• A suitable resection method should be selected to ensure a curative operation for lung cancer adjacent to honeycomb lung. Meanwhile, more accurate diagnostic tools to determine the extension of lung cancer into the adjacent honeycomb lung should be developed.
Introduction
The incidence of lung cancer in the patients with idiopathic interstitial pneumonias (IIPs) is reportedly as high as 15% (1). IIPs, especially idiopathic pulmonary fibrosis (IPF), are characterized by honeycombing resulting from alveolar fibrosis (2). Sakai et al. showed that lung cancer in the honeycomb lung progressed along with reticulated fibrotic lung tissue and collapsed alveoli, so the border between the tumor and fibrotic lung tissue was unclear radiologically (3). Therefore, it is difficult to estimate tumor extension adjacent to fibrotic lung tissue through computed tomography (CT) images (4). Fukui et al. showed the risk of preoperative underestimation of tumor size in patients with IIPs was high (5).
Patients with IIPs may sometimes have limited respiratory function, often resulting in surgeons selecting a limited resection such as sub-lobar resection to treat lung cancer complicated by IIPs (6,7). In addition, postoperative acute exacerbation (AE) of IIPs is a life-threatening complication (6). Sato et al. advocated for a risk-scoring system to predict AEs of IIPs after pulmonary resection for lung cancer, in which surgical procedures, other than wedge resection, were one of the major predictors for AE (8). They suggested that a limited resection was recommended for high-risk patients to reduce the risk of AE (8). However, the possible preoperative underestimation of tumor size could result in incomplete tumor resection (5).
The lung fields of the patients with IIPs show not only honeycombing but also reticulation and emphysema; they may even be normal (9). It is unclear which findings of the lung field adjacent to cancer segments may affect tumor size assessment. Therefore, this study aimed to investigate whether findings of lung fields adjacent to cancer segments, including honeycombing, could cause underestimation of tumor size on CT images when compared to the pathological specimen. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1115/rc).
Methods
Study design
This retrospective observational study was conducted in accordance with the ethical standards of the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Review Board of Fujita Health University (CI21-392). The patients viewed an online version of the consent document and were enrolled in the study using the opt-out method.
Patient population
We investigated 896 patients with primary lung cancer who underwent lung resection between January 2015 and June 2020 in the Department of Thoracic Surgery, Fujita Health University Hospital, Japan. This study size was reasonable considering frequency of surgical intervention for patients who have honeycomb lung in our institution. Patients who received preoperative chemotherapy and/or radiotherapy were excluded. Patients who had radiological findings other than normal, emphysematous, reticulated, or honeycombing areas adjacent to the tumor were excluded.
Definition of preoperative underestimate of tumor size
We defined it as the underestimation of ≥10 mm in pathological maximum tumor dimension compared with radiological one.
Preoperative radiological assessment and background lung assessment
Two board certified chest radiologists with more than 20 years experiences reviewed all CT images and classified the background lung according to eight patterns; normal lung, bronchiectasis, consolidation, emphysema, ground-glass opacity, honeycombing, nodular lesion, reticulation based on the glossary of Flesichner Society (10). Preoperative tumor size was determined based on radiological reports from at least two radiologists. The largest diameters were measured on 0.5–7.0-mm CT images at axial plane with or without 0.5–5.0-mm multiplanar reconstruction (MPR) images at coronal or sagittal planes with lung window setting (width, 1,600 HU; level, −600 HU). Tumors close to the chest wall and mediastinum were also measured at a mediastinal window setting (width, 300 HU; level, 30 HU). These measurements were also checked in our thoracic surgeon conferences. We restaged the cases after 2018 according to the 7th Edition of TNM in Lung Cancer of International Association for Study of Lung Cancer (IASLC).
Pathological assessment
Ten percent buffered formalin was injected into wedge resection specimens using needle, or into segmentectomy, lobectomy, and pneumonectomy specimens from the bronchial stump soon after excision. After inflation of the lung, the specimen was soaked in 10% buffered formalin. All specimens were evaluated by three pathologists. Pathological tumor size was evaluated with hematoxylin and eosin staining (H&E) microscopically. We reviewed all pathological reports and restaged the cases after 2018 according to the 7th Edition of TNM in IASLC.
Statistical analysis
We divided the patients by each radiological finding and the presence or absence of underestimated tumor size. The difference between these groups was compared using chi-square tests, student t-tests, Bonferroni correction, Spearman’s rank correlation coefficient, and logistic regression analysis. All P values were two-sided, and P values of 0.05 or less were considered statically significant. A Bland-Altman plot (11) was constructed with Excel for Mac version 16.62 (Microsoft Corporation, Redmond, WA, USA). All other statistical analyses were performed with EZR (12) (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (version 4.0.0, The R Foundation for Statistical Computing, Vienna, Austria). More precisely, it is a modified version of R commander (version 2.6-2) designed to add statistical functions frequently used in biostatistics.
Results
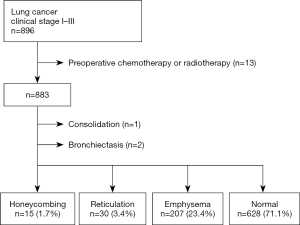
Overall, 883 patients were classified into four groups according to radiological findings of the lung field adjacent to lung cancer on preoperative chest CT images (honeycombing, n=15, 1.7%; reticulation, n=30, 3.4%; emphysema, n=207, 23.4%; and normal, n=628, 71.1%) (Figure 1). Patients with consolidation (n=1) or bronchiectasis (n=2) in the lung field adjacent to lung cancer were excluded from the subjects. The clinicopathological characteristics of the patients in the four groups are shown in Table 1.
Table 1
| Variables | Honeycombing (n=15) | Reticulation (n=30) | Emphysema (n=207) | Normal (n=628) |
|---|---|---|---|---|
| Age (years) | 74.8±5.4 | 72.3±4.7 | 70.0±8.0 | 68.6±9.9 |
| Sex | ||||
| Female | 3 (20.0%) | 8 (26.7%) | 22 (10.6%) | 309 (49.2%) |
| Male | 12 (80.0%) | 22 (73.3%) | 185 (89.4%) | 319 (50.8%) |
| Smoking history, pack-year | ||||
| <30 | 2 (13.3%) | 14 (46.7%) | 26 (12.6%) | 418 (67.3%) |
| ≥30 | 13 (86.7%) | 16 (53.3%) | 180 (87.4%) | 203 (32.7%) |
| Tumor location | ||||
| Upper or middle lobe | 6 (40.0%) | 6 (20.0%) | 153 (73.9%) | 398 (63.4%) |
| Lower lobe | 9 (60.0%) | 24 (80.0%) | 54 (26.1%) | 230 (36.6%) |
| Imaging plane | ||||
| Axial plane | 5 (33.3%) | 4 (13.3%) | 31 (15.0%) | 84 (13.4%) |
| MPRa | 10 (66.7%) | 26 (86.7%) | 176 (85.0%) | 544 (86.6%) |
| Most fine slice width of CT images (mm) | 1.1±1.1 | 1.9±1.7 | 1.2±1.1 | 1.2±1.1 |
| Surgical procedure | ||||
| Wedge resection | 8 (53.3%) | 5 (16.7%) | 22 (10.6%) | 62(9.9%) |
| Segmentectomy | 1 (6.7%) | 0 | 5 (2.4%) | 14 (2.2%) |
| Lobectomy | 6 (40.0%) | 24 (80.0) | 174 (84.1%) | 545 (86.8%) |
| Pneumonectomy | 0 | 1 (3.3%) | 6 (2.9%) | 7 (1.1%) |
| Interval between CT and surgery (days) | 40.5±35.7 | 32.0±19.1 | 36.2±28.5 | 37.6±31.7 |
| Radiological maximum tumor size (mm) | 20.5±6.1 | 31.1±18.7 | 29.7±16.1 | 24.1±14.1 |
| Pathological maximum tumor size (mm) | 35.3±28.0 | 29.4±14.9 | 28.6±16.7 | 22.3±13.8 |
| Histological type | ||||
| Adenocarcinoma | 9 (60.0%) | 15 (60.0%) | 120 (62.2%) | 545 (89.3%) |
| Squamous cell carcinoma | 6 (40.0%) | 10 (40.0%) | 73 (37.8%) | 65 (10.7%) |
| Lymphovascular invasion | ||||
| Positive | 7 (46.7%) | 15 (50.0%) | 73 (35.3%) | 137 (21.8%) |
| Vascular invasion | ||||
| Positive | 7 (46.7%) | 16 (53.3%) | 89 (43.0%) | 176 (28.0%) |
| Pleural invasion | ||||
| Positive | 6 (40.0%) | 9 (30.0%) | 51 (24.6%) | 78 (12.4%) |
| Histological grade | ||||
| 1 | 2 (13.3%) | 2 (6.7%) | 21 (10.1%) | 175 (27.9%) |
| 2 | 8 (53.3%) | 22 (73.3%) | 132 (63.8%) | 395 (62.9%) |
| 3 | 4 (26.7%) | 5 (16.7%) | 42 (20.3%) | 49 (7.8%) |
| 4 | 1 (6.7%) | 1 (3.3%) | 12 (5.8%) | 9 (1.4%) |
| %VC | 101.6±17.6 | 107.6±21.3 | 116.2±76.0 | 112.2±18.4 |
| FEV1/FVC (%) | 77.6±8.4 | 72.7±14.0 | 66.3±12.7 | 74.2±12.6 |
| %DLCO | 77.3±20.0 | 87.1±27.7 | 87.2±23.8 | 107.0±24.4 |
| Underestimation of tumor sizeb (mm) | 14.7±28.3 | −1.7±9.3 | −1.1±10.0 | −1.7±9.7 |
Values are shown as number of patients (percentage) or mean ± SD. a, axial, coronal or sagittal plane on multiplanar reconstruction; b, pathological maximum tumor size minus radiological maximum tumor size on preoperative CT images. SD, standard deviation; CT, computed tomography; %VC, %vital capacity; FEV1, forced expiratory volume in 1 second; FVC, forced expiratory volume; %DLCO, %diffusing capacity for carbon monoxide.
Radiological findings adjacent to lung cancer and tumor size underestimation
Preoperative radiological tumor size underestimation of 10 mm or more occurred in 5 patients (33.3%) in the honeycombing group, whereas in 2 (6.7%), 15 (7.2%), and 47 patients (7.5%) in the reticulation, emphysema, and normal lung groups respectively (P=0.003) (Table 2). Comparison between the honeycombing group and the group without honeycombing (including the reticulation, emphysema, and normal lung groups) revealed that tumor size underestimation was significantly more frequent in the patients with honeycomb lung adjacent to the tumor (5 out of 15, 33.3%) than in those without honeycombing (64 out of 865, 7.4%) (P=0.004) (Table 3).
Table 2
| Variables | Honeycombing (n=15) | Reticulation (n=30) | Emphysema (n=207) | Normal (n=628) | P value |
|---|---|---|---|---|---|
| Underestimateda | 5 (33.3%) | 2 (6.7%) | 15 (7.2%) | 47 (7.5%) | 0.003 |
| Not underestimated | 10 (66.7%) | 28 (93.3%) | 192 (92.8%) | 581 (92.5%) |
a, defined as 10 mm or more in pathological maximum tumor size compared to radiological maximum tumor size on preoperative CT images. CT, computed tomography.
Table 3
| Variables | Honeycombing (n=15) | Without honeycombing (n=865) | P value |
|---|---|---|---|
| Underestimateda | 5 (33.3%) | 64 (7.4%) | 0.004 |
| Not underestimated | 10 (66.7%) | 801 (92.6%) |
a, defined as 10 mm or more in pathological maximum tumor size compared to radiological maximum tumor size on preoperative CT images. CT, computed tomography.
Risk factors for tumor size underestimation
The results of univariate analysis for the characteristics of the patients with or without tumor size underestimation are shown in Table 4. Age, sex, smoking history, tumor location, imaging plane (only axial or axial, coronal or sagittal plane on MPR images), most fine slice width of CT images, surgical procedure (wedge resection or anatomical resection), interval between CT and surgery, histological type of the tumor (adenocarcinoma or squamous cell carcinoma), pathological lymph-vascular invasion, vascular invasion, histological grade, and preoperative pulmonary function [%vital capacity (%VC), forced expiratory volume in 1 second (FEV1)/forced expiratory volume (FVC), and %diffusing capacity for carbon monoxide (%DLCO)] were not significantly different between the patients with and without tumor size underestimation. Honeycombing adjacent to the tumor on preoperative CT images was substantially more frequent in the patients with tumor size underestimation than in those without underestimation (P=0.004). The patients with tumor size underestimation had significantly larger radiological (P=0.0013) and pathological (P<0.001) tumor sizes than did those without tumor size underestimation. Pathological pleural invasion of the tumor tended to be observed more frequently in patients with tumor size underestimation than in those without underestimation.
Table 4
| Variables | Underestimationa (n=69) | Without underestimation (n=811) | P value |
|---|---|---|---|
| Age | 70 [49, 86] | 71 [27, 91] | 0.681 |
| Sex | 0.521 | ||
| Female | 24 (34.8%) | 318 (39.2%) | |
| Male | 45 (65.2%) | 493 (60.8%) | |
| Smoking history, pack-year | 0.131 | ||
| <30 | 30 (43.5%) | 430 (53.5%) | |
| ≥30 | 39 (56.5%) | 373 (46.5%) | |
| Tumor location | 0.434 | ||
| Upper or middle lobe | 41 (59.4%) | 522 (64.4%) | |
| Lower lobe | 28 (40.6%) | 289 (35.6%) | |
| Radiological findingsb | 0.004 | ||
| Honeycombing | 5 (7.2%) | 10 (1.2%) | |
| Without honeycombing | 64 (92.8%) | 801 (98.8%) | |
| Imaging plane | 0.469 | ||
| Axial plane | 117 (14.4%) | 7 (10.1%) | |
| MPRc | 694 (85.6%) | 62 (89.9%) | |
| Most fine slice width of CT images (mm) | 1.14 [0.50, 5.00] | 1.22 [0.30, 7.00] | 0.554 |
| Surgical procedure | 0.421 | ||
| Wedge resection | 5 (7.2%) | 91 (11.2%) | |
| Anatomical resectiond | 64 (92.8%) | 720 (88.8%) | |
| Interval between CT and surgery (days) | 36 [1, 167] | 27 [0, 37] | 0.226 |
| Radiological maximum tumor size (mm) | 25.0 [11.0, 84.0] | 21.0 [5.0, 111.0] | 0.013 |
| Pathological maximum tumor size (mm) | 41.0 [25.0, 120.0] | 20.0 [1.0, 80.0] | <0.001 |
| Histological type of lung cancer | 0.621 | ||
| Adenocarcinoma | 53 (79.1%) | 636 (82.0%) | |
| Squamous cell carcinoma | 14 (20.9%) | 140 (18.0%) | |
| Lymphovascular invasion | |||
| Positive | 20 (29.0%) | 212 (26.1%) | 0.669 |
| Vascular invasion | |||
| Positive | 25 (36.2%) | 263 (32.4%) | 0.507 |
| Pleural invasion | |||
| Positive | 17 (24.6%) | 127 (15.7%) | 0.062 |
| Histological grade | 0.141 | ||
| 1 | 9 (13.0%) | 191 (23.6%) | |
| 2 | 49 (71.0%) | 508 (62.6%) | |
| 3 | 8 (11.6%) | 92 (11.3%) | |
| 4 | 3 (4.3%) | 20 (2.5%) | |
| %VC | 110.4 [76.6, 158.1] | 111.4 [55.3, 175.0] | 0.141 |
| FEV1/FVC (%) | 72.9 [40.3, 129.3] | 72.4 [29.7, 134.7] | 0.419 |
| %DLCO | 101.1 [53.5, 198.3] | 100.6 [39.1, 199.10] | 0.953 |
| Underestimation of tumor sizee (mm) | 14.0 [10.0, 99.0] | −2.0 [−83.0, 9.0] | <0.001 |
Values are shown as number of patients (percentage) or median value [minimum value, maximum value]. a, defined as 10 mm or more in pathological maximum tumor size compared to radiological maximum tumor size on preoperative CT images; b, radiological findings of the lung field adjacent to the tumor on preoperative CT images; c, axial, coronal or sagittal plane on multiplanar reconstruction; d, segmentectomy, lobectomy, or pneumonectomy; e, pathological maximum tumor size minus radiological maximum tumor size on preoperative CT images. %VC, %vital capacity; FEV1, forced expiratory volume in 1 second; FVC, forced expiratory volume; %DLCO, %diffusing capacity for carbon monoxide. CT, computed tomography.
Multivariate analysis using the logistic regression model revealed that honeycombing adjacent to the tumor was a statistically significant predictor for tumor size underestimation (odds ratio, 8.58; 95% CI: 2.49–29.60; P=0.000659) (Table 5).
Table 5
| Variables | Odds ratio | 95% CI | P value |
|---|---|---|---|
| Honeycombing adjacent to the tumor | 8.58 | 2.49–29.60 | 0.000659 |
| Surgical procedure (wedge resection) | 0.47 | 0.16–1.35 | 0.158 |
| Radiological maximum tumor size | 1.07 | 0.91–1.26 | 0.419 |
| Tumor location (lower lobe) | 1.19 | 0.71–1.99 | 0.505 |
| Pathological pleural invasion, positive | 1.54 | 0.83–2.86 | 0.168 |
| Most fine slice width of CT images | 0.99 | 0.72–1.36 | 0.930 |
| Imaging plane (MPR) | 1.46 | 0.52–4.15 | 0.475 |
CI, confidence interval; CT, computed tomography; MPR, multiplanar reconstruction.
Pathological causes of tumor size underestimation in the honeycombing group
Two representative cases with honeycombing in the lung field adjacent to the tumor, in whom underestimation of tumor size occurred, are shown in Figure 2. Histopathological analysis of the first case revealed that adenocarcinoma infiltrated along the surface of the dilated airway in the honeycomb lung (Figure 2B,2C). The radiological maximum tumor size assessed by sagittal plane on the preoperative MPR images was 21 mm (Figure 2A), whereas pathological examination showed the actual tumor size was 120 mm. In histology of the second case, adenocarcinoma extended to fibrotic lung tissue adjacent to honeycomb lung (Figure 2E,2F). The radiological maximum tumor size assessed by coronal plane on the preoperative MPR images was 23 mm (Figure 2D), whereas the actual pathological tumor size was 60 mm. The pathological causes for tumor size underestimation in the five cases with honeycombing adjacent to the tumor were tumor infiltration to honeycomb lung in 2 patients, including in Figure 2A-2C, tumor extension to fibrotic lung tissue adjacent to honeycomb lung in 2 cases, including in Figure 2D-2F, and tumor invasion into a subpleural cyst in 1 case (Table 6).
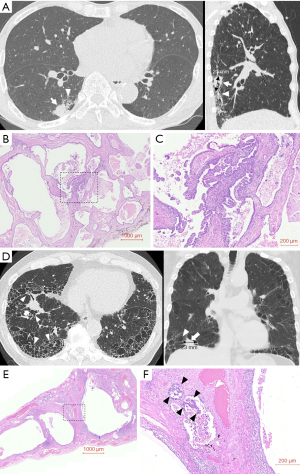
Table 6
| Age (years) | Sex | Surgical procedure | Radiological size (mm)a | Pathological size (mm)b | Histological type of lung cancer | Pathological cause for tumor size underestimation |
|---|---|---|---|---|---|---|
| 78 | Male | RLLd | 21 | 120 | Adenocarcinoma | Infiltration to honeycomb lung |
| 69 | Male | Wedgec | 11 | 65 | Adenocarcinoma | Infiltration to honeycomb lung |
| 69 | Male | RLLd | 23 | 60 | Adenocarcinoma | Infiltration to fibrotic lung tissue adjacent to honeycomb lung |
| 81 | Female | LLLe | 19 | 30 | Adenocarcinoma | Infiltration to fibrotic lung tissue adjacent to honeycomb lung |
| 79 | Male | RULf | 13 | 26 | Adenocarcinoma | Invasion to subpleural cyst |
a, radiological maximum tumor size on preoperative CT images; b, pathological maximum tumor size; c, wedge resection; d, right lower lobectomy; e, left lower lobectomy; f, right upper lobectomy. CT, computed tomography.
Spearman’s correlation analysis and Bland-Altman analysis of pathological and radiological maximum tumor sizes
Next, we created a scatter diagram for the radiological maximum tumor size on the preoperative CT images and the pathological tumor size in the patient groups with (Figure 3A) and without (Figure 3B) honeycombing adjacent to the tumor. The correlation between the radiological and pathological maximum tumor sizes in the patient group without honeycombing was satisfactory (Spearman’s correlation rho =0.831, P<0.0001), but not that with honeycombing (Spearman’s correlation rho =0.294, P=0.287).
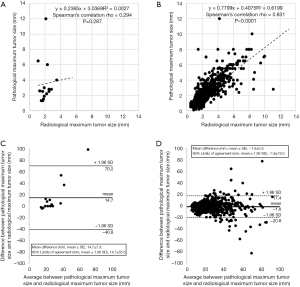
The mean difference and 95% limits of agreement between the pathological and radiological maximum tumor sizes in two groups of patients with (Figure 3C) and without (Figure 3D) honeycombing were determined using Bland-Altman analysis. The mean difference between the pathological and radiological maximum tumor sizes in the group with honeycombing (14.7±7.3 mm) was significantly more when compared with that in the group without honeycombing (−1.6±0.3 mm) (P=0.0013). The 95% limits of agreement between the pathological and radiological maximum tumor sizes were 14.7±55.5 (−40.8 to 70.2) mm in the group with honeycombing, whereas −1.6±19.0 (−20.6 to 17.4) mm in the group without honeycombing.
Discussion
The principal findings of this study are as follows: (I) preoperative radiological tumor size underestimation of 10 mm or more on CT images occurred significantly more frequently in the patients with honeycomb lung adjacent to the tumor than in those without honeycombing (i.e., with reticulation, emphysema, or normal lung); (II) multivariate analysis revealed that honeycombing adjacent to the tumor was a statistically significant predictor for preoperative radiological tumor size underestimation; (III) the pathological causes for tumor size underestimation was mainly due to tumor infiltration to honeycomb lung or fibrotic lung tissue adjacent to honeycomb lung, and (IV) Bland-Altman analysis determined the 95% limits of agreement between the pathological and radiological maximum tumor sizes were 14.7±55.5 (−40.8 to 70.2) mm in the patients with honeycombing adjacent to the tumor.
Patients with IPF have a greater risk for lung cancer development (13,14), with relative risks of 7.31–14.1 than the general population, even after accounting for common risk factors, such as older age and smoking history (1). Limited resection is sometimes selected for the patients with lung cancer complicated with IIPs due to the poor pulmonary function or high risk of AE of IIPs post-surgery (8). In sub-lobar surgery, such as partial resection or segmental resection, we need to focus on accurate assessment of tumor extension and ensuring adequate surgical margins without residual cancer are essential.
Usual interstitial pneumonia is the hallmark radiologic and histopathological pattern of IPF (9). The diagnostic criterion of usual interstitial pneumonia is a low magnification appearance of patchy dense fibrosis that: (I) causes remodeling of lung architecture; (II) often results in honeycomb change; and (III) alternates with areas of less-affected parenchyma (9). Honeycombing refers to clustered cystic airspaces of typically consistent diameter with thick, well-defined walls, and is usually accompanied by a reticular pattern (10). It has been shown that lung cancer in honeycombed lungs progressed along with reticulated fibrotic lung tissue and collapsed alveoli, until the border between the tumor and fibrotic lung tissue was unclear radiologically (3). Moreover, many patients with IPF also have other comorbid conditions, including emphysema (15), which can cause tumor size underestimation on radiological measurements when compared with pathological ones (16). Therefore, in this study, two radiologists classified the lung background on CT images into four patterns; normal, emphysematous, reticulated, or honeycombing. Subsequently, the ratio of preoperative radiological tumor size underestimation in each group was investigated. The normal, emphysema, and reticulation groups had almost the same low incidence of the tumor size underestimation (7.5%, 7.2%, and 6.7%, respectively). Fukui et al. reported that maximum tumor dimension was underestimated (defined as 10 mm or more in pathological tumor dimension compared with radiological ones by preoperative computed axial tomography) in 3.2% of patients without IIPs (5). Park et al. compared maximal tumor diameters between fresh pathology specimens and CT images in lung adenocarcinoma and found that postoperative up-staging occurred in 12.3% and 1.4% of tumors on performing radiological staging using axial and multiplanar reformatted CT images (17). In contrast, the honeycombing group in this study had a significantly higher ratio of the tumor size underestimation (33.3%) than did the other groups. Univariate analysis showed that the radiological and pathological maximum tumor sizes were predictive factors for preoperative tumor size underestimation in addition to honeycombing adjacent to the tumor; similar to findings in the study regarding pulmonary emphysema and tumor size underestimation (16). However, multivariate analysis clearly showed that honeycombing adjacent to the tumor was the only statistically significant predictor for the tumor size underestimation.
Sakai et al. reviewed the CT scans and pathologic specimens of 57 lung cancer cases in 47 patients with diffuse pulmonary fibrosis for the first time and found that seven tumors invaded the adjacent honeycomb lung histologically and lacked distinct margins on CT images (3). In our study, five cases had tumors that were contiguous with the honeycombed lung and were radiologically underestimated preoperatively; four of these showed tumor infiltration to honeycomb lung or to fibrotic lung tissue adjacent to honeycombed lung on histopathology.
Meanwhile, to discuss causes of the radiological tumor size underestimation in cases of the normal lung group, we divided 628 subjects with normal parenchyma adjacent to the tumor into ones with (n=47) and without (n=581) tumor size underestimation and performed univariate analysis of several risk factors. Pleural invasion of the tumor was significantly more frequent in the patients with tumor size underestimation [12/47 (25.5%)] than in those without underestimation [66/581 (11.4%)] (P=0.01). We examined histopathology of several cases with pleural invasion of the tumor and found that pleural invasion itself did not lead to cause an underestimation of tumor size. The tumors with pleural invasion in the patients with normal parenchyma (n=78) showed significantly larger radiological and pathological maximum tumor sizes than those without pleural invasion (n=550) (radiological, 32.4±15.8 versus 22.9±13.4 mm, P<0.001; pathological, 30.9±15.1 versus 21.1±13.1 mm, P<0.001). At the same time, the patients with tumor size underestimation had significantly larger radiological and pathological tumor sizes than did those without tumor size underestimation (radiological, 29.9±14.2 versus 23.6±13.9 mm, P<0.001; pathological, 47.4±16.4 versus 20.3±11.3 mm, P<0.001). It has been reported that larger tumor size was a risk for radiological tumor size underestimation probably due to difficulties in obtaining the largest cross section of tumors on the CT images (16). Moreover, in other underestimated cases, tumors sometimes extended into narrow lung parenchyma parallel to relatively large sized broncho-pulmonary arterial bundle or into lymphatic vessels along interlobular septum or beneath the pleura, which were not usually visualized on the CT images.
Bland-Altman analysis revealed that the 95% limits of agreement between the pathological and radiological maximum tumor sizes in the patients with honeycombing were 14.7±55.5 mm, i.e., −40.8 to 70.2 mm, indicating that, in 95% of patients with honeycombing adjacent to lung cancer, preoperative radiological underestimation of tumor size can be nearly 70.2 mm. When sub-lobar resection is selected, circumferential margin of at least 35 mm from the tumor should be secured.
Our investigation showed the large difference between lung cancer extent on histopathology and on CT images; hence, it is not favorable to perform limited surgery for tumors adjacent to honeycombed lungs. Although formalin fixation could affect the tumor size in histopathology (18), this report is the first to show that honeycombing adjacent to the tumor is the major risk of preoperative tumor size underestimation by preoperative examination on CT images. Radiological tumor size underestimation is also important in patients who do not undergo surgery since it could affect the prediction of the patients’ prognosis. In the future, we need more accurate diagnostic tools to determine the extension of lung cancer into the adjacent honeycombed lung. A possible candidate for this is the recently advanced dynamic contrast-enhanced MRI with three-dimensional gradient echo (GRE) and ultrashort echo time (UTE) (19,20).
There are some limitations to this study. First, this was a retrospective study conducted at a single institution. Second, the sample size for patients with honeycombing was small. A larger sample size would make the association between the tumor size underestimation and honeycombing more robust. Third, we measured preoperative tumor size on different-sized slices of CT images; mainly on 0.5 mm slices of a CT image, but in some cases, on a 5.0–7.0 mm slices. To discuss a little more about this issue, we divided the subjects into two groups of patients whose tumors were assessed by imaging planes with slice width ≤2 mm (n=786) and ≥5 mm (n=55) and assessed the incidence of preoperative radiological tumor size underestimation in each group. As a result, the incidences of tumor size underestimation in two groups were comparable [≤2 versus ≥5 mm, 64/786 (8.1%) versus 3/55 (5.5%), no statistically significant difference]. However, there is still a possibility of measurement error of the tumor size because of reconstruction thickness. In addition to that, MPR images were available in only some cases. It was reported that tumor size measured on MPR images was more strongly correlated with pathological tumor size than that measured only on axial images (17). Up to thirty-three percent of patients with honeycombing were analyzed only with images in the axial plane, whereas only 13.3%, 15.0%, and 13.4% of patients with reticulation, emphysema, and normal parenchyma were analyzed with the axial images. It might has caused higher incidence of tumor size underestimation in the patients with honeycombing than in those with the other findings. However, as shown in Table 3, the proportions of the axial plane (versus MPR) utilized for the analysis of tumor size in the patients with and without tumor size underestimation were comparable (14.4% versus 10.1%, no statistically significant difference). Moreover, multivariate analysis revealed that the imaging plane (only axial plane versus MPR) was not a predictor for tumor size underestimation in this study.
Conclusions
Honeycomb lung adjacent to the tumor is the major risk factor for preoperative radiological tumor size underestimation in patients with lung cancer. It is difficult to accurately measure the extension of tumor into the honeycombed lung areas on CT images. Hence, a suitable resection method should be selected to ensure a curative operation.
Acknowledgments
The authors thank the doctors of the radiology, diagnostic pathology and public health departments of Fujita Health University.
Funding: This work was supported by Fujita Health University research foundation 2021.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1115/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1115/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1115/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1115/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Review Board of Fujita Health University (CI21-392). The patients viewed an online version of the consent document and were enrolled in the study using the opt-out method.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Raghu G, Nyberg F, Morgan G. The epidemiology of interstitial lung disease and its association with lung cancer. Br J Cancer 2004;91:S3-10. [Crossref] [PubMed]
- Hubbard R, Venn A, Lewis S, et al. Lung cancer and cryptogenic fibrosing alveolitis. A population-based cohort study. Am J Respir Crit Care Med 2000;161:5-8. [Crossref] [PubMed]
- Sakai S, Ono M, Nishio T, et al. Lung cancer associated with diffuse pulmonary fibrosis: CT-pathologic correlation. J Thorac Imaging 2003;18:67-71. [Crossref] [PubMed]
- Lampen-Sachar K, Zhao B, Zheng J, et al. Correlation between tumor measurement on Computed Tomography and resected specimen size in lung adenocarcinomas. Lung Cancer 2012;75:332-5. [Crossref] [PubMed]
- Fukui M, Takamochi K, Matsunaga T, et al. Risk of the preoperative underestimation of tumour size of lung cancer in patients with idiopathic interstitial pneumonias. Eur J Cardiothorac Surg 2016;50:428-32. [Crossref] [PubMed]
- Sato T, Teramukai S, Kondo H, et al. Impact and predictors of acute exacerbation of interstitial lung diseases after pulmonary resection for lung cancer. J Thorac Cardiovasc Surg 2014;147:1604-11.e3. [Crossref] [PubMed]
- Kumar P, Goldstraw P, Yamada K, et al. Pulmonary fibrosis and lung cancer: risk and benefit analysis of pulmonary resection. J Thorac Cardiovasc Surg 2003;125:1321-7. [Crossref] [PubMed]
- Sato T, Kondo H, Watanabe A, et al. A simple risk scoring system for predicting acute exacerbation of interstitial pneumonia after pulmonary resection in lung cancer patients. Gen Thorac Cardiovasc Surg 2015;63:164-72. [Crossref] [PubMed]
- Raghu G, Remy-Jardin M, Myers JL, et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med 2018;198:e44-68. [Crossref] [PubMed]
- Hansell DM, Bankier AA, MacMahon H, et al. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008;246:697-722. [Crossref] [PubMed]
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1:307-10.
- Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant 2013;48:452-8. [Crossref] [PubMed]
- Tzouvelekis A, Gomatou G, Bouros E, et al. Common Pathogenic Mechanisms Between Idiopathic Pulmonary Fibrosis and Lung Cancer. Chest 2019;156:383-91. [Crossref] [PubMed]
- Brown SW, Dobelle M, Padilla M, et al. Idiopathic Pulmonary Fibrosis and Lung Cancer. A Systematic Review and Meta-analysis. Ann Am Thorac Soc 2019;16:1041-51. [Crossref] [PubMed]
- Raghu G, Amatto VC, Behr J, et al. Comorbidities in idiopathic pulmonary fibrosis patients: a systematic literature review. Eur Respir J 2015;46:1113-30. [Crossref] [PubMed]
- Sugawara H, Watanabe H, Kunimatsu A, et al. Tumor size in patients with severe pulmonary emphysema might be underestimated on preoperative CT. Eur Radiol 2022;32:163-73. [Crossref] [PubMed]
- Park CH, Kim TH, Lee S, et al. Correlation between maximal tumor diameter of fresh pathology specimens and computed tomography images in lung adenocarcinoma. PLoS One 2019;14:e0211141. [Crossref] [PubMed]
- Hsu PK, Huang HC, Hsieh CC, et al. Effect of formalin fixation on tumor size determination in stage I non-small cell lung cancer. Ann Thorac Surg 2007;84:1825-9. [Crossref] [PubMed]
- Hatabu H, Ohno Y, Gefter WB, et al. Expanding Applications of Pulmonary MRI in the Clinical Evaluation of Lung Disorders: Fleischner Society Position Paper. Radiology 2020;297:286-301. [Crossref] [PubMed]
- Ciliberto M, Kishida Y, Seki S, et al. Update of MR Imaging for Evaluation of Lung Cancer. Radiol Clin North Am 2018;56:437-69. [Crossref] [PubMed]