
Impact of anesthetic factors on prognosis of patients with non-small cell lung cancer after surgery
Highlight box
Key findings
• Perioperative opioid and glucocorticoid exposure were independent predictors for outcomes.
What is known and what is new?
• Perioperative anesthesia management affects the prognosis of patients with lung cancer after surgery to some extent.
• This study found that perioperative opioid exposure seems to be beneficial to long-term survival, but has unfavorable effect on short-term outcomes of patients with non-small cell lung cancer after surgery.
What is the implication, and what should change now?
• This study suggested that the rational use of opioids should be able to balance the pros and cons of opioids, and clinicians should take into account of the cancer subtypes and the specific features of each individual patient on the development of perioperative analgesia strategies for patients with non-small cell lung cancer.
Introduction
According to the global cancer statistics in 2020, there were 2,206,771 new cases of lung cancer worldwide, and at least 1,766,144 patients died of lung cancer (1). One of the leading causes of cancer mortality in both sexes is lung cancer. According to the American Cancer Society for Lung Cancer in the United States for 2020, it accounted for 25% of all cancer deaths (1). Non-small cell lung cancer (NSCLC) accounts for about 84% of all lung cancer cases and there has been a revolution of new advances in the management of NSCLC with remarkable progresses in screening, diagnosis, and treatment since the past decade (2). If the patient condition is feasible, surgical resection remains the single most recommended and successful option for cure (3). However, some patients experience recurrence and metastasis of lung cancer after surgery (4). The perioperative period constitutes an important stage of disease management in cancer surgery because circulating tumor cells shed from the primary tumor into the patient’s bloodstream might lead to the formation of new micro-metastases (5). Experimental and observational research suggests that the immune function of patients is blunted due to the cytotoxic effect of natural killer (NK) cells and the reaction of T cells attenuated by perioperative traumatic stress response during the perioperative period (6). Various perioperative anesthetic factors, including anesthesia techniques, anesthetic agents, intraoperative blood transfusion and so forth, have been implicated in tumor recurrence or metastasis through effects on cancer cell signaling, the immune response, or modulation of the neuroendocrine stress response (7), but it seems to be difficult to reach a conclusion whether they promote or inhibit the progression of cancer. The previous study has revealed the perioperative administration of dexamethasone and flurbiprofen axetil, which is a kind of nonselective cyclooxygenase (COX) inhibitor with high binding affinity to the site of lesion, may improve patients’ long-term survival after surgery for NSCLC (8).
Several literatures have also shown that the association of perioperative anesthesia management and postoperative complications (9,10). Postoperative pulmonary complications (PPCs) which refers to a series of lung complications that occur after surgery such as atelectasis and pneumonia, are the most frequently observed complications following lung resection (11). PPCs have a significant clinical impact associated with the short-term rehabilitation of patients (12). The decrease of complications is conducive to promote postoperative rehabilitation and improve the short-term prognosis of patients (13).
So far, there are not many retrospective studies on NSCLC with the follow-up period of over 10 years and a combination of long-term survival with short-term outcomes to comprehensively analyze the correlation between perioperative factors and prognosis of patients. The aim of this study was to retrospectively investigate the prognostic factors of patients with NSCLC undergoing surgery through analysis of 10-year follow-up data, and to evaluate the effect of perioperative anesthesia management on postoperative short-term rehabilitation and long-term survival of patients, in order to provide a theoretical basis for optimizing perioperative management and improving the prognosis of cancer patients. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1812/rc).
Methods
Patients
The electronic medical records system of Peking University Cancer Hospital was reviewed to screen consecutive patients undergoing surgical procedures between January 1, 2006 and December 31, 2009. The inclusion criteria were: (I) pathologically confirmed NSCLC; (II) underwent segmentectomy, lobectomy or total pneumonectomy. The exclusion criteria were as follows: (I) combined with other primary malignant tumors; (II) recurrent lung tumor; (III) long-term steroid exposure; (IV) impossible follow-up because of missing data. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Clinical Research Ethics Committee of Peking University Cancer Hospital approved the study (No. 2019YJZ22-GZ02) and waived the need for written informed consent given that all patients were operated on many years ago and lived all over the country. But oral consent was obtained from each patient included in the study.
Anesthesia method
Patients was induced with intravenous anesthetics (propofol and/or etomidate) and opioids (fentanyl or sufentanil). Anesthesia was maintained with inhalational anesthetics (sevoflurane or isoflurane, with or without nitrous oxide) and opioids (fentanyl and/or sufentanil). In some cases, combined epidural anesthesia was also administered with local anesthetics (lidocaine and/or ropivacaine) according to the preference of anesthesiologists. After surgery, postoperative analgesia was provided with patient-controlled analgesia pumps, which were performed with ropivacaine for epidural analgesia or opioids (morphine or sufentanil) for intravenous analgesia.
Data collection
Information of patients were collected and sorted through the medical record system as follows: demographic characteristics (age, gender, height and weight), preoperative information (comorbidity, American Society of Anesthesiologists classification, tumor location, lymph node metastasis according to imageological examination, history of smoking and drinking, and preoperative chemotherapy), anesthesia-related information (anesthesia technique, anesthetics administration, intraoperative fluid infusion, intraoperative blood transfusion, perioperative total amount of fentanyl equivalents, perioperative glucocorticoids, and nonsteroidal anti-inflammatory drugs administration), surgical information (surgical procedure, mediastinal lymph node dissection, duration of surgery), and postoperative information (PPCs, maximal tumor size, pathological diagnosis, grade of tumor cell differentiation, chemotherapy and/or radiotherapy). The consumption of opioids was involved in preoperative sedation, intraoperative anesthesia, patient control analgesia and remedial medications due to insufficient postoperative analgesia, and eventually converted to fentanyl equivalents. The fentanyl equivalents conversion is sufentanil 0.1 µg, remifentanil 1 µg, or 110 µg morphine for 1 µg fentanyl. Data assignment was conducted with reference to the previously published study (8).
Follow-up
All patients were followed up by specially assigned personnel through outpatient review and telephone inquiry. Patients were followed up every 6 months within 1 year after surgical procedures, and once a year thereafter. Tumor recurrence and patient survival were confirmed during each follow-up. The time of tumor recurrence and death was recorded. Tumor recurrence including local recurrence or distant recurrence/metastasis was confirmed by imageological examination. The time of recurrence was the earliest date of clinical diagnosis made by surgeons or radiologists according to imageological evidence. The time of death was the date appeared in the medical certificate of death. The termination of follow-up were death, loss of follow-up or completed follow-up of 10 years. Last follow-up date was on July 1, 2020.
Endpoints and statistical analysis
The primary endpoint was overall survival (OS), which is defined as the duration in years from surgery to death from any cause. The secondary endpoint was recurrence-free survival (RFS), which was defined as the duration in years from surgery to recurrence or death, whichever happened first, and PPCs, including prolonged air leakage over 5 days, pleural effusion requiring drainage, atelectasis and pneumonia, were diagnosed by postoperative imageological examination and clinicians.
Numeric data with abnormal distribution were presented as median [interquartile range (IQR)]. Categorical data were presented as numbers (%). Time-to-event endpoints including RFS and OS were estimated using the Kaplan-Meier univariable analyses, and were compared using log-rank tests between layers. For continuous variables, cutoff values between layers were selected based on clinical significance, literatures, or median values. Relevant variables with P value <0.10 in Kaplan-Meier survival analyses were included into the Cox proportional hazard model for multivariable analyses to identify independent factors that were associated with RFS or OS. Univariable and multivariable logistic regression were performed to estimate the association between perioperative factors and PPCs. Variables that were possibly associated with the PPCs (P value <0.05) in univariable logistic regression analyses were included in the multivariable model to determine potentially independent risk factors for PPCs. Statistical analysis was performed using SPSS19.0 (SPSS, Inc., Chicago, IL, USA). Statistical significance was set at a two-sided P value <0.05.
Results
Patient recruitment and follow-up
A total of 659 consecutive patients with NSCLC who had undergone lung resection between January 1, 2006 and December 31, 2009 were retrospectively reviewed in the study. With in depth consideration of the data, 22 cases were excluded, among which 15 cases were complicated with other primary malignant tumors, and 7 cases had missing clinical data. During the follow-up period, 49 patients were lost to follow-up, and the loss rate was 7.4% (49/659). In total, 588 patients were enrolled in the statistical analysis finally (Figure 1).
The postoperative follow-up was terminated on June 30, 2020. The median duration of postoperative follow-up was 7.4 (IQR, 3.3–10.2) years. By the end of follow-up, 342 of 588 patients died, with a total mortality rate of 58.2% and an overall recurrence/mortality rate of 61.7%. The overall RFS was 4.4 (IQR, 1.1–10.1) years, and the OS was 6.2 (IQR, 2.4–10.2) years (Table 1). The cumulative RFS rate and OS rate at 1, 3, 5, and 10 years were 65.0%, 51.4%, 43.6 %, 37.7% and 79.3%, 62.1%, 52.2%, 40.4% respectively (Table 2).
Table 1
| Variables | Median [IQR] or n (%) |
|---|---|
| Age (years) | 61 [53–67] |
| BMI (kg/m2) | 24.2 [22.2–26.2] |
| Gender | |
| Female | 214 (36.4) |
| Male | 374 (63.6) |
| Preoperative complication | |
| Coronary heart disease | 37 (6.3) |
| Hypertension | 156 (26.5) |
| Arrhythmia | 41 (7.0) |
| Diabetes | 62 (10.5) |
| Smoking | 313 (53.2) |
| Drinking | 135 (23.0) |
| Preoperative chemotherapy | 60 (10.2) |
| ASA | |
| I | 215 (36.6) |
| II | 373 (63.4) |
| Tumor stage† | |
| I | 265 (45.1) |
| II | 114 (19.4) |
| III | 161 (27.4) |
| IV | 48 (8.1) |
| Anesthesia technique | |
| General anesthesia | 448 (76.2) |
| Combined epidural-general anesthesia | 140 (23.8) |
| Duration of surgery (h) | 3.8 [3.1–4.4] |
| Total intraoperative fluid volume (mL) | 1,600 [2,000–2,500] |
| Intraoperative blood transfusion | 9 (1.5) |
| Perioperative fentanyl equivalents (μg/kg) | 28.2 [7.7–35.9] |
| Perioperative NSAIDs administration | 332 (53.5) |
| Perioperative glucocorticoids administration | 353 (56.8) |
| Surgical procedure | |
| Segmentectomy | 131 (22.3) |
| Lobectomy | 436 (74.1) |
| Total pneumectomy | 21 (3.6) |
| Mediastinal lymph node dissection | 527 (89.6) |
| Grade of tumor cell differentiation | |
| Undifferentiation | 81 (13.8) |
| Poor differentiation | 75 (12.8) |
| Moderate differentiation | 364 (61.9) |
| High differentiation | 68 (11.6) |
| Maximal tumor size (cm) | 3 [2.0–4.2] |
| PPCs | 413 (70.2) |
| Prolonged air leakage over 5 days | 359 (61.1) |
| Pleural effusion requiring drainage | 74 (12.6) |
| Atelectasis | 27 (4.5) |
| Pneumonia | 21 (3.7) |
| Postoperative radiotherapy | 24 (4.1) |
| Postoperative chemotherapy | 285 (48.5) |
| Death | 342 (58.2) |
| Recurrence or death | 363 (61.7) |
| Overall survival (years) | 6.2 [2.4–10.2] |
| Recurrence-free survival (years) | 4.4 [1.1–10.1] |
| Postoperative follow-up (years) | 7.4 [3.3–10.2] |
†, tumor stage according to the 8th edition of TNM staging system for lung cancer. ASA, American Society of Anesthesiologists; BMI, body mass index; NSAIDs, non-steroid anti-inflammatory drugs; IQR, interquartile range; PPCs, postoperative pulmonary complications.
Table 2
| Survival rate | OS (%) | RFS (%) |
|---|---|---|
| Survival rate at 1 year | 79.3 (75.7–82.3) | 65.0 (61.0–68.7) |
| Survival rate at 3 years | 62.1 (58.0–65.9) | 51.4 (47.3–55.3) |
| Survival rate at 5 years | 52.2 (48.0–56.1) | 43.6 (39.6–47.6) |
| Survival rate at 10 years | 40.4 (36.2–44.5) | 37.7 (33.7–41.7) |
Results are mean (95% confidence interval). OS, overall survival; RFS, recurrence-free survival.
Predictor analysis
OS
Kaplan-Meier univariable analyses was used to screen out fifteen factors (P<0.10), including age, BMI, hypertension history, preoperative chemotherapy, tumor stage, surgery procedure, mediastinal lymph node dissection, duration of surgery, intraoperative blood transfusion, perioperative fentanyl equivalents, perioperative glucocorticoids administration, tumor grade, maximal tumor size, postoperative radiotherapy and chemotherapy (Table 3, Figure 2).
Table 3
| Variables | N | RFS | OS | |||
|---|---|---|---|---|---|---|
| n (%) | P value | n (%) | P value | |||
| Age (years) | 0.123 | 0.053 | ||||
| <60 | 258 | 112 (43.4) | 122 (47.3) | |||
| ≥60 | 330 | 113 (34.2) | 124 (37.6) | |||
| Gender | 0.962 | 0.118 | ||||
| Female | 214 | 81 (37.9) | 96 (44.9) | |||
| Male | 374 | 144 (38.5) | 150 (40.1) | |||
| BMI (kg/m2) | 0.083 | 0.031 | ||||
| <18.5 | 19 | 4 (21.1) | 5 (26.3) | |||
| 18.5–24.9 | 343 | 123 (35.9) | 135 (39.4) | |||
| 25–27.9 | 170 | 67 (39.4) | 72 (42.4) | |||
| ≥28 | 56 | 31 (55.4) | 34 (60.7) | |||
| Coronary heart disease | 0.139 | 0.657 | ||||
| No | 551 | 208 (37.7) | 229 (41.6) | |||
| Yes | 37 | 17 (45.9) | 17 (45.9) | |||
| Hypertension | <0.001 | 0.002 | ||||
| No | 432 | 147 (34.0) | 164 (38.0) | |||
| Yes | 156 | 78 (50.0) | 82 (52.6) | |||
| Arrythmia | 0.476 | 0.398 | ||||
| No | 547 | 212 (38.8) | 231 (42.2) | |||
| Yes | 41 | 13 (31.7) | 15 (36.6) | |||
| Diabetes | 0.093 | 0.189 | ||||
| No | 526 | 195 (37.1) | 215 (40.9) | |||
| Yes | 62 | 30 (48.4) | 31 (50.0) | |||
| Smoking | 0.734 | 0.221 | ||||
| No | 275 | 103 (37.5) | 120 (43.6) | |||
| Yes | 313 | 122 (39.0) | 126 (40.3) | |||
| Drinking | 0.900 | 0.472 | ||||
| No | 453 | 172 (38.0) | 192 (42.4) | |||
| Yes | 135 | 53 (39.3) | 54 (40.0) | |||
| Preoperative chemotherapy | 0.063 | 0.028 | ||||
| No | 528 | 206 (39.0) | 226 (42.8) | |||
| Yes | 60 | 41 (31.7) | 20 (33.3) | |||
| ASA | 0.342 | 0.303 | ||||
| I | 215 | 81 (37.7) | 86 (40.0) | |||
| II | 373 | 144 (38.6) | 160 (42.9) | |||
| Tumor stage† | <0.001 | <0.001 | ||||
| I | 265 | 146 (55.1) | 158 (59.6) | |||
| II | 114 | 44 (38.6) | 46 (40.4) | |||
| III | 161 | 29 (18.0) | 35 (21.7) | |||
| IV | 48 | 6 (12.5) | 7 (14.6) | |||
| Surgical procedure | <0.001 | <0.001 | ||||
| Segmentectomy | 131 | 36 (27.5) | 38 (29.0) | |||
| Lobectomy | 436 | 178 (40.8) | 197 (45.2) | |||
| Total pneumectomy | 21 | 11 (52.4) | 11 (52.4) | |||
| Mediastinal lymph node dissection | <0.001 | <0.001 | ||||
| No | 61 | 10 (16.4) | 11 (18.0) | |||
| Yes | 527 | 215 (40.8) | 235 (44.6) | |||
| Anesthesia technique | 0.168 | 0.399 | ||||
| General anesthesia | 448 | 166 (37.1) | 184 (41.1) | |||
| Combined epidural-general anesthesia | 140 | 59 (42.1) | 62 (44.3) | |||
| Duration of surgery (h) | 0.019 | 0.012 | ||||
| ≤3.0 | 129 | 40 (31.0) | 42 (32.6) | |||
| >3.0 | 459 | 185 (40.3) | 204 (44.4) | |||
| Total intraoperative fluid volume (mL) | 0.492 | 0.659 | ||||
| ≤2,000 | 306 | 116 (37.9) | 127 (41.5) | |||
| >2,000 | 282 | 109 (38.7) | 119 (42.2) | |||
| Intraoperative blood transfusion | 0.099 | 0.027 | ||||
| No | 579 | 224 (38.7) | 245 (42.3) | |||
| Yes | 9 | 1 (11.1) | 1 (11.1) | |||
| Perioperative fentanyl equivalents (μg/kg) | 0.081 | 0.007 | ||||
| ≤28.2 | 294 | 101 (34.4) | 110 (37.4) | |||
| >28.2 | 294 | 117 (42.2) | 136 (46.3) | |||
| Perioperative NSAIDs administration | 0.591 | 0.251 | ||||
| No | 267 | 97 (36.3) | 105 (39.3) | |||
| Yes | 321 | 128 (39.9) | 141 (43.9) | |||
| Perioperative glucocorticoids administration | 0.016 | 0.047 | ||||
| No | 256 | 90 (35.2) | 98 (38.3) | |||
| Yes | 332 | 135 (40.7) | 148 (44.6) | |||
| Grade of tumor cell differentiation | 0.001 | <0.001 | ||||
| Undifferentiation | 81 | 36 (44.4) | 38 (46.9) | |||
| Poor differentiation | 75 | 19 (25.3) | 21 (28.0) | |||
| Moderate differentiation | 364 | 132 (36.3) | 144 (39.6) | |||
| High differentiation | 68 | 38 (55.9) | 43 (63.2) | |||
| Maximal tumor size (cm) | <0.001 | <0.001 | ||||
| ≤3.0 | 327 | 142 (43.4) | 157 (48.0) | |||
| >3.0 | 261 | 83 (31.8) | 89 (34.1) | |||
| PPCs | 0.080 | 0.117 | ||||
| No | 175 | 78 (44.6) | 83 (47.4) | |||
| Yes | 413 | 147 (35.6) | 163 (39.5) | |||
| Postoperative radiotherapy | <0.001 | 0.024 | ||||
| No | 564 | 223 (39.5) | 242 (42.9) | |||
| Yes | 24 | 2 (8.3) | 4 (16.7) | |||
| Postoperative chemotherapy | <0.001 | <0.001 | ||||
| No | 303 | 146 (48.2) | 150 (49.5) | |||
| Yes | 285 | 79 (27.7) | 96 (33.7) | |||
†, tumor stage according to the 8th edition of TNM staging system for lung cancer. P<0.05 was considered statistically significant. RFS, recurrence-free survival; OS, overall survival; BMI, body mass index; ASA, American Society of Anesthesiologists; NSAIDs, Non-Steroid Anti-Inflammatory Drugs; PPCs, Postoperative pulmonary complications.
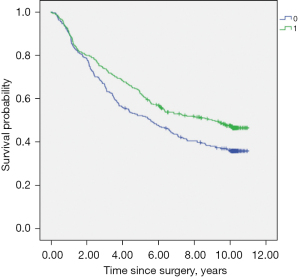
All the fifteen factors were taken into consideration in multivariable Cox regression model and 8 independent factors were eventually identified. Among them, age ≥60 years (HR =1.372, 95% CI: 1.096–1.719, P=0.006), advanced tumor stage (HR =1.525, 95% CI: 1.361–1.710, P<0.001), maximal tumor size >3 cm (HR =1.364, 95% CI: 1.092–1.703, P=0.006) were associated with shortened survival, whereas high BMI grade (HR =0.796, 95% CI: 0.679–0.933, P=0.005), mediastinal lymph node dissection (HR =0.588, 95% CI: 0.400–0.862, P=0.007), perioperative fentanyl equivalents >28.2 µg/kg (HR =0.779, 95% CI: 0.619–0.980, P=0.033), and high tumor grade (HR =0.853, 95% CI: 0.749–0.971, P=0.017) were associated with prolonged survival (Table 4).
Table 4
| Variable | RFS | OS | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age ≥60 years | – | – | 1.372 (1.096–1.719) | 0.006 | |
| BMI† | 0.825 (0.704–0.967) | 0.017 | 0.796 (0.679–0.933) | 0.005 | |
| Preoperative hypertension | 0.927 (0.707–1.214) | 0.580 | 0.843 (0.640–1.110) | 0.223 | |
| Preoperative diabetes | 0.827 (0.653–1.048) | 0.116 | – | – | |
| Preoperative chemotherapy | 1.011 (0.716–1.429) | 0.950 | 1.221 (0.862–1.729) | 0.260 | |
| Tumor stage‡ | 1.526 (1.363–1.708) | <0.001 | 1.525 (1.361–1.710) | <0.001 | |
| Surgical procedure§ | 0.865 (0.678–1.103) | 0.243 | 0.844 (0.655–1.087) | 0.190 | |
| Duration of surgery >3.0 h | 1.166 (0.883–1.540) | 0.278 | 1.099 (0.830–1.456) | 0.511 | |
| Intraoperative blood transfusion | 1.360 (0.645–2.867) | 0.419 | 1.808 (0.867–3.772) | 0.115 | |
| Mediastinal lymph node dissection | 0.612 (0.421–0.889) | 0.010 | 0.588 (0.400–0.862) | 0.007 | |
| Perioperative fentanyl equivalents >28.2 μg/kg | 0.926 (0.741–1.158) | 0.502 | 0.779 (0.619–0.980) | 0.033 | |
| Perioperative glucocorticoids administration | 0.791 (0.632–0.989) | 0.040 | 0.812 (0.643–1.024) | 0.078 | |
| Grade of tumor cell differentiation¶ | 0.914 (0.808–1.034) | 0.153 | 0.853 (0.749–0.971) | 0.017 | |
| Maximal tumor size >3 cm | 1.179 (0.948–1.465) | 0.138 | 1.364 (1.092–1.703) | 0.006 | |
| PPCs | 1.130 (0.890–1.434) | 0.316 | – | – | |
| Postoperative radiotherapy | 1.688 (1.050–2.714) | 0.031 | 1.300 (0.798–2.115) | 0.292 | |
| Postoperative chemotherapy | 1.524 (1.223–1.899) | <0.001 | 1.112 (0.885–1.396) | 0.363 | |
†, BMI was divided into four classes: underweight (<18.5 kg/m2), normal (18.5–24.9 kg/m2), overweight (25–28 kg/m2) and obesity (>28 kg/m2). The next class was compared to the last level. ‡, tumor stage was divided into four stages: I, II, III and IV. The next stage was compared to the last stage. §, surgical procedure was divided into three categories: segmentectomy, lobectomy and total pneumectomy. Total pneumectomy was compared to lobectomy or segmentectomy. ¶, grade of tumor cell differentiation was divided into four grades: undifferentiation, poor differentiation, moderate differentiation and high differentiation. The next grade was compared to the last grade. P<0.05 was considered statistically significant. RFS, recurrence-free survival; OS, overall survival; HR, hazard ratio; CI, confidence interval; BMI, body mass index; PPCs, postoperative pulmonary complications.
RFS
The Kaplan-Meier univariable analysis indicated that the following factors (P<0.10) were significantly associated with RFS: BMI, hypertension history, diabetes, preoperative chemotherapy, tumor stage, surgery procedure, mediastinal lymph node dissection, duration of surgery, intraoperative blood transfusion, perioperative fentanyl equivalents, perioperative glucocorticoids administration, tumor grade, maximal tumor size, PPCs, postoperative radiotherapy and chemotherapy (Table 3, Figure 3).
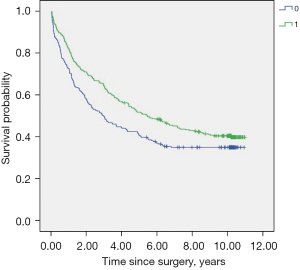
The sixteen factors above were included into multivariable Cox regression model and 6 independent factors were eventually identified. Upon multivariable analyses, advanced tumor stage (HR =1.526, 95% CI: 1.363–1.708, P<0.001), postoperative radiotherapy (HR =1.688, 95% CI: 1.050–2.714, P=0.031), and postoperative chemotherapy (HR =1.524, 95% CI: 1.223–1.899, P<0.001) were associated with early recurrence, whereas BMI (HR =0.825, 95% CI: 0.704–0.967, P=0.017), mediastinal lymph node dissection (HR =0.612, 95% CI: 0.421–0.889, P=0.010) and perioperative glucocorticoids administration (HR =0.791, 95% CI: 0.632–0.989, P=0.040) were associated with delayed recurrence (Table 4).
PPCs
Six factors (P<0.05) which were screened by univariable logistic regression analyses were included in the multivariable model (Table 5). Multivariable logistic regression analyses identified two independent factors: advanced tumor stage (OR =1.205, 95% CI: 1.002–1.448, P=0.047) and perioperative fentanyl equivalents >28.2 μg/kg (OR =1.623, 95% CI: 1.123–2.346, P=0.010) were associated with an increased PPCs risk (Table 5).
Table 5
| Variable | Univariable | Multivariable | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Age ≥60 years | 1.227 (0.860–1.750) | 0.259 | – | – | |
| Male gender | 1.814 (1.263–2.605) | 0.001 | 1.384 (0.844–2.271) | 1.198 | |
| BMI† | 1.021 (0.795–1.311) | 0.871 | – | – | |
| Preoperative hypertension | 0.863 (0.581–1.282) | 0.466 | – | – | |
| Preoperative coronary heart disease | 1.880 (0.810–4.365) | 0.142 | – | – | |
| Preoperative arrythmia | 1.168 (0.571–2.386) | 0.671 | – | – | |
| Preoperative diabetes | 0.962 (0.659–1.403) | 0.839 | – | – | |
| Smoking | 1.697 (1.188–2.424) | 0.004 | 1.136 (0.683–1.889) | 0.622 | |
| Drinking | 1.941 (1.219–3.091) | 0.005 | 1.454 (0.859–2.461) | 0.163 | |
| Preoperative chemotherapy | 2.007 (1.017–3.959) | 0.044 | 1.456 (0.722–2.936) | 0.293 | |
| ASA II | 0.838 (0.578–1.215) | 0.350 | – | – | |
| Tumor stage‡ | 1.236 (1.034–1.477) | 0.020 | 1.205 (1.002–1.448) | 0.047 | |
| Surgical procedure§ | 1.347 (0.929–1.951) | 0.116 | – | – | |
| Combined epidural-general anesthesia | 1.239 (0.809–1.897) | 0.324 | – | – | |
| Duration of surgery >3 h | 1.079 (0.706–1.648) | 0.726 | – | – | |
| Total intraoperative fluid volume (mL) | 1.340 (0.938–1.913) | 0.107 | – | – | |
| Intraoperative blood transfusion | 1.491 (0.307–7.252) | 0.620 | – | – | |
| Mediastinal lymph node dissection | 1.493 (0.861–2.591) | 0.154 | – | – | |
| Perioperative fentanyl equivalents >28.2 μg/kg | 1.556 (1.089–2.223) | 0.015 | 1.623 (1.123–2.346) | 0.010 | |
| Perioperative NSAIDs administration | 1.160 (0.814–1.654) | 0.411 | – | – | |
| Perioperative glucocorticoids administration | 0.994 (0.696–1.420) | 0.972 | – | – | |
| Grade of tumor cell differentiation¶ | 1.219 (0.992–1.498) | 0.059 | – | – | |
| Maximal tumor size >3 cm | 1.582 (1.100–2.275) | 0.013 | 1.323 (0.898–1.950) | 0.157 | |
†, BMI was divided into four classes: underweight (<18.5 kg/m2), normal (18.5–24.9 kg/m2), overweight (25–28 kg/m2) and obesity (>28 kg/m2). The next class was compared to the last level. ‡, tumor stage was divided into four stages: I, II, III and IV. The next stage was compared to the last stage. §, surgical procedure was divided into three categories: segmentectomy, lobectomy and total pneumectomy. Total pneumectomy was compared to lobectomy or segmentectomy. ¶, grade of tumor cell differentiation was divided into four grades: undifferentiation, poor differentiation, moderate differentiation and high differentiation. The next grade was compared to the last grade. P<0.05 was considered statistically significant. OR, odd ratio; CI, confidence interval; BMI, body mass index; ASA, American Society of Anesthesiologists; NSAIDs, non-steroid anti-inflammatory drugs.
Discussion
NSCLC is one of the most common causes of cancer-deaths worldwide (14). Surgical treatment is the most effective method for early NSCLC. Previous literatures have demonstrated that tumor resection promotes local recurrence and distant metastasis (15,16). Explanations for this could include inadvertent spread of tumor cells due to surgical procedures, inhibited cell-mediated immunity in surgical stress response, and increased levels of vascular endothelial growth factor (VEGF) induced by surgery, which is closely related to tumor proliferation (17-19). For lung cancer patients, many perioperative factors apart from surgery may also affect patients’ outcomes (20). Identifying these factors may provide valuable information for better strategy planning for perioperative management.
Patient-related and surgical factors
Lung cancer is a typical age-related disease. In the United States, the median age for lung cancer has reached 71 years old (21). In this cohort, the median age of patients with NSCLC was 61 (IQR, 53–67) years, and patients aged 60 years old or above accounted for 56.1% (Tables 1,3). Legras et al. reported that patients younger than 61 years old survived significantly longer than older patients (22). The findings from the study also suggested that age ≥60 years was associated with shortened survival, attributed to the declining function of the organs of the elderly and the disordered compensatory of vital organs induced by surgical stress stimulation (23). BMI is another important personal factor. A link between BMI and survival in cancer has always been sought since the past decade. It is reported that BMI <18.5 kg/m2 was associated with shortened survival of lung cancer, and a decrease in BMI was associated with an increase in risk of death in all subsets of NSCLC (24,25). This is also found to be the case in this study.
TNM staging system is valid for defining prognosis and prognosis-related criteria in NSCLC (26). Advanced tumor stage reflects poor outcomes of patients with NSCLC. In the present study, advanced tumor stage showed a meaningful relationship in association with shortened survival (HR =1.525, 95% CI: 1.361–1.710, P<0.001), early recurrence (HR =1.526, 95% CI: 1.363–1.708, P<0.001), and high risk of PPCs (OR =1.205, 95% CI: 1.002–1.448, P=0.047), in accord with previous researches (27,28). Furthermore, metastasis to the lymph nodes is a main path of lung cancer metastasis and related to poor outcomes (29), while mediastinal lymph node dissection decreases the postoperative recurrence and death of patients with NSCLC (30). In this study, complete mediastinal lymph node dissection also prolonged survival and delayed recurrence. Meanwhile, larger tumor size indicates advanced clinical stages in some sense (31). A tumor size of 3 cm in diameter has been regarded as the prognostic threshold in the staging of bronchogenic carcinoma since 1974 (32). Rena et al. found Tumor size ≤3 cm, adenocarcinoma histologic type and negative bronchial resection margins were associated with a more favorable outcome in their patient population (33). In this cohort, the tumor size over 3cm significantly shortened the OS rate. In addition, histological grading may be helpful for lung cancer management and deciding its prognosis (34). Similar to others, the results from this study found that high grade tumor-cell differentiation was associated with prolonged survival (34,35). It should also be mentioned that postoperative radiotherapy and chemotherapy were found to be associated with early recurrence in the current study. A possible reason is that most patients who received radiotherapy and chemotherapy were in advanced stages.
Perioperative opioids exposure
The perioperative use of opioids forms a crucial part of daily clinical practice in anesthesia. Opioids such as morphine, fentanyl, and sufentanil are the most widely used and effective analgesics (36), and previous study implicated that opioid impacted the oncological endpoint (37). Overall, reports on the relationship between opioid exposure and oncologic outcomes are contradictory and appear to reflect the influence of multiple factors of tumor biology and drug administration (38). For instance, morphine, the main component of opium, has the dual effect of on tumor development. Morphine may reduce NK cell activity and T-lymphocyte proliferation as well as cytokine secretion, and eventually promote tumor growth and metastasis (39). On the other hand, morphine was shown to inhibit the migration of tumor-infiltrating leukocytes and suppress angiogenesis associated with tumor growth (40). Silagy et al. and Zylla et al. reported opioid exposure was associated with shortened progression-free survival and OS in advanced-stage prostate cancer and renal cell carcinoma (41,42), whereas Du et al. and Montagna et al. demonstrated that opioids were protective against recurrence and associated with improved RFS in squamous oesophageal cancer and triple-negative breast cancer (43,44).
As far as lung cancer is concerned, we noted opioids were generally considered to have a negative effect on cancer and linked to worse oncologic outcomes in most of the literatures. Preclinical and retrospective data demonstrate that opioids promote lung cancer progression and metastasis and reduce survival (45). However, there are also different voices in recent studies. An in vitro and human lung tissue study suggested that lung cancer tissues and cell lines express opioid growth factor receptor (OGFR), a negative regulator of cell proliferation, was a binding site of morphine and involved in subsequent morphine-induced lung cancer growth suppression (46). Oh et al. investigated 1,009 medical records of patients and found intraoperative and postoperative use of opioid does not affect the risk of recurrence or death due to lung cancer (47). Hasegawa et al. drew a similar conclusion in a prospective cohort study of patients with NSCLC and suggested opioid dose does not shorten the survival of patients with advanced NSCLC (48). In this study, the association between perioperative fentanyl equivalents and OS, RFS and PPCs for NSCLC was evaluated and the median of perioperative fentanyl equivalents was selected as the cut-off value referring to the previous study (8). The results revealed that perioperative fentanyl equivalents >28.2 μg/kg prolonged the OS and improved long-term patient outcomes (HR =0.779, 95% CI: 0.619–0.980, P=0.033). In the case of small sample size and retrospective nature, the value of exceeding 28.2 μg/kg for perioperative fentanyl equivalent needed to be confirmed by prospective studies, but it suggested a trend that to some extent, increasing perioperative opioid dosage might be beneficial for the survival of patients. The effects of opioids probably varied according to cancer subtype, because the different expression of opioid receptors in lung cancer cells would be a crucial factor for opioid effects on oncologic outcomes. For example, lung adenocarcinoma showed a higher OGFR expression than squamous cell carcinoma (46). Additionally, unlike other retrospective data, sufentanil accounted for the majority of opioids in this cohort, therefore we hypothesized the biological effects that opioid drugs had on tumors were possibly related to the different drug type, dose and mode of administration (49-51). What’s more, previous evidence for the use of opioids impact on long-term survival of lung cancer cells are retrospective, and follow-up rarely exceeds 5 years in these studies (49,50). Hence, it is reasonable to speculate that different conclusions may be drawn with the extension of follow-up time, and further studies should be conducted to confirm the conclusions of those aforementioned literatures.
In contrast, the findings from this study also indicated that perioperative fentanyl equivalents >28.2 µg/kg significantly increased the risk of PPCs, was is related to unfavorable short-term outcomes, and affect the postoperative recovery of patients (OR =1.623, 95% CI: 1.123–2.346, P=0.010). There have been evidences that opioids may have adverse effects on the lungs and increase the risk of PPCs associated with opioid use (52), which may be partly attributed to potential side effects of respiratory depression and increased inhalation risk. In addition, Plein et al. and Roy et al. reported that opioids damage macrophages and NK cells and reduce migration of macrophages and neutrophils (53,54). Therefore, opioid may inhibit the immune system, and, as a result, increase the risk of postoperative pneumonia and pleural effusion. In general, as noted above, it was prudent to consider that opioids might affect PPCs primarily through indirect effects, including respiratory depression that increases the occurrence of pulmonary atelectasis, and immunosuppression that promotes inflammatory responses in the lungs and thorax, partly leading to pneumonia and pleural effusion, and further affecting the healing of prolonged air leakage. However, results from the study added evidence for increased risk for PPCs in the patients with opioid exposure alike. Increased perioperative fentanyl equivalents may be detrimental factor for recovery of patients after surgery.
From an evolving perspective, the overall decrease in opioid is not a universal end point, but should be target at specific cancer subtypes or ultimately specific patients. These findings should be considered when formulating perioperative analgesic strategies for patients with NSCLC, and supports the judicious use of opioids to balance the favorable and unfavorable effect.
Perioperative glucocorticoids exposure
In this cohort, results suggested perioperative glucocorticoids (mainly dexamethasone) were associated with delayed recurrence. Dexamethasone is frequently used to prevent postoperative nausea and vomiting (PONV) in doses of 8–10 mg (55). The effect of perioperative glucocorticoids on the outcome of cancer patients is controversial. Previous research showed that perioperative dexamethasone was associated with an increased risk of recurrence in patients after colorectal cancer surgery (56). On the other hand, perioperative dexamethasone was found that it inhibited the proliferation of breast cancer cells (57), and improved the long-term survival of patients with pancreatic cancer (58). In the previous research, the results showed that dexamethasone prolonged RFS and OS after lung cancer surgery, and that perioperative NSAIDs and glucocorticoids might produce a “synergic” effect in improving survival (8). Nevertheless, these effects require further clarification in the future.
Limitations
This study comes with several limitations. Firstly, the data of intraoperative body temperature was not recorded because this parameter was not routinely monitored during operation. Several researches reported intraoperative hypothermia was associated with increased early complications and shortened long-term survival (59). Secondly, in addition to PPCs, postoperative complications also include arrhythmias, myocardial infarction and so on (60). Nevertheless, considering the low incidence of these complications in this study, they were not set as observation variables. Thirdly, as a mono-center study, the results may not be extrapolated to other patients’ population in other centers. Finally, although epidural opioids entered the systemic circulation, the accuracy of this conversion ratio for epidural opioids was unclear (47). Therefore, differences in the blood half-lives of opioids and method of administration (bolus or continuous) might lead to unresolved bias in the analysis.
Conclusions
Taken together, though there were no significant associations observed between other perioperative anesthetic factors such as anesthesia technique with patient’s outcomes in this study, the findings suggested that opioid and glucocorticoid exposure may affect the prognosis of patients with NSCLC after surgery anyway. If perioperative fentanyl equivalents were over 28.2 µg/kg, it could be a favorable factor for OS, but a negative factor for short-term rehabilitation of patients with NSCLC undergoing resection. However, these findings need to be validated by further prospective studies and must be interpreted with some caution because of the small cohort size and retrospective design.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1812/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1812/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1812/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1812/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Clinical Research Ethics Committee of Peking University Cancer Hospital approved the study (No. 2019YJZ22-GZ02) and waived the need for written informed consent given that all patients were operated on many years ago and lived all over the country. But oral consent was obtained from each patient included in the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7-30. [Crossref] [PubMed]
- Alexander M, Kim SY, Cheng H. Update 2020: Management of Non-Small Cell Lung Cancer. Lung 2020;198:897-907. [Crossref] [PubMed]
- Molina JR, Yang P, Cassivi SD, et al. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc 2008;83:584-94. [Crossref] [PubMed]
- Wang J, Liu L, Song Y, et al. Current Understanding on Perioperative Management in Lung Cancer: Implications for Anesthetic Considerations. Drug Des Devel Ther 2021;15:835-42. [Crossref] [PubMed]
- Müller SD, Ziegler JSH, Piegeler T. Local Anesthetics and Recurrence after Cancer Surgery-What's New? A Narrative Review. J Clin Med 2021;10:719. [Crossref] [PubMed]
- Orriach JLG, Raigon-Ponferrada A, Buggy DJ. Can Anaesthesia and Analgesia Interventions During Cáncer Surgery Influence Recurrence or Metástasis? Curr Pharm Des 2019;25:2997. [Crossref] [PubMed]
- Byrne K, Levins KJ, Buggy DJ. Can anesthetic-analgesic technique during primary cancer surgery affect recurrence or metastasis? Can J Anaesth 2016;63:184-92. [Crossref] [PubMed]
- Huang WW, Zhu WZ, Mu DL, et al. Perioperative Management May Improve Long-term Survival in Patients After Lung Cancer Surgery: A Retrospective Cohort Study. Anesth Analg 2018;126:1666-74. [Crossref] [PubMed]
- Lee EK, Ahn HJ, Zo JI, et al. Paravertebral Block Does Not Reduce Cancer Recurrence, but Is Related to Higher Overall Survival in Lung Cancer Surgery: A Retrospective Cohort Study. Anesth Analg 2017;125:1322-8. [Crossref] [PubMed]
- Lemme NJ, Glasser JL, Yang DS, et al. Chronic Obstructive Pulmonary Disease Associated with Prolonged Opiate Use, Increased Short-Term Complications, and the Need for Revision Surgery following Total Knee Arthroplasty. J Knee Surg 2023;36:335-43. [Crossref] [PubMed]
- Li S, Zhou K, Lai Y, et al. Estimated intraoperative blood loss correlates with postoperative cardiopulmonary complications and length of stay in patients undergoing video-assisted thoracoscopic lung cancer lobectomy: a retrospective cohort study. BMC Surg 2018;18:29. [Crossref] [PubMed]
- Agostini PJ, Lugg ST, Adams K, et al. Risk factors and short-term outcomes of postoperative pulmonary complications after VATS lobectomy. J Cardiothorac Surg 2018;13:28. [Crossref] [PubMed]
- Agostini P, Lugg ST, Adams K, et al. Postoperative pulmonary complications and rehabilitation requirements following lobectomy: a propensity score matched study of patients undergoing video-assisted thoracoscopic surgery versus thoracotomy†. Interact Cardiovasc Thorac Surg 2017;24:931-7. [Crossref] [PubMed]
- Takahashi Y, Suzuki S, Hamada K, et al. Sarcopenia is poor risk for unfavorable short- and long-term outcomes in stage I non-small cell lung cancer. Ann Transl Med 2021;9:325. [Crossref] [PubMed]
- Shurin MR, Baraldi JH, Shurin GV. Neuroimmune Regulation of Surgery-Associated Metastases. Cells 2021;10:454. [Crossref] [PubMed]
- Market M, Tennakoon G, Auer RC. Postoperative Natural Killer Cell Dysfunction: The Prime Suspect in the Case of Metastasis Following Curative Cancer Surgery. Int J Mol Sci 2021;22:11378. [Crossref] [PubMed]
- Liu X, Wang Q. Application of Anesthetics in Cancer Patients: Reviewing Current Existing Link With Tumor Recurrence. Front Oncol 2022;12:759057. [Crossref] [PubMed]
- Mao L, Lin S, Lin J. The effects of anesthetics on tumor progression. Int J Physiol Pathophysiol Pharmacol 2013;5:1-10. [PubMed]
- Yan T, Zhang GH, Wang BN, et al. Effects of propofol/remifentanil-based total intravenous anesthesia versus sevoflurane-based inhalational anesthesia on the release of VEGF-C and TGF-β and prognosis after breast cancer surgery: a prospective, randomized and controlled study. BMC Anesthesiol 2018;18:131. [Crossref] [PubMed]
- Santander Ballestín S, Lanuza Bardaji A, Marco Continente C, et al. Antitumor Anesthetic Strategy in the Perioperatory Period of the Oncological Patient: A Review. Front Med (Lausanne) 2022;9:799355. [Crossref] [PubMed]
- Vora N, Reckamp KL. Non-small cell lung cancer in the elderly: defining treatment options. Semin Oncol 2008;35:590-6. [Crossref] [PubMed]
- Legras A, Mordant P, Arame A, et al. Long-term survival of patients with pN2 lung cancer according to the pattern of lymphatic spread. Ann Thorac Surg 2014;97:1156-62. [Crossref] [PubMed]
- Zhang J, Xue ZQ, Chu XY, et al. Surgical treatment and prognosis of octogenarians with non-small cell lung cancer. Asian Pac J Trop Med 2012;5:465-8. [Crossref] [PubMed]
- Alifano M, Daffré E, Iannelli A, et al. The Reality of Lung Cancer Paradox: The Impact of Body Mass Index on Long-Term Survival of Resected Lung Cancer. A French Nationwide Analysis from the Epithor Database. Cancers (Basel) 2021;13:4574. [Crossref] [PubMed]
- Shepshelovich D, Xu W, Lu L, et al. Body Mass Index (BMI), BMI Change, and Overall Survival in Patients With SCLC and NSCLC: A Pooled Analysis of the International Lung Cancer Consortium. J Thorac Oncol 2019;14:1594-607. [Crossref] [PubMed]
- Pfannschmidt J, Muley T, Hoffmann H, et al. Dtsch Med Wochenschr 2006;131:2643-8. [Prognosis after complete surgical resection for non-small cell lung cancer based on the staging classification]. [Crossref] [PubMed]
- Pfannschmidt J, Muley T, Bülzebruck H, et al. Prognostic assessment after surgical resection for non-small cell lung cancer: experiences in 2083 patients. Lung Cancer 2007;55:371-7. [Crossref] [PubMed]
- Okami J, Higashiyama M, Asamura H, et al. Pulmonary resection in patients aged 80 years or over with clinical stage I non-small cell lung cancer: prognostic factors for overall survival and risk factors for postoperative complications. J Thorac Oncol 2009;4:1247-53. [Crossref] [PubMed]
- Lee K, Kim HR, Kim DK, et al. Post-recurrence survival analysis of stage I non-small-cell lung cancer. Asian Cardiovasc Thorac Ann 2017;25:623-9. [Crossref] [PubMed]
- Saji H, Tsuboi M, Yoshida K, et al. Prognostic impact of number of resected and involved lymph nodes at complete resection on survival in non-small cell lung cancer. J Thorac Oncol 2011;6:1865-71. [Crossref] [PubMed]
- Zhang J, Gold KA, Lin HY, et al. Relationship between tumor size and survival in non-small-cell lung cancer (NSCLC): an analysis of the surveillance, epidemiology, and end results (SEER) registry. J Thorac Oncol 2015;10:682-90. [Crossref] [PubMed]
- López-Encuentra A, Duque-Medina JL, Rami-Porta R, et al. Staging in lung cancer: is 3 cm a prognostic threshold in pathologic stage I non-small cell lung cancer? A multicenter study of 1,020 patients. Chest 2002;121:1515-20. [Crossref] [PubMed]
- Rena O, Oliaro A, Cavallo A, et al. Stage I non-small cell lung carcinoma: really an early stage? Eur J Cardiothorac Surg 2002;21:514-9. [Crossref] [PubMed]
- Yasukawa M, Sawabata N, Kawaguchi T, et al. Histological Grade: Analysis of Prognosis of Non-small Cell Lung Cancer After Complete Resection. In Vivo 2018;32:1505-12. [Crossref] [PubMed]
- Kadota K, Miyai Y, Katsuki N, et al. Nuclear grade based on transbronchial cytology is an independent prognostic factor in patients with advanced, unresectable non-small cell lung cancer. Cancer Cytopathol 2016;124:630-40. [Crossref] [PubMed]
- Schug SA, Chandrasena C. Pain management of the cancer patient. Expert Opin Pharmacother 2015;16:5-15. [Crossref] [PubMed]
- Ramirez MF, Gorur A, Cata JP. Opioids and cancer prognosis: A summary of the clinical evidence. Neurosci Lett 2021;746:135661. [Crossref] [PubMed]
- Tuerxun H, Cui J. The dual effect of morphine on tumor development. Clin Transl Oncol 2019;21:695-701. [Crossref] [PubMed]
- Zhang XY, Liang YX, Yan Y, et al. Morphine: double-faced roles in the regulation of tumor development. Clin Transl Oncol 2018;20:808-14. [Crossref] [PubMed]
- Koodie L, Yuan H, Pumper JA, et al. Morphine inhibits migration of tumor-infiltrating leukocytes and suppresses angiogenesis associated with tumor growth in mice. Am J Pathol 2014;184:1073-84. [Crossref] [PubMed]
- Silagy AW, Hannum ML, Mano R, et al. Impact of intraoperative opioid and adjunct analgesic use on renal cell carcinoma recurrence: role for onco-anaesthesia. Br J Anaesth 2020;125:e402-4. [Crossref] [PubMed]
- Zylla D, Gourley BL, Vang D, et al. Opioid requirement, opioid receptor expression, and clinical outcomes in patients with advanced prostate cancer. Cancer 2013;119:4103-10. [Crossref] [PubMed]
- Du KN, Feng L, Newhouse A, et al. Effects of Intraoperative Opioid Use on Recurrence-Free and Overall Survival in Patients With Esophageal Adenocarcinoma and Squamous Cell Carcinoma. Anesth Analg 2018;127:210-6. [Crossref] [PubMed]
- Montagna G, Gupta HV, Hannum M, et al. Intraoperative opioids are associated with improved recurrence-free survival in triple-negative breast cancer. Br J Anaesth 2021;126:367-76. [Crossref] [PubMed]
- Zylla D, Kuskowski MA, Gupta K, et al. Association of opioid requirement and cancer pain with survival in advanced non-small cell lung cancer. Br J Anaesth 2014;113:i109-16. [Crossref] [PubMed]
- Kim JY, Ahn HJ, Kim JK, et al. Morphine Suppresses Lung Cancer Cell Proliferation Through the Interaction with Opioid Growth Factor Receptor: An In Vitro and Human Lung Tissue Study. Anesth Analg 2016;123:1429-36. [Crossref] [PubMed]
- Oh TK, Jeon JH, Lee JM, et al. Investigation of opioid use and long-term oncologic outcomes for non-small cell lung cancer patients treated with surgery. PLoS One 2017;12:e0181672. [Crossref] [PubMed]
- Hasegawa T, Oguri T, Osawa T, et al. Opioid Dose and Survival of Patients with Incurable Nonsmall Cell Lung Cancer: A Prospective Cohort Study. J Palliat Med 2018;21:1436-41. [Crossref] [PubMed]
- Maher DP, Wong W, White PF, et al. Association of increased postoperative opioid administration with non-small-cell lung cancer recurrence: a retrospective analysis. Br J Anaesth 2014;113:i88-94. [Crossref] [PubMed]
- Cata JP, Keerty V, Keerty D, et al. A retrospective analysis of the effect of intraoperative opioid dose on cancer recurrence after non-small cell lung cancer resection. Cancer Med 2014;3:900-8. [Crossref] [PubMed]
- Luan T, Li Y, Sun L, et al. Systemic immune effects of anesthetics and their intracellular targets in tumors. Front Med (Lausanne) 2022;9:810189. [Crossref] [PubMed]
- Park YJ, Yo CH, Hsu WT, et al. Use of Opioids and Outcomes of Pneumonia: Results From the US Nationwide Inpatient Sample. J Acute Med 2021;11:113-28. [PubMed]
- Plein LM, Rittner HL. Opioids and the immune system - friend or foe. Br J Pharmacol 2018;175:2717-25. [Crossref] [PubMed]
- Roy S, Ninkovic J, Banerjee S, et al. Opioid drug abuse and modulation of immune function: consequences in the susceptibility to opportunistic infections. J Neuroimmune Pharmacol 2011;6:442-65. [Crossref] [PubMed]
- Chen W, Li G, Jiang K, et al. Dexamethasone for Postoperative Nausea and Vomiting in Papillary Thyroid Carcinoma Patients: A Randomized Clinical Trial. J Am Coll Surg 2022;235:454-67. [Crossref] [PubMed]
- Yu HC, Luo YX, Peng H, et al. Avoiding perioperative dexamethasone may improve the outcome of patients with rectal cancer. Eur J Surg Oncol 2015;41:667-73. [Crossref] [PubMed]
- Buxant F, Kindt N, Laurent G, et al. Antiproliferative effect of dexamethasone in the MCF-7 breast cancer cell line. Mol Med Rep 2015;12:4051-4. [Crossref] [PubMed]
- Call TR, Pace NL, Thorup DB, et al. Factors associated with improved survival after resection of pancreatic adenocarcinoma: a multivariable model. Anesthesiology 2015;122:317-24. [Crossref] [PubMed]
- Yi J, Lei Y, Xu S, et al. Intraoperative hypothermia and its clinical outcomes in patients undergoing general anesthesia: National study in China. PLoS One 2017;12:e0177221. [Crossref] [PubMed]
- Nakada T, Noda Y, Kato D, et al. Risk factors and cancer recurrence associated with postoperative complications after thoracoscopic lobectomy for clinical stage I non-small cell lung cancer. Thorac Cancer 2019;10:1945-52. [Crossref] [PubMed]



