Serum vitamin D3 deficiency can affect the efficacy of sublingual immunotherapy in children with allergic rhinitis: a retrospective cohort study
Highlight box
Key findings
• Serum vitamin D3 deficiency can affect the efficacy of SLIT in children with allergic rhinitis.
What is known and what is new?
• SLIT is effective, tolerable, and convenient for many allergic patients but is still ineffective for many children with allergic rhinitis.
• To identify the independent factors influencing the efficacy of SLIT, this study analyzed the relationship between vitamin D3 and SLIT efficacy.
What is the implication, and what should change now?
• The main question under investigation in this study is whether vitamin D3 supplementation can improve the efficacy of SLIT.
Introduction
Allergic rhinitis (AR) is a common atopic and chronic disease in childhood. Globally, between 10% and 40% of children suffer from AR, and its prevalence has increased markedly over the past two decades (1). Through etiological research, researchers have found that AR is a complex disease with multiple factors, mainly including genetic as well as environmental factors, such as air pollution, infection, diet, and nutritional status. In recent years, the relationship between vitamin D3 and allergic diseases has attracted our attention and become the focus of several studies.
In allergy-mediated allergic diseases, allergen-specific immunotherapy (SIT) targets sensitized allergens and is the only treatment that can change the natural progression of the disease (2). Based on the method of allergen administration, allergen-SIT is classified into two types: subcutaneous immunotherapy (SCIT) and sublingual immunotherapy (SLIT) (3). At present, numerous international studies on the efficacy and safety of SLIT in children with AR have been conducted. Patients with poor SLIT efficacy usually receive individual dose adjustments to further improve its efficacy but there are still some children with poor efficacy who cease SLIT treatment. The incidence of low response (LR) in SLIT treatment varies significantly among the reports of many studies. This study provides a new treatment idea for patients with poor efficacy of SLIT, and vitamin D3 supplementation may become a powerful method to enhance the efficacy of SLIT in the future. The present study aimed to identify the independent factors affecting the efficacy of SLIT and provide new ideas for improving the efficacy of SLIT in children with AR. We present the following article in accordance with the STARD reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1883/rc).
Methods
Patients
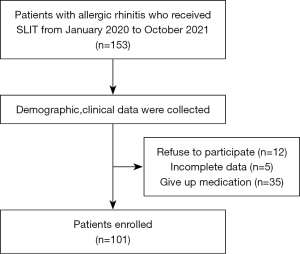
In this study, 153 patients with AR who received SLIT with Dermatophagoides farinae (D. farinae) drops in the Otorhinolaryngology Department of Tianjin Children’s Hospital from January 2020 to October 2021 were selected as the study objects. The patient’s sex, age, serum vitamin D3 levels, family history of allergic disease, food allergies, their caregiver’s education level, asthma status, whether they had used acarid products, etc. were collected retrospectively. All patients included in this study recorded their visual simulation scores, symptom scores, medication scores, and quality of life scores to evaluate the efficacy of SLIT. The primary endpoint of this study was the efficacy of patients treated with SLIT, and the independent influencing factors affecting the efficacy of SLIT were analyzed.
The enrolled patients were required to fully meet the following conditions: (I) diagnosed with AR, with or without other allergic diseases; (II) 3–14 years old, male or female; (III) allergen detection results: dust mites were positive, closely related to clinical symptoms and were the main allergen; (IV) allergens could be avoided as much as possible during treatment; (V) willing to receive SLIT and able to comply with the program and receive follow-up. The exclusion criteria were as follows: (I) patients with infectious rhinitis; (II) patients who could not understand the purpose of this study and refused to cooperate with follow-up; (III) those with incomplete data. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Tianjin Children’s Hospital (No. KY2020-47) and informed consent was taken from all the patients’ guardians.
Diagnostic criteria
The diagnostic criteria applied in this study are based on the Guidelines for the Diagnosis and Treatment of Allergic Rhinitis in Children (2022, revised edition) (4), which include the following typical symptoms of AR: watery nose, itching, stuffy nose, sneezing (note: two or more items needed to be met, and had to last or accumulate for more than 1 hour every day). At the same time, the following were considered typical signs of AR: pale and edema of the nasal mucosa and watery secretion of the nasal cavity. Allergen detection was determined as follows: dust mite serum-specific immunoglobulin E (IgE) was positive.
Detection of serum allergen-specific immunoglobulin E (sIgE)
We collected 3 mL of venous blood to separate the serum for use and used the Fubok allergen detector and its allergen diagnostic reagent produced by Jiangsu Haooubo Biological Medicine Co., Ltd. (Suzhou, Jiangsu Province, China) to determine the specific IgE of the corresponding allergen in the serum. The system adopts the enzyme-linked immunostaining method to detect the serum-specific IgE content, which is a quantitative detection. Inhalation allergens include house dust mites, dust mites, mugwort, ragweed, cockroaches, cat epithelia, dog epithelia, house dust, Alternaria, and willow. Food allergens include peanut, egg, milk, cod, wheat flour, shrimp, soybean, crab, beef, and mutton.
Determination of 25 hydroxyvitamin D3 [25(OH)D3] in serum
The content of 25- hydroxyvitamin D3 [25(OH)D3] in the serum was detected by high-performance liquid chromatography (tandem mass spectrometry). An isotopic internal standard was added to 10 microliter serum samples. The protein in samples was precipitated with anhydrous ethanol and the target chemicals were extracted using n-hexane. After extraction, the supernatants were evaporated to dryness under a stream of nitrogen. The residue underwent the derivation process and the derivatizing products were redissolved and chromatographically separated and quantified by mass spectrometry. The liquid chromatographic column was InfinityLab Poroshell 120 EC-C18 (4.6 mm × 50 mm, 2.7 µm), C18 guard column (Phenomenex, USA). The LC-20AD liquid chromatography-API 3200MD triple quadrupole mass spectrometer (Shimadzu, AB Sciex, USA) with an automatic injector was used.
Medication method and dosage of SLIT
AR patients were treated by sublingual administration of D. farinae drops (Changdi, produced by Zhejiang Wowu Biotechnology Co., Ltd., China). SLIT with D. farinae drops can be divided into two stages: the increasing stage and the dose maintenance stage. Children under 14 years of age used No. 1–4, among which the No. 1–3 preparation was used in the incremental phase and No. 4 preparation was used in the maintenance phase. Table 1 shows the dosage schedule of D. farinae drops for children. To administer the medication, it should be swallowed 1–3 minutes after it is under the tongue. If the number of drops is too large, it can be taken several times. Patients could eat and drink normally 5 minutes after taking the medicine. Concurrent with desensitization treatment, symptomatic drugs must be used together as needed to control symptoms. The drug regimen should follow Global Initiative for Asthma (GINA) and allergic rhinitis and its impact on asthma (ARIA), and the patient’s condition should be evaluated regularly. When the patient’s condition improves, symptomatic drugs can be used for degrading treatment.
Table 1
| Week(s) | Drug No. | Dose, mL | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 day | 2 days | 3 days | 4 days | 5 days | 6 days | 7 days | ||
| 1 | 1 | 0.05 | 0.1 | 0.15 | 0.20 | 0.30 | 0.40 | 0.50 |
| 2 | 2 | 0.05 | 0.1 | 0.15 | 0.20 | 0.30 | 0.40 | 0.50 |
| 3 | 3 | 0.05 | 0.1 | 0.15 | 0.20 | 0.30 | 0.40 | 0.50 |
| 4 | 4 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 |
| ≥5 | 4 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 |
Follow-up
The follow-up plan was divided into two methods: telephone and outpatient follow-up, and Internet APP and WeChat follow-up. In principle, telephone follow-ups should be conducted once a month for the enrolled patients. The follow-up should include answering the patients’ questions about sublingual medication, reminding them about the medication, and asking them about the use of symptomatic medication and their rhinitis symptoms. An outpatient follow-up visit should be conducted every 3 months. The follow-up content is the same as above, and the patient should be given a continuation of the prescription. Also, the latest knowledge about AR and precautions for SLIT should be popularized on the Internet APP. The symptom and medication questionnaire survey were conducted through the Internet APP to follow up on the improvement of symptoms and medications of the children and monitor the adverse drug reactions to guide parents in their use of the drugs. A WeChat group should also be established, through which regular health lectures on AR can be given. We can also listen to the feedback of the children’s parents, follow up on symptoms and medication, promote communication and interaction between families, and further enhance compliance.
Efficacy evaluation criteria
The patients were followed up after 6 months of treatment. According to the improvement rate of combined symptom medication score (CSMS), the patients were divided into high response (HR) and LR groups. Patients with CSMS improvement rates greater than 50% were defined as HR, while those with CSMS improvement rates between 20% and 50% were defined as LR (5).
Statistical analysis
A receiver operating characteristic (ROC) curve was drawn, the optimal cut-off point was determined according to the Youden index, and vitamin D3 was converted into a classification variable. The Chi-square test was used to compare the categorical variables between groups. Univariate and multivariate logistic regression were performed to analyze the relationship between serum vitamin D3 and the efficacy of SLIT. Statistically significant variables in univariate logistic regression analysis were included in multivariate regression analysis (using Enter method). Odds ratio (OR) value is used to measure the magnitude of the correlation effect. Two-tailed P values <0.05 were considered statistically significant. All statistical analyses were performed using SPSSv26.0 (IBM Corporation, Armonk, NY, USA).
Results
Patient characteristics
A total of 153 patients were included in this study. Among them, 12 cases refused to participate in the study, 5 cases had incomplete data, and 35 cases ceased medication. The details are shown in Figure 1. The mean vitamin D3 level of this patient cohort was (20.42±7.48) ng/mL. The optimal cutoff point for vitamin D3 for the efficacy of SLIT (Figure 2) was determined according to Youden’s index (cutoff value, 22.25 ng/mL). Vitamin D3 was then transformed into a classification variable according to this cutoff value (≤22.25, n=66; >22.25, n=35).
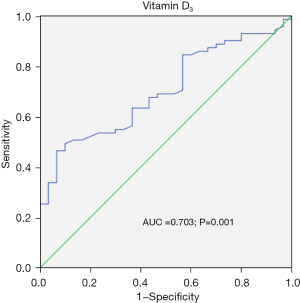
Table 2 shows the patients’ baseline clinical characteristics according to vitamin D3. The efficacy of SLIT among the different vitamin D3 groups was statistically significant (P<0.001). No significant differences existed between the vitamin D3 groups in terms of the other clinical characteristics.
Table 2
| Characteristics | Total | Vitamin D3 | P value | |
|---|---|---|---|---|
| ≤22.25 ng/mL | >22.25 ng/mL | |||
| Total, n (%) | 101 (100.0) | 66 (65.3) | 35 (34.7) | |
| Sex, n (%) | 0.629 | |||
| Male | 78 (77.2) | 50 (75.8) | 28 (80.0) | |
| Female | 23 (22.8) | 16 (24.2) | 7 (20.0) | |
| Age, years, n (%) | 0.524 | |||
| ≤6 | 39 (38.6) | 24 (36.4) | 15 (42.9) | |
| >6 | 62 (61.4) | 42 (63.6) | 20 (57.1) | |
| Family history of allergic disease, n (%) | 0.341 | |||
| No | 26 (25.7) | 15 (22.7) | 11 (31.4) | |
| Yes | 75 (74.3) | 51 (77.3) | 24 (68.6) | |
| Food allergy, n (%) | 0.421 | |||
| No | 43 (42.6) | 30 (45.5) | 13 (37.1) | |
| Yes | 58 (57.4) | 36 (54.5) | 22 (62.9) | |
| Education level of caregivers, n (%) | 0.825 | |||
| Higher education | 39 (38.6) | 26 (39.4) | 13 (37.1) | |
| Not receiving higher education | 62 (61.4) | 40 (60.6) | 22 (62.9) | |
| Follow-up mode, n (%) | 0.938 | |||
| Internet medical APP | 64 (63.4) | 42 (63.6) | 22 (62.9) | |
| Traditional method | 37 (36.6) | 24 (36.4) | 13 (37.1) | |
| Asthma, n (%) | 0.361 | |||
| No | 61 (60.4) | 42 (63.6) | 19 (54.3) | |
| Yes | 40 (39.6) | 24 (36.4) | 16 (45.7) | |
| Use of acarid products, n (%) | 0.993 | |||
| No | 52 (51.5) | 34 (51.5) | 18 (51.4) | |
| Yes | 49 (48.5) | 32 (48.5) | 17 (48.6) | |
| SLIT efficacy, n (%) | <0.001 | |||
| High response | 71 (70.3) | 38 (57.6) | 33 (94.3) | |
| Low response | 30 (29.7) | 28 (42.4) | 2 (5.7) | |
SLIT, sublingual immunotherapy.
Finally, a total of 101 children with AR were included in this study. Among them, 78 cases (77.2%) were male, 39 cases (38.6%) were 3–6 years old, 75 cases (74.3%) had a family history of allergic diseases, 58 cases (57.4%) had food allergies, the main caregivers of 62 cases (61.4%) had no higher education, 64 cases (63.4%) had Internet APP follow-up, 40 cases (39.6%) had asthma, and 49 cases (48.5%) had acarid product use in their families. Also, 71 patients (70.3%) were classified into the HR group and 30 patients (29.7%) were in the LR group.
Logistic regression analysis
Univariate logistic regression analysis of the efficacy of SLIT (Table 3) showed that whether the patient also had food allergy (P<0.001) or asthma (P=0.011), whether they had used of acarid products (P=0.002), and whether vitamin D3 is sufficient (P=0.001) were significantly related to the efficacy of SLIT, with statistically significant differences.
Table 3
| Variables | Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | ||
| Sex | 1.26 | 0.44–3.59 | 0.67 | ||||
| Age | 0.47 | 0.18–1.20 | 0.11 | ||||
| Family history of allergic disease | 2.10 | 0.71–6.23 | 0.18 | ||||
| Food allergy | 10.95 | 3.88–30.90 | <0.001 | 12.13 | 3.57–41.18 | <0.001 | |
| Education level of caregivers | 2.13 | 0.84–5.43 | 0.11 | ||||
| Follow up mode | 0.67 | 0.28–1.60 | 0.37 | ||||
| Asthma | 3.68 | 1.34–10.08 | 0.011 | 0.43 | 0.03–5.45 | 0.75 | |
| Use of acarid products | 4.76 | 1.81–12.55 | 0.002 | 8.80 | 0.76–101.79 | 0.08 | |
| Whether vitamin D3 was sufficient | 12.16 | 2.69–54.95 | 0.001 | 22.21 | 4.04–122.30 | <0.001 | |
SLIT, sublingual immunotherapy; OR, odds ratio; CI, confidence interval.
Subsequently, the above four variables were included in the multivariate analysis. Multivariate logistic regression analysis was performed to determine the independent factors influencing SLIT efficacy. Multivariate logistic regression analysis (Table 3) showed that after adjusting for whether the patient also had asthma and whether they had used acarid products, whether the patient also had a food allergy (OR: 12.13, 95% CI: 3.57–41.18, P<0.001) and whether vitamin D3 is sufficient (OR: 22.21, 95% CI: 4.04–122.30, P<0.001) were independent factors affecting the efficacy of SLIT.
Discussion
SLIT can be used to treat allergic diseases. It is no longer based on the failure of symptomatic drug treatment but is currently recommended as the initial first-line treatment for allergic respiratory diseases (6). SLIT is considered a disease improvement therapy that can prevent sensitization by new allergens and the progression of respiratory allergies (7). The first studies on the efficacy and safety of SLIT for children and adults with AR in China were published in 2007 and 2011 respectively (8,9). Therefore, as a disease improvement treatment scheme with good safety and tolerance, SLIT is currently recommended as the first-line treatment of AR in China (10). To guide and improve the effectiveness and safety of SLIT in clinical practice, the Chinese guidelines for SLIT of AR and asthma were published in 2019. These guidelines include the epidemiology, molecular mechanism, indications, and contraindications of SLIT, standardized allergen preparation, efficacy evaluation, and adverse event management of AR and asthma (11).
Sublingual administration of D. farinae drops improves the clinical symptoms of children with AR caused by dust mites and also enhances their immune function (12). Studies have confirmed the efficacy and safety of SLIT in children with AR coupled with single and multiple allergies and can achieve long-term efficacy (13-15). A study has shown that SLIT can effectively reduce the dose of inhaled corticosteroids (ICS) in adolescents with mild to moderate allergic asthma, with a good safety profile (16). Additionally, SLIT also improves disease severity and prevents new sensitization in children with an atopic dermatitis allergy to dust mites (17). Despite its good curative effect, SLIT still cannot improve the disease condition of some patients. For patients with poor SLIT efficacy, individual dose adjustment is usually applied to further improve the efficacy (5); however, some children still have poor efficacy and cease SLIT treatment. Therefore, identifying the factors affecting the efficacy of SLIT is crucial.
Vitamin D3 deficiency is positively correlated with the prevalence of allergy; A study has found that the OR remained significant after adjusting the model according to age, gender, race, smoking, drinking, and education (18). Vitamin D3 deficiency is a risk factor for allergic diseases, especially in children (19). A study has shown that vitamin D3 supplements can reduce the severity of atopic dermatitis and AR in children (20). Through animal experiments, some scholars have revealed that vitamin D3 has a preventive and therapeutic effect on AR, which provides theoretical guidance for practical application (21). Comprehensive metabolomic analysis has demonstrated the links between vitamin D-related metabolites and the gut microbiome as well as immunosensitivity responses associated with childhood allergy (22). A systematic review and meta-analysis showed that vitamin D levels were not associated with the occurrence of AR but lower vitamin D levels were only associated with a higher prevalence of AR in children. Thus, there is insufficient evidence to support vitamin D supplementation to prevent AR.
However, doctors should consider assessing vitamin D deficiency in patients during AR treatment, especially in children (23). The incidence of vitamin D deficiency and insufficiency in Korean primary school students is very high. Low vitamin D levels are related to the recent symptoms of atopic dermatitis (AD) and AR (24). A study has shown that vitamin D deficiency is negatively related to mite-specific IgE levels, which may increase the susceptibility to early childhood allergies (including rhinitis and asthma) (25). A study has shown that adequate vitamin D levels are associated with a reduced risk of asthma attacks over a 3-year period (26). Compared with the healthy group, vitamin D deficiency was often observed in Egyptian children. A significant negative correlation was also identified between vitamin D levels and the severity of AR disease (27). Consistent with the above study, our study found that after adjusting for asthma and the use of acarid products, the adequacy of vitamin D3 is an independent factor affecting the efficacy of SLIT. We also found that whether the patient also had a food allergy is an independent factor affecting the efficacy of SLIT. It may be that food allergies lead to dietary restrictions, which is related to the insufficient intake of vitamin D orally.
Chinese scholars have found that a certain proportion of people of all ages in Hainan has vitamin D deficiency and insufficiency. Formal recommendation for vitamin D supplementation should be considered, especially in children over 7 years of age in autumn and winter (28). The common international classification of vitamin D nutritional status is as follows: vitamin D concentration less than 10 ng/mL is considered a severe deficiency; 10–25 ng/mL is considered a deficiency; 25–30 ng/mL is considered insufficiency; 30–100 ng/mL is considered normal; 100–150 ng/mL is considered excessive; and over 150 ng/mL is considered poisoning (29). The cutoff value of vitamin D3 in this study was 22.25 ng/mL, which is basically consistent with the international reference standard and is convenient for clinical application. In the clinical application of SLIT for the treatment of AR in children, we should pay more attention to children with vitamin D deficiency. In future research, we will attempt to observe whether vitamin D supplementation can enhance the efficacy of SLIT in children with poor efficacy.
Jerzyńska et al. found that vitamin D 1,000 IU daily supplementation as a supplementary treatment of grass pollen allergy in children with pollen seasonal AR significantly reduced the symptom/drug score. They revealed the immunotherapeutic effect of vitamin D on AR in children (30). A study has found that vitamin D supplementation can enhance the efficacy of antihistamines in AR patients with vitamin D deficiency (31). Vitamin D3 can also enhance the efficacy of SLIT by stimulating allergen-specific helper T cells (TH1) and regulatory T cells (Treg) (32). Although some studies have shown that vitamin D supplementation may play a role in the prevention of allergic diseases, other studies do not support this hypothesis. It is not possible to determine the effect of vitamin D on allergic diseases. Further research should focus on large, multicenter human studies to explore the dose of vitamin D supplementation at different stages of life and identify the key factors affecting efficacy.
This study has some limitations that should be considered. Due to the limited resources and number of patients, the small sample size may limit the generalizability of the results of this study to a larger population. In addition, the short follow-up period is another drawback, and we will extend the follow-up period in the future to enhance the reliability of our conclusions.
Conclusions
The present study found that whether vitamin D3 is sufficient is an independent factor affecting the efficacy of SLIT. Therefore, serum vitamin D3 deficiency can affect the efficacy of SLIT in children with AR. This study provided a new therapeutic approach for SLIT patients with poor efficacy, and vitamin D3 supplementation may be a powerful way to enhance SLIT efficacy in the future.
Acknowledgments
Funding: The study was supported by Science and Technology Project of Tianjin Municipal Health Commission (grant No. ZC20135).
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1883/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1883/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1883/coif). All authors report that the study was supported by Science and technology project of Tianjin Municipal Health Commission (grant No. ZC20135). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Tianjin Children’s Hospital (No. KY2020-47) and informed consent was taken from all the patients’ guardians.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nasser M, Fedorowicz Z, Aljufairi H, et al. Antihistamines used in addition to topical nasal steroids for intermittent and persistent allergic rhinitis in children. Cochrane Database Syst Rev 2010;2010:CD006989. [Crossref] [PubMed]
- Jacobsen L, Wahn U, Bilo MB. Allergen-specific immunotherapy provides immediate, long-term and preventive clinical effects in children and adults: the effects of immunotherapy can be categorised by level of benefit -the centenary of allergen specific subcutaneous immunotherapy. Clin Transl Allergy 2012;2:8. [Crossref] [PubMed]
- Qin YE, Mao JR, Sang YC, et al. Clinical efficacy and compliance of sublingual immunotherapy with Dermatophagoides farinae drops in patients with atopic dermatitis. Int J Dermatol 2014;53:650-5. [Crossref] [PubMed]
- Subspecialty Group of Rhinology, Editorial Board of Chinese Journal of Otorhinolaryngology Head and Neck Surgery. Subspecialty Groups of Rhinology and Pediatrics, Society of Otorhinolaryngology Head and Neck Surgery, Chinese Medical Association. Guideline for diagnosis and treatment of pediatric allergic rhinitis (2022, revision). Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2022;57:392-404. [Crossref] [PubMed]
- Gao Y, Lin X, Ma J, et al. Enhanced Efficacy of Dust Mite Sublingual Immunotherapy in Low-Response Allergic Rhinitis Patients after Dose Increment at 6 Months: A Prospective Study. Int Arch Allergy Immunol 2020;181:311-9. [Crossref] [PubMed]
- Canonica GW, Cox L, Pawankar R, et al. Sublingual immunotherapy: World Allergy Organization position paper 2013 update. World Allergy Organ J 2014;7:6. [Crossref] [PubMed]
- Pajno GB, Bernardini R, Peroni D, et al. Clinical practice recommendations for allergen-specific immunotherapy in children: the Italian consensus report. Ital J Pediatr 2017;43:13. [Crossref] [PubMed]
- Cao LF, Lu Q, Gu HL, et al. Clinical evaluation for sublingual immunotherapy of allergic asthma and atopic rhinitis with Dermatophagoides Farinae Drops. Zhonghua Er Ke Za Zhi 2007;45:736-41.
- Li TY, Chen DH, Lin ZB, et al. Efficacy of sublingual immunotherapy with dermatophagoides farinae drops in patients with allergic rhinitis. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2011;46:859-62.
- Cheng L, Zhou WC. Sublingual immunotherapy of house dust mite respiratory allergy in China. Allergol Immunopathol (Madr) 2019;47:85-9. [Crossref] [PubMed]
- Li H, Chen S, Cheng L, et al. Chinese guideline on sublingual immunotherapy for allergic rhinitis and asthma. J Thorac Dis 2019;11:4936-50. [Crossref] [PubMed]
- Yu W, Mao L, Pan Q, et al. Efficacy of Sublingual Administration of Dermatophagoides Farinae Drops for Treatment of Pediatric Allergic Rhinitis Accompanied by Adenoid Hypertrophy and Improvement of Immune Function. Med Sci Monit 2019;25:333-40. [Crossref] [PubMed]
- Zhang YZ, Luo J, Wang ZH, et al. Efficacy and safety of sublingual dust mite drops in children with mono- or polysensitized allergic rhinitis. Am J Otolaryngol 2019;40:755-60. [Crossref] [PubMed]
- Cui L, Li J, Li Y, et al. Long-Term Efficacy of Sublingual Mite Immunotherapy in Monosensitized and Polysensitized Children with Allergic Rhinitis: A 7-Year Prospective Study. Int Arch Allergy Immunol 2019;180:144-9. [Crossref] [PubMed]
- Kulalert P, Phinyo P, Lao-Araya M. Efficacy and safety of house dust mite sublingual immunotherapy tablets in allergic rhinitis: A systematic review and meta-analysis. World Allergy Organ J 2022;15:100691. [Crossref] [PubMed]
- Wongsa C, Phinyo P, Sompornrattanaphan M, et al. Efficacy and Safety of House Dust Mite Sublingual Immunotherapy Tablet in Allergic Asthma: A Systematic Review of Randomized Controlled Trials. J Allergy Clin Immunol Pract 2022;10:1342-1355.e24. [Crossref] [PubMed]
- Kim M, Lee E, Yoon J, et al. Sublingual immunotherapy may be effective in reducing house dust mite allergies in children with atopic dermatitis. Acta Paediatr 2022;111:2142-8. [Crossref] [PubMed]
- Frieri M, Valluri A. Vitamin D deficiency as a risk factor for allergic disorders and immune mechanisms. Allergy Asthma Proc 2011;32:438-44. [Crossref] [PubMed]
- Litonjua AA. Vitamin D deficiency as a risk factor for childhood allergic disease and asthma. Curr Opin Allergy Clin Immunol 2012;12:179-85. [Crossref] [PubMed]
- Li Q, Zhou Q, Zhang G, et al. Vitamin D Supplementation and Allergic Diseases during Childhood: A Systematic Review and Meta-Analysis. Nutrients 2022;14:3947. [Crossref] [PubMed]
- Li B, Zhang X, Sun Z, et al. A Novel Strategy for the Treatment of Allergic Rhinitis: Regulating Treg/Th17 and Th1/Th2 Balance In Vivo by Vitamin D. Comput Math Methods Med 2022;2022:9249627. [Crossref] [PubMed]
- Chang YH, Yeh KW, Huang JL, et al. Metabolomics analysis reveals molecular linkages for the impact of vitamin D on childhood allergic airway diseases. Pediatr Allergy Immunol 2022;33:e13785. [Crossref] [PubMed]
- Kim YH, Kim KW, Kim MJ, et al. Vitamin D levels in allergic rhinitis: a systematic review and meta-analysis. Pediatr Allergy Immunol 2016;27:580-90. [Crossref] [PubMed]
- Yang HK, Choi J, Kim WK, et al. The association between hypovitaminosis D and pediatric allergic diseases: A Korean nationwide population-based study. Allergy Asthma Proc 2016;37:64-9. [Crossref] [PubMed]
- Chiu CY, Su KW, Tsai MH, et al. Longitudinal vitamin D deficiency is inversely related to mite sensitization in early childhood. Pediatr Allergy Immunol 2018;29:254-9. [Crossref] [PubMed]
- Li Q, Zhou Q, Zhang G, et al. Long-term effects of vitamin D on exacerbation rate, health care utilization and lung function in children with asthma. Ann Transl Med 2022;10:1094. [Crossref] [PubMed]
- Saad K, Abdelmoghny A, Aboul-Khair MD, et al. Vitamin D Status in Egyptian Children With Allergic Rhinitis. Ear Nose Throat J 2020;99:508-12. [Crossref] [PubMed]
- Li HA, Zou SQ, Li BT, et al. Serum vitamin D status among healthy children in Hainan, South China: a multi-center analysis of 10,262 children. Transl Pediatr 2022;11:1010-7. [Crossref] [PubMed]
- Institute of Medicine Standing Committee on the Scientific Evaluation of Dietary Reference I. The National Academies Collection: Reports funded by National Institutes of Health. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. Washington (DC): National Academies Press (US); National Academy of Sciences; 1997.
- Jerzyńska J, Stelmach W, Rychlik B, et al. Clinical and immunological effects of vitamin D supplementation during the pollen season in children with allergic rhinitis. Arch Med Sci 2018;14:122-31. [Crossref] [PubMed]
- Bakhshaee M, Sharifian M, Esmatinia F, et al. Therapeutic effect of vitamin D supplementation on allergic rhinitis. Eur Arch Otorhinolaryngol 2019;276:2797-801. [Crossref] [PubMed]
- Sadeghi M, Keshavarz Shahbaz S, Dehnavi S, et al. Current possibilities and future perspectives for improving efficacy of allergen-specific sublingual immunotherapy. Int Immunopharmacol 2021;101:108350. [Crossref] [PubMed]
(English Language Editor: A. Kassem)