
Initial Asian experience of the branched E-vita open NEO in complex aortic pathologies
Highlight box
Key findings
• The novel hybrid prosthesis, E-vita Open NEO, was implanted in 25 patients from two Asian centers for total arch replacement with frozen elephant trunk and showed minimal bleeding and no overall 30-day mortality.
What is known and what is new?
• Total arch replacement with frozen elephant trunk has developed to simultaneously repair complex aortic pathologies.
• Current retrospective cohort study is a first Asian multicenter study on the clinical outcome of E-vita Open NEO.
What is the implication, and what should change now?
• This multi-center study reported a feasible initial experience of using the E-vita Open NEO and showed safety of promising initial clinical outcomes.
Introduction
Aortic arch pathology often requires staged procedures for multiple aortic segments. Concerns of cerebral, spinal, and visceral malperfusion add to the complexity of surgical management. Total aortic arch replacement with frozen elephant trunk (TAR FET) procedure has developed over the past decades to simultaneously tackle ascending aorta, aortic arch, and descending aorta pathologies (1-4). This technique not only allows a single-stage procedure for arch pathologies, but also facilitates subsequent second-stage endovascular (5-7) and open procedures (5,6,8). The E-Vita Open™ was the first commercially available straight-type hybrid prosthesis in Europe (3,4) followed by the introduction of a branched type of hybrid prosthesis, Thoraflex™ hybrid device, by Vascutek Terumo® in 2012. Owing to concerns over the designs of commercially available hybrid prostheses from the surgical community, new generations of hybrid grafts were developed (9-12). The novel Jotec E-vita Open NEO™ hybrid prosthesis (Artivions Inc., Atlanta, Georgia) demonstrated a more accommodating branch for suturing collar distance (13) and a conventional endovascular stent graft component that might be associated with fewer distal stent graft-induced new entry tears (9).
In this retrospective cohort study, we aimed to share our experience on the prosthesis selection strategies, surgical techniques, anastomosis-bleeding and graft-oozing control methods, and early clinical outcomes of the E-vita Open NEO™ branched device from two Asian institutes. We present the following article in accordance with the STORBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1055/rc).
Methods
Patients
We reviewed all consecutive patients who underwent TAR FET with the Jotec E-vita Open NEO™ hybrid prosthesis for complex aortic arch disease in two different Asian institutes, Gangnam Severance Hospital (GSH) and Prince of Wales Hospital (PWH), between October 2020 and August 2021. Preoperative characteristics of the patients are shown in Table 1.
Table 1
| Demographics | Overall, n=25 | PWH, n=10 | GSH, n=15 | P value |
|---|---|---|---|---|
| Age, years, mean ± SD | 65.9±11.8 | 65.2±8.5 | 66.3±13.8 | 0.820 |
| Male, n (%) | 18 (72.0) | 6 (60.0) | 12 (80.0) | 0.378 |
| Preop BSA, m2, mean ± SD | 1.8±0.2 | 1.8±0.2 | 1.8±0.2 | 0.981 |
| Aortic disease, n (%) | 0.336 | |||
| Aortic dissection/IMH | ||||
| Acute | ||||
| Acute type I dissection | 2 (8.0) | 0 | 2 (13.3) | |
| Acute type I IMH | 1 (4.0) | 1 (10.0) | 0 | |
| Acute type III dissection, Non A Non B | 2 (8.0) | 0 | 2 (13.3) | |
| Aortic aneurysm | 11 (44.0) | 4 (40.0) | 7 (46.7) | |
| Aortic dissecting aneurysm | ||||
| Acute | ||||
| Acute type I dissecting aneurysm | 1 (4.0) | 0 | 1 (6.7) | |
| Chronic | ||||
| Chronic type III dissecting aneurysm | 8 (32.0) | 5 (50.0) | 3 (20.0) | |
| Operative priority | 0.061 | |||
| Emergency, n (%) | 5 (20.0) | 0 | 5 (33.3) | |
| Elective, n (%) | 20 (80.0) | 10 (100.0) | 10 (66.7) | |
| Previous surgery, n (%) | 2 (8.0) | 0 | 2 (13.3) | 0.142 |
| Presentation, n (%) | 0.041* | |||
| Acute pain | 8 (32.0) | 1 (10.0) | 7 (46.7) | |
| Chronic back pain | 1 (4.0) | 1 (10.0) | 0 | |
| Ortner syndrome | 2 (8.0) | 2 (20.0) | 0 | |
| Radiological progression | 14 (56.0) | 6 (60.0) | 8 (53.3) |
*, P value <0.05. PWH, Prince of Wales Hospital; GSH, Gangnam Severance Hospital; SD, standard deviation; BSA, body surface area; IMH, intramural hematoma.
Indications of TAR FET in both institutions align with the European Association for Cardio-Thoracic Surgery position statement on the conduct of the FET procedure (10,11). The indications include: (I) acute type A aortic dissection or intramural hematoma with entry tear in the aortic arch or proximal descending thoracic aorta, (II) descending aortic dissection type B without adequate landing zone and/or when endovascular treatment is contraindicated and/or with non-A non-B dissection (14), or (III) acute rupture of arch aneurysm, or (IV) ascending thoracic aortic aneurysm or arch aneurysm with descending thoracic aortic pathologies such as dissection, aneurysm . In both institutions, computed tomography (CT) aortogram prior to the surgery and a follow-up CT aortogram after chest drain removal postoperatively were performed.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The institutional review boards of both institutes approved this retrospective cohort study (PWH IRB No. 2021.573, 4/11/2021; GSH IRB No. 2021-0421-010 3-2021-0359), and individual consent for this retrospective analysis was waived.
Prosthesis selection strategies
GSH, Seoul, Korea
In GSH, the stent graft diameter is decided according to aortic pathologies. In acute aortic dissection, stent graft diameter oversizing is 0% of the diameter of true lumen of the intended distal landing zone or stent graft diameter downsizing is 10% of the overall aortic diameter of the intended distal landing zone. On the other hand, in chronic aortic dissection, stent graft diameter oversizing is 10% of the diameter of true lumen of intended distal landing zone in case of chronic aortic dissection. In case of chronic aortic aneurysm, stent graft diameter oversizing is 10–20% of the diameter of the intended distal landing zone.
The longer the stent graft length, the lower the type-Ib endoleak and distal stent graft-induced new entry (SINE). However, long stent graft coverage induces a risk of paraplegia. Therefore, we decide the stent graft length to ensure sufficient coverage over the entry site, re-entry site, or aneurysmal sac and the distal landing zone located above T8–T10.
PWH, Hong Kong SAR, China
In PWH, the strategy of FET sizing is decided based on the chronicity of pathology as previously described (15). In acute aortic syndromes, there is no oversizing measurement of the aorta with the true perpendicular diameter measurement of the outer-to-outer wall under the aid of multiplanar reconstruction. Similar maximal diameter is measured for aortic aneurysm cases, and 10–20% oversizing of the FET is selected. In chronic dissection, the average diameter by the longest and shortest diameter of the true lumen was measured for FET sizing with 10–20% oversizing. Given that the length of FET for E-vita Open NEOTM was either 120 or 130 mm, distal landing is also planned superior to T8 with most appropriate aortic quality according to preoperative CT scans.
Operative techniques
GSH, Seoul, Korea
In GSH, the use of a guidewire was contemplated pre-operatively based on vascular anatomy from the pre-operative CT. The guidewire was placed under fluoroscopy in a hybrid theatre before sternal incision, when indicated. The surgical strategies involve establishing a unilateral antegrade cerebral perfusion through a right axillary cannulation, moderate hypothermic circulatory arrest at 28 ℃, “Y-incision” extended median sternal incision (16), and anastomotic reinforcement by interrupted pledgets. The operation is performed in the order of distal anastomosis, left subclavian artery anastomosis, left carotid artery anastomosis, proximal anastomosis, and right brachiocephalic artery anastomosis after releasing aortic cross-clamping. In the case of aortic dissection, a neomedia layer was additionally created on the false lumen using Teflon™ (Chemours, Wilmington, Delaware, USA) felt for false lumen obliteration, a procedure called Teflon felt neomedia formation.
The position and deployment of the FET is confirmed with intraoperative transesophageal echocardiography.
PWH, Hong Kong SAR, China
The technique of PWH has been previously described (15,17). Standard median sternotomy is the routine of PWH, with the routine arterial cannulation via the ascending aorta in cases of aneurysm or via the femoral artery in cases of dissections. The targeted core temperature during moderate hypothermic circulatory arrest was at 25 ℃; selected antegrade cerebral protection was routinely given to all 3 supra-aortic branches to ensure complete cerebral and spinal protection during circulatory arrest. The proximal aortic anastomosis is performed immediately following the distal anastomosis reducing the cardiac ischemic time, followed by the anastomosis of the supra-aortic branches.
Anastomosis-bleeding and graft-oozing control methods
GSH, Seoul, Korea
In GSH, distal anastomosis is performed with marking of the sewing collar and native aorta at every quadrant to ensure precise anastomotic ratio. Further, all around the sewing collar is trimmed 5 mm away from the circumference of the native aorta to avoid folding of the collar, called dog-ear phenomenon for hemostasis of distal anastomosis before distal anastomosis. After continuous suture, the reinforcement sutures are always made all around the anastomosis site with 3-0 pledgeted Prolene™.
Minimizing the graft manipulation is important to prevent damage to uncoated graft and reduce the graft oozing, including clamping the site as far away from the joint as possible and the site of incision.
In GSH, not only in TAR FET, but also in total arch replacement, the plant-based hemostatic powder agent was used for hemostasis with satisfactory results. If more control on graft oozing is required, the graft-oozing point is sutured with 5-0 Prolene™ pledgeted sutures.
PWH, Hong Kong SAR, China
In PWH, interrupted suture aortic anastomosis and reinforcement have been deployed to minimize anastomotic bleeding. The sewing collar is trimmed when the discrepancy was considered large, preventing the dog-ear phenomenon. Minimizing graft manipulation with least possible clamping was also particularly considered; hence, the first side branch of the E-vita Open NEOTM was used for de-airing to prevent graft puncture. Pre-emptive BioglueTM priming of the graft was also used to tackle graft oozing for the non-coated graft materials of the E-vita Open NEOTM (18). In general, 4-0 sutures were used for proximal and distal aortic anastomosis and 5-0 sutures were used for anastomosis of the supra-aortic side branches.
Statistical analysis
Data were collected prospectively by JYKH and CHK from electrical medical records. Categorical and continuous variables were listed as percentage and mean ± standard deviation (SD), respectively. The primary outcome was overall 30-day mortality, and the secondary outcomes were operative complications. Independent t-test was used to compare continuous variables between two cohorts and Chi-square test, Fischer’s exact test were used to compare categorical variables. Analyses were performed using IBM SPSS (IBM Corp. 2016. Version 24.0., Armonk, New York, USA), and GraphPad Prism (Version 8.4.2 for Windows, GraphPad Software, La Jolla California, USA).
Results
Perioperative data
Between October 2020 and August 2021, a total of 25 consecutive patients with complex aortic pathologies listed in Table 1 who underwent TAR FET with E-vita Open NEOTM in two Asian centers were recruited. Patients’ baseline characteristics and presenting pathology are shown in Table 1. Emergency surgeries within 24 hours of presentation were performed in five patients.
Intraoperative data are listed in Table 2. Overall mean operative, cardiopulmonary bypass, hypothermic circulatory arrest, and selective antegrade cerebral perfusion times were 353.4±80.5, 183.2±39.6, 57.2±14.7, and 138.2±28.6 minutes, respectively. The vascular graft diameter, stent graft diameter, and stent graft length were 27.8±1.9, 28.5±5.1, and 133.6±23.0 mm, respectively. Distal anastomoses were performed in zones 1, 2, and 3 in 1 (4.0%), 18 (72.0%), and 6 (24.0%) patients, respectively. Coverage of FET was at or distal to T8 in eight (32.0%) patients and proximal to T8 in 17 (68.0%) patients. Hybrid theatre was used in 80.0% of all cases with 52.0% of all patients having FET deployed under guidewire guidance.
Table 2
| Variables | Overall, n=25 | PWH, n=10 | GSH, n=15 | P value |
|---|---|---|---|---|
| Operative time, min, mean ± SD | 353.4±80.5 | 345.5±80.5 | 358.7±82.8 | 0.697 |
| Cardiopulmonary bypass time, min, mean ± SD | 183.2±39.6 | 211.8±37.1 | 164.2±28.8 | <0.001* |
| Aortic cross-clamp time, min, mean ± SD | 118.0±27.6 | 119.3±34.5 | 117.1±23.3 | 0.848 |
| Moderate hypothermic circulatory arrest time, min, mean ± SD | 57.2±14.7 | 55.5±19.0 | 58.3±11.7 | 0.655 |
| Antegrade cerebral perfusion time, min, mean ± SD | 138.2±28.6 | 154.1±27.3 | 127.6±24.9 | 0.020* |
| Hybrid prosthesis size, mm, mean ± SD | ||||
| Vascular graft diameter | 27.8±1.9 | 27.4±1.9 | 28.0±1.9 | 0.440 |
| Stent graft diameter | 28.5±5.1 | 28.3±5.9 | 28.7±4.7 | 0.865 |
| Stent graft length | 133.6±23.0 | 128.7±18.7 | 137.7±25.4 | 0.331 |
| Zone of distal anastomosis, n (%) | <0.001* | |||
| Zone 1 | 1 (4.0) | 0 | 1 (6.7) | |
| Zone 2 | 18 (72.0) | 4 (40.0) | 14 (93.3) | |
| Zone 3 | 6 (24.0) | 6 (60.0) | 0 | |
| Extent of coverage of FET, n (%) | 0.667 | |||
| Coverage at or distal to T8 | 8 (32.0) | 4 (40.0) | 4 (26.7) | |
| Coverage at proximal to T8 | 17 (68.0) | 6 (60.0) | 11 (73.3) | |
| Intraoperative blood products, units, mean ± SD | ||||
| Packed red blood cell | 2.5±2.5 | 4.1±2.8 | 1.5±1.8 | 0.008* |
| Platelet concentrate | 10.5±4.3 | 8.2±3.6 | 12.0±4.1 | 0.026* |
| Fresh frozen plasma | 5.1±2.3 | 5.0±3.3 | 5.1±1.4 | 0.907 |
| Cryoprecipitate | 3.0±4.0 | 7.4±2.5 | 0 | <0.001* |
| Hybrid theatre use, n (%) | 20 (80.0) | 5 (50.0) | 15 (100.0) | 0.005* |
| Guidewire deployment of FET, n (%) | 13 (52.0) | 4 (40.0) | 9 (60.0) | 0.428 |
| Concomitant procedures, n (%) | 0.141 | |||
| None | 17 (68.0) | 7 (70.0) | 10 (66.7) | |
| AVR | 1 (4.0) | 1 (10.0) | 0 | |
| Bentall procedure | 1 (4.0) | 0 | 1 (6.7) | |
| CABG | 2 (8.0) | 2 (20.0) | 0 | |
| TEVAR | 3 (12.0) | 0 | 3 (20.0) | |
| Pulmonary artery fistula repair | 1 (4.0) | 0 | 1 (6.7) |
*, P value <0.05. PWH, Prince of Wales Hospital; GSH, Gangnam Severance Hospital; SD, standard deviation; FET, frozen elephant trunk; AVR, aortic valve replacement; CABG, coronary artery bypass grafting; TEVAR, thoracic endovascular aortic repair.
Overall mean use of blood products, in terms of packed red blood cells, platelet concentrate, and fresh frozen plasma, was 2.5±2.5, 10.5±4.3, and 5.1±2.3 units, respectively (Table 2).
Postoperative outcomes
The overall and 30-day mortality were both 0% as listed in Table 3. Mean intensive care unit (ICU) stay of this series was 3.4±5.0 days and the length of stay was 20.2±37.5 days with no significant difference between the two centers. No patient required re-sternotomy for postoperative bleeding hemostasis in any cohort. Permanent spinal cord injury (SCI) with residual weakness upon discharge was recorded in one patient (4.0%), and one patient had transient lower limb weakness that resolved after spinal drainage for 72 hours. One patient (4.0%) required transient renal dialysis during ICU stay and none required renal replacement therapy upon discharge. All cases of FET stent deployment were successful at the intended location without unexpected procedure. None of the patients developed postoperative vocal cord palsy.
Table 3
| Variables | Overall, n=25 | PWH, n=10 | GSH, n=15 | P valve |
|---|---|---|---|---|
| In-hospital mortality, n (%) | 0 | 0 | 0 | NA |
| Overall 30-days mortality, n (%) | 0 | 0 | 0 | NA |
| Drain output, day of surgery, mL, mean ± SD | 461.9±344.9 | 305.5±256.1 | 566.1±364.2 | 0.063 |
| Drain output, postoperative day 1, mean ± SD | 399.5±235.4 | 304.0±161.2 | 463.1±259.7 | 0.098 |
| Intensive care unit stay, day, mean ± SD | 3.4±5.0 | 3.4±7.2 | 3.5±2.9 | 0.975 |
| Length of stay, day, mean ± SD | 20.2±37.5 | 30.1±59.2 | 13.5±6.3 | 0.401 |
| Redo-sternotomy for hemostasis, n (%) | 0 | 0 | 0 | NA |
| Stroke, n (%) | 0 | 0 | 0 | NA |
| Spinal cord injury, n (%) | 2 (8%) | 2 (20%) | 0 | 0.150 |
| Permanent | 1 (4%) | 1 (10%) | 0 | 0.400 |
| Transient | 1 (4%) | 1 (10%) | 0 | 0.400 |
| New postoperative renal dialysis, n (%) | 1 (4%) | 1 (10%) | 0 | 0.400 |
| New vocal cord paralysis, n (%) | 0 | 0 | 0 | NA |
| Stent mal-deployment, n (%) | 0 | 0 | 0 | NA |
| Sternal wound infection, n (%) | 0 | 0 | 0 | NA |
| Stent graft shortening, n (%) | 0 | 0 | 0 | NA |
PWH, Prince of Wales Hospital; GSH, Gangnam Severance Hospital; SD, standard deviation; NA, not available.
Discussion
Complex aortic pathologies involve extensive and multi-segments of aortic disease imposing vast surgical challenges. In the past decades, the hybrid device of TAR FET offered single-stage management and facilitated multi-stage procedures in complex aortic disease. Kato and colleagues (1) first introduced the modification of the conventional elephant trunk procedure with the deployment of a distal endovascular stent graft, while Karck and colleagues (2) named it as the ‘‘frozen elephant trunk.” As a commercial FET prosthesis that was first developed in 2005, the new generation E-vita Open NEO™ was designed to accommodate a variety of aortic arch anatomy and surgical preferences. Potential benefits of the latest generation include the three arch branch designs and configurations, collar-to-stent distances for left subclavian artery (13,18), and a flexible delivery system with the conventional thoracic endovascular aortic repair (TEVAR) stent graft design, which possibly prevents the distal stent graft-induced new entry tear (12).
A previous systematic review on TAR FET by Tian and colleagues (19) reported weighted average rates of mortality, stroke, SCI, and re-surgery due to bleeding as 8.3%, 4.9%, 5.1%, and 7.8%, respectively. In a more recent review by Papakonstantinou and colleagues (20), a pooled 30-day or hospital mortality rate and rates of stroke, paraplegia, and re-surgery for bleeding were reported as 5.04%, 2.38%, 0.63%, and 7.5%, respectively. A more recent systematic review reported on the comparison of the previous version of JOTEC E-vita OpenTM (Artivions Inc., Atlanta, Georgia) and ThoraflexTM (VASCUTEK, Terumo, Inchinnan, Germany), reported on the limited and heterogeneous datasets, suggesting E-vita Open possibly had a lower mortality and morbidity risks in thoracic aortic aneurysm surgery (21). In the present study demonstrating the initial experience of a novel TAR FET hybrid prosthesis from two centers, the 30-day and in-hospital mortalities were 0%. Recent surveys reported that aortic repair at high-volume aortic centers was associated with lower mortality (22), echoing the results of the current study. Both centers are high-volume aortic centers, including approximately 100 cases in PWH and 300 cases in GSH of open and endovascular aortic surgery annually. Both centers have a dedicated aortic team for TAR FET procedures, and this is the cornerstone for achieving good results in performing complex aortic procedures.
SCI related to FET is multifactorial, dependent on the aortic tissue quality, aortic dissection anatomy, stent coverage affected by the distal anastomotic sites. There were two cases of SCI in this cohort, both from the PWH cohort. One of the two patients had an extreme “shaggy” aorta with frail atherosclerotic debris, this patient had the 180 mm length of FET stent chosen to achieve best possible distal FET landing. The other patient whom had a transient SCI, recovered completely after spinal drainage post operation had no specific risk factor for SCI. Hence, there are important considerations of SCI in TAR FET, concerning the aortic quality preoperatively and to note the distal landing particularly when a longer version of FET stent or same stage TEVAR is planned. There could also be concerns related to removal of air in the circulatory pathway of the lower body (12); upon resuming distal perfusion from the femoral cannulation, air could possibly migrate proximally toward the vertebral or collateral arteries; leading to stroke and SCI. We suggest a meticulous distal perfusion and de-airing can lower the rate of stroke and SCI.
The conventional Z-shape design of the stent-graft component provides longitudinal stability and even distribution of radial force (9). In our study, postoperative CT showed no shortening, endoleak, or distal stent-graft induced new entry tear despite the short follow-up period.
There was no re-sternotomy for post-operative bleeding in the current cohort. Both centers adopted the strategy of full circumferential reinforcement sutures of the distal and proximal anastomoses with interrupted pledgeted sutures (Figure 1). The experience of TAR FET from PWH with the same emphasis as that of the anastomotic technique also reported a low rate of re-sternotomy for bleeding at 4.9% previously (15). In comparison to earlier reports on the use of ThoraflexTM trifurcated graft in re-sternotomy for bleeding at 13% (23), it is believed that the meticulous hemostasis starting from the cautious anastomotic technique is pivotal.
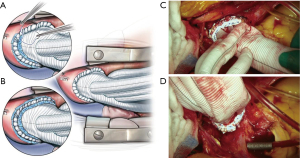
Excessive oozing of the graft material had been a concern since the graft body is not coated and detrimental bleeding was previously reported in Europe (18,24). We experienced three cases of excessive graft oozing of the novel hybrid prosthesis from the PWH cohort. The oozing phenomenon was attributed to the coating of the graft body; however, the proper use of ROTEM had been reported (25) and a pre-emptive coating by BioGlue has been described (18). In the presenting cohort, platelet concentrates were more used in the GSH (GSH vs. PWH; 12.0±4.1 vs. 8.2±3.6 units, P=0.026); cryoprecipitate was only used in the PWH cohort, and the PWH cohort was infused with more packed red blood cells intraoperatively. Jakob et al. (25) also reported that three cases of graft oozing used 6 units, 4 units, and 0 unit of platelet concentrates in each case. This suggests that giving more platelet concentrates may help reduce graft oozing. Despite the portrayed concerns (26) and uncorroborated ex-vivo experiments (27), this combined real-world experience from two Asian centers demonstrated the hemostatic status that is manageable with promising outcomes as an initial report of experience.
Rylski et al. (28) reported that the descending aorta diameter was increased by approximately 10% after acute aortic dissection. Therefore, it is possible that stent graft diameter downsizing is 10% of the overall aortic diameter of the intended distal landing zone in case of acute aortic dissection containing severely collapsed true lumen.
Generally, the larger the stent graft diameter, the lower the type-Ib endoleak rate. In TEVAR experience of GSH, oversizing the diameter to more than 12% is a risk factor of distal SINE in chronic dissection. Therefore, to reduce type-Ib endoleak and distal SINE, stent graft diameter oversizing is considered as 10% of the diameter of the intended distal landing zone in chronic dissection.
Finally, since aneurysm has various shapes, from the subgroup of only acute aortic dissection, the mean vascular graft diameter, stent graft diameter, were 26.4±0.9, 25.2±1.8 mm respectively. All stent graft length was 120 mm long and all distal anastomosis was performed in zone 2. In that situation, extent of coverage of FET in all except one patient is above T8. Therefore, if a stent graft 120 mm is used for Asians and zone 2 anastomosis is performed, most of the distal landing zone will be above T8. It is not consistent with 100 mm, but is consistent with results suggested by Preventza et al. (29) that SCI event occurred more frequently in distal landing zone at T8 or below T8. Therefore, even though 120 mm stent graft is used for Asian, most distal landing zone will not be at T8 or below T8, so SCI event will not occur more frequently, as our results. Additionally, this reflected that the 26-26-120 or 26-24-120 size TAR FET prosthesis may be the most commonly used grafts in the Asian groups for acute aortic dissection.
The small number of patients of current study is an obvious limitation. However, this is the first multi-center cohort on the initial experience of the only commercially available hybrid prosthesis in Korea and a widely use version of E-vita Open prosthesis in Hong Kong, SAR. Based on the promising outcomes from current study, it would bring further insights on the use and development of TAR FET.
Conclusions
TAR FET is an important technique to address complex arch and descending aortic pathologies. This multicenter study reported the initial experience of implementing TAR FET with a new branched hybrid prosthesis device and demonstrated acceptable mortality and morbidity in centers with dedicated aortic teams. The technique of circumferential reinforcement of vascular anastomosis for hemostasis may be one of the methods for lowering the rates of re-sternotomy for hemostasis, and proper surgical or transfusion strategies would overcome the excessive oozing of the prosthesis. Long-term follow-up is required for further evaluation of aortic pathology progression, secondary intervention rates, and survival rates.
Acknowledgments
The authors would like to thank Medical Illustration & Design, part of the Medical Research Support Services of Yonsei University College of Medicine, for all artistic support related to this work.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1055/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1055/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1055/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1055/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The institutional review boards of both institutes approved this retrospective cohort study (PWH IRB No. 2021.573, 4/11/2021; GSH IRB No. 2021-0421-010 3-2021-0359), and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kato M, Ohnishi K, Kaneko M, et al. New graft-implanting method for thoracic aortic aneurysm or dissection with a stented graft. Circulation 1996;94:II188-93.
- Karck M, Chavan A, Hagl C, et al. The frozen elephant trunk technique: a new treatment for thoracic aortic aneurysms. J Thorac Cardiovasc Surg 2003;125:1550-3. [Crossref] [PubMed]
- Di Marco L, Pantaleo A, Leone A, et al. The Frozen Elephant Trunk Technique: European Association for Cardio-Thoracic Surgery Position and Bologna Experience. Korean J Thorac Cardiovasc Surg 2017;50:1-7. [Crossref] [PubMed]
- Jakob H, Idhrees M, Bashir M. From E-VITA open plus to E-VITA NEO and E-NOVIA. J Card Surg 2021;36:1814-7. [Crossref] [PubMed]
- Leone A, Beckmann E, Martens A, et al. Total aortic arch replacement with frozen elephant trunk technique: Results from two European institutes. J Thorac Cardiovasc Surg 2020;159:1201-11. [Crossref] [PubMed]
- Berger T, Weiss G, Voetsch A, et al. Multicentre experience with two frozen elephant trunk prostheses in the treatment of acute aortic dissection†. Eur J Cardiothorac Surg 2019;56:572-8. [Crossref] [PubMed]
- Wong RHL, Yu PSY, Kwok MWT, et al. Endovascular Fenestration for Distal Aortic Sealing After Frozen Elephant Trunk With Thoraflex. Ann Thorac Surg 2017;103:e479-82. [Crossref] [PubMed]
- Fujikawa T, Ho JYK, Wong HMK, et al. Open descending aortic replacement after Thoraflex™ hybrid graft implantation. Eur J Cardiothorac Surg 2019;56:612-4. [Crossref] [PubMed]
- Kreibich M, Bünte D, Berger T, et al. Distal Stent Graft-Induced New Entries After the Frozen Elephant Trunk Procedure. Ann Thorac Surg 2020;110:1271-9. [Crossref] [PubMed]
- Shrestha M, Bachet J, Bavaria J, et al. Current status and recommendations for use of the frozen elephant trunk technique: a position paper by the Vascular Domain of EACTS. Eur J Cardiothorac Surg 2015;47:759-69. [Crossref] [PubMed]
- Czerny M, Schmidli J, Adler S, et al. Current options and recommendations for the treatment of thoracic aortic pathologies involving the aortic arch: an expert consensus document of the European Association for Cardio-Thoracic surgery (EACTS) and the European Society for Vascular Surgery (ESVS). Eur J Cardiothorac Surg 2019;55:133-62. [Crossref] [PubMed]
- Czerny M, Rylski B, Kari FA, et al. Technical details making aortic arch replacement a safe procedure using the Thoraflex™ Hybrid prosthesis. Eur J Cardiothorac Surg 2017;51:i15-9. [Crossref] [PubMed]
- Ho JYK, Bashir M, Jakob H, et al. Management of left subclavian artery in total arch replacement and frozen elephant trunk procedure. JTCVS Tech 2021;7:36-40. [Crossref] [PubMed]
- Howard C, Ponnapalli A, Shaikh S, et al. Non-A non-B aortic dissection: A literature review. J Card Surg 2021;36:1806-13. [Crossref] [PubMed]
- Ho JYK, Chow SCY, Kwok MWT, et al. Total Aortic Arch Replacement and Frozen Elephant Trunk. Semin Thorac Cardiovasc Surg 2021;33:656-62. [Crossref] [PubMed]
- Kazui T. Total arch replacement with separated graft technique and selective antegrade cerebral perfusion. Ann Cardiothorac Surg 2013;2:353-7. [Crossref] [PubMed]
- Wong RHL, Ho JYK, Bashir M, Jakob H. First In Man implantation of Evita Open Neo in Hong Kong 2020. Available online: https://ctsnet.figshare.com/articles/media/First_In_Man_implantation_of_Evita_Open_Neo_in_Hong_Kong/13373762/1
- Ho JYK, Lim K, Fujikawa T, et al. Pre-emptive BioGlue priming of the branched Evita Open NEO hybrid prosthesis: tackling excessive oozing. Eur J Cardiothorac Surg 2022;61:728-9. [Crossref] [PubMed]
- Tian DH, Wan B, Di Eusanio M, et al. A systematic review and meta-analysis on the safety and efficacy of the frozen elephant trunk technique in aortic arch surgery. Ann Cardiothorac Surg 2013;2:581-91. [Crossref] [PubMed]
- Papakonstantinou NA, Antonopoulos CN, Baikoussis NG, et al. Frozen Elephant Trunk: An Alternative Surgical Weapon Against Extensive Thoracic Aorta Disease. A Three-Year Meta-Analysis. Heart Lung Circ 2019;28:213-22. [Crossref] [PubMed]
- Harky A, Fok M, Bashir M. Which is the Optimal Frozen Elephant Trunk? A Systematic Review and Meta-Analysis of Outcomes in 2161 Patients Undergoing Thoracic Aortic Aneurysm Surgery Using E-vita OPEN PLUS Hybrid Stent Graft versus Thoraflex™ Hybrid Prosthesis. Braz J Cardiovasc Surg 2020;35:427-36. [Crossref] [PubMed]
- Andersen ND, Ganapathi AM, Hanna JM, et al. Outcomes of acute type a dissection repair before and after implementation of a multidisciplinary thoracic aortic surgery program. J Am Coll Cardiol 2014;63:1796-803. [Crossref] [PubMed]
- Beckmann E, Martens A, Korte W, et al. Open total arch replacement with trifurcated graft and frozen elephant trunk. Ann Cardiothorac Surg 2020;9:170-7. [Crossref] [PubMed]
- Czerny M, Beyersdorf F, Murana G, et al. Excessive oozing through the fabric of the branched Cryolife-Jotec Evita Open NEO hybrid prosthesis. Eur J Cardiothorac Surg 2021;60:423-4. [Crossref] [PubMed]
- Jakob H, Ho JYK, Wong RHL, et al. Paving the way for E-vita open NEO hybrid prosthesis implantation for complex aortic arch disease in Asia-Pacific. J Card Surg 2021;36:3963-7. [Crossref] [PubMed]
- Jubouri M, Abdelhaliem A. BioGlue and E-Vita Open NEO graft oozing: Long-term solution or band aid? J Card Surg 2022;37:561-2. [Crossref] [PubMed]
- Tan SZCP, Bashir M. Prevention versus cure: Is BioGlue priming the optimal strategy against E-Vita NEO graft oozing? J Card Surg 2022;37:555-60. [Crossref] [PubMed]
- Rylski B, Blanke P, Beyersdorf F, et al. How does the ascending aorta geometry change when it dissects? J Am Coll Cardiol 2014;63:1311-9. [Crossref] [PubMed]
- Preventza O, Liao JL, Olive JK, et al. Neurologic complications after the frozen elephant trunk procedure: A meta-analysis of more than 3000 patients. J Thorac Cardiovasc Surg 2020;160:20-33.e4. [Crossref] [PubMed]


