
Minimally invasive repair of pectus excavatum in adults: a review article of presentation, workup, and surgical treatment
Introduction
Background
Pectus comprises 90% of all chest wall deformities, with pectus excavatum (Pex) being the most common congenital deformity of the chest wall having an occurrence between 1 in 400 and 1 in 1,000 live births (1,2). Pectus deformities have been reported to occur three to five times more often in males than in females (3); however, this may be due to a failure to diagnose in females as breast tissue may conceal the severity of the defect (4-6). A recent study involving over 2,600 thoracic computed tomography imaging studies highlights this point noting Pex being more prevalent in their female cohort (7). The deformity causes a backward displacement of the sternum compressing most commonly the right chambers of the heart, leading to reduced stroke volume and restrictive deficits (Figure 1) (8-11). Depending on the severity of the depression, presentation of Pex may range from a minor cosmetic issue to disabling cardiopulmonary symptoms (8,9,12). As the patient advances in age, the chest wall can become less flexible leading to progression of symptoms with patient maturing (13-15). Development of symptoms in the 4th and 5th decade was reported in nearly half of adult patients in one report, with significant improvement after surgical repair (14).
Rationale and knowledge gap
Surgical correction of the deformity with open resection of cartilage goes back in history to the 1940’s with controversial outcomes especially in very young children (16). In 1998, the “Nuss” procedure or minimally invasive repair for pectus excavatum (MIRPE) was published (17). The procedure was quicker to perform with excellent cosmetic outcomes, rapidly making it the standard of care for treatment of pediatric and adolescent patients. Although the procedure has become standard of care in young patients, the use of MIRPE in adults has been slower to adopt and more controversial due to the increased difficulty of repair in this population and the higher reported rates of complications due to the less flexible mature chest wall (18-21). This is likely due to the increased calcification and rigidity of the chest wall that occurs with aging leading to more complicated and difficult sternal elevation, greater pressure levels distributed to bars, and higher risks of bar displacement. Despite these issues in the adult population, the use of MIRPE for adult pectus repair has continued to increase. Many surgeons have modified the original Nuss techniques to allow for safe and successful adult repair even in advanced patient ages. However, no comprehensive review of the most recent techniques and their impact on outcomes and complications has been performed and this review could provide practical information for surgeons performing MIRPE on the challenging adult population.
Objective
The aim of this manuscript was to perform a review of the major articles addressing surgical techniques and outcomes of adult patients undergoing MIRPE, as well as describe our institution’s experience.
Methods
Extensive literature review was performed including PubMed/MEDLINE publications that described surgical repair of Pex utilizing minimally invasive repair techniques in adult patients. Search terms (Nuss OR thoracoscopic pectus OR minimally invasive pectus) were utilized and the references from pulled publications were also reviewed for additional sources. Included manuscripts were both retrospective and prospective observational studies, randomized control trials, and large case series (more than 10 patients); timeframe considered was from January 2004 until March 2023. According to the objectives of this review, authors MRA and JMF selected articles discussing the preoperative evaluation (including diagnostic strategies and cardiopulmonary evaluation), modifications and updates in the surgical techniques, surgical outcomes, quality of life evaluation, and pain management. Selected articles were conferred with author DEJ and discrepancies were solved by consensus among the authors.
Adult presentation: symptoms and cardiopulmonary effects of pectus excavatum
An adult patient presenting for surgery may do so secondary to a new onset of symptoms in adulthood after having been completely asymptomatic without noticeable physiologic effects from the defect when they were younger (22-26). Others may have had milder symptoms as an adolescent but developed definitive worsening of their symptoms with age (23). Adult symptoms most commonly include exercise intolerance, increasing levels of fatigue, dyspnea on exertion, tachycardia, and compensatory tachypnea (10,11,23,27-30). Most adult patients have lived with the cosmetic deformity for many years and the appearance of their chest generally was not the primary reason for undergoing surgical repair. Despite this, visible physical differences do increase the Pex patient’s risk for body image and interpersonal difficulties and should not be discounted (29,31).
The majority of patients complain of cardiopulmonary type symptoms including exertional dyspnea, tachycardia/palpitations, and chest pain (32), yet the cardiopulmonary effects of Pex on adult patients remains a topic of debate (33-35) due to a paucity of reports evaluating adult patients (36,37). The inward deformity of the anterior chest wall causing right heart compression and displacement into the left chest to various degrees can have negative cardiopulmonary consequences on adult patients as supported by recent data (Figure 2) (9,25). Direct cardiac compression has been shown to not only reduce right heart chamber’s dimensions, but also stroke volume, cardiac output, and diastolic and systolic function as well as strain (38). This may explain the cause of accelerated fatigue and compensatory tachycardia (9,22,39-41). Mocchegiani et al. (42) reported that the right ventricular outflow tract in Pex patients was significantly narrower and right ventricle end-diastolic and-systolic areas were significantly smaller. Töpper et al. (43) evaluated cardiovascular function of adult Pex patients using cardiac magnetic resonance and found that right ventricle ejection fraction was reduced in this population, with a significant improvement after MIRPE. Chao et al. (9) demonstrated an immediate improvement in right heart chambers size, and right and left ventricle systolic function (using strain techniques) after surgical repair by using intraoperative transesophageal echocardiography. Negative cardiopulmonary effects of Pex in 68% (of 392 preop patients) had significantly improved physiological benefits of MIRPE [130 patients with post op cardiopulmonary exercise testing (CPET)]. This has been demonstrated by Jaroszewski et al. (44) in a recent publication using CPET in adult cases. One small underpowered study (only 15 patients completed follow up) by Udholm et al. (10) found no significant improvement (but a trend towards improvement) at one year after MIRPE in adult patients. Sternal compression is also suspected to decrease the thoracic volume, which can reduce the mixed venous oxygen saturation, exercise tolerance, tidal volume, and vital capacity (34,44,45). A summary of the major publications with evidence related to the effects of MIRPE on cardiopulmonary function in adult patients can be found in Table 1.
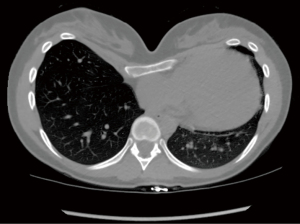
Table 1
| Study | N | Age, year | Haller index | Test used | Cardiopulmonary outcomes |
|---|---|---|---|---|---|
| Farina et al. (46), 2023 | 127 | Median 29.0 (IQR 15.4) |
Median 4.2 (IQR 1.7) | Intraoperative transesophageal echocardiography | Significant improvements in right ventricular stroke volume and diastolic function as measured by hepatic vein waves velocities were seen after pectus repair (P<0.001 for all comparisons) |
| Preoperatively, 5.5% of patients had constrictive-like physiology (end-diastolic retrograde flow) which normalized after surgical correction (P=0.016) | |||||
| Patients with more proximal cardiac compression had greater improvements in hepatic vein velocities after repair | |||||
| Jaroszewski et al. (44), 2022 |
392 | Mean 32.4 (SD 10.0) | Mean 4.6 (SD 2.2) |
Cardiopulmonary exercise testing | Post-repair tests were performed immediately before bar removal procedure |
| 130 completed pre- and post-operative evaluation | Intraoperative transesophageal echocardiography | A significant improvement (P<0.001) in cardiopulmonary outcomes (VO2max, O2 pulse, anaerobic threshold, and maximal ventilation) was seen in the post-repair evaluations | |||
| In a sub-analysis of 39 patients who also underwent intraoperative transesophageal echocardiography at repair and at bar removal, a significant (P<0.001) increase in RV stroke volume was found | |||||
| Skoczyński et al. (47), 2022 |
55 | Mean 21.1 (SD 3.0) | NA | Pulmonary function tests | Participants who underwent MIRPE had normal pulmonary function and exercise capacity 7 years after the intervention |
| Compared with matched controls, patients who underwent MIRPE had higher RV%, TLC, and FEV1/VC ratio | |||||
| Chao et al. (38), 2018 | 165 | Mean 33 (range, 18–71) | Mean 5.7 (SD 3.1) |
Intraoperative transesophageal echocardiography | Right heart chambers size (right atrium, tricuspid annulus, and RVOT dimensions) significantly (P<0.001) increased after surgery |
| RV and LV systolic function (evaluated with strain techniques) significantly (P<0.001) improved immediately after surgical repair | |||||
| Udholm et al. (10), 2016 | 19 | Mean 32 | NA | Cardiopulmonary exercise testing | Surgical correction in adult patients did not improve the cardiopulmonary function (VO2max, cardiac output, FEV1) one year after surgery |
| 15 completed follow-up | |||||
| Töpper et al. (43), 2016 | 38 | Mean 21 (SD 8.3) | Mean 9.64 | Cardiac magnetic resonance | Pectus was associated with reduced RVEF in the preoperative tests, which significantly (P<0.001) improved after surgical correction (mean follow-up 472 days after surgery) |
| LVEF was between normal limits before surgery, but also increased significantly after correction (P=0.016) | |||||
| Chao et al. (9), 2015 | 168 | Mean 33 (range, 18–71) | Mean 5.7 (SD 3.1) | Intraoperative Transesophageal Echocardiography | Surgical correction caused a significant (P<0.001) improvement in right heart chambers size (right atrium, tricuspid annulus and right ventricle outflow tract) and cardiac output immediately after surgical repair |
| Szydlik et al. (48), 2013 | 44 | Mean 16 (range, 10–32) |
NA | Pulmonary function test | A significant improvement (P<0.001) in lung function (FVC, FEV1, FEF25, and FEV1/VC ratio) was seen in patients who underwent Nuss procedure |
IQR, interquartile range; SD, standard deviation; NA, not applicable; VO2max, maximal oxygen consumption; RV, right ventricle; MIRPE, minimally invasive repair of pectus excavatum; RV%, residual volume; TLC, total lung capacity; FEV1, forced expiratory volume in 1 s; VC, vital capacity; RVOT, right ventricle outflow tract; LV, left ventricle; RVEF, right ventricle ejection fraction; LVEF, LV ejection fraction; FVC, forced vital capacity; FEF25, forced expiratory flow for 25%.
Diagnostic work up
The preoperative evaluation of an adult patient presenting with clinically symptomatic Pex warrants anatomical and functional workup to assess the significance of the defect and necessity for repair. Many of the presenting symptoms can be caused by a variety of cardiopulmonary diseases and a thorough workup to eliminate these causes is critical. Additional analyses based on patient comorbidities are obtained as deemed fit by the physician and symptoms dictate (32). A complete history and physical examination, including findings suggestive of connective tissue diseases such as Marfan Familial Syndrome is critical (17,23-26).
Thoracic imaging with either a non-contrast computerized tomography (CT) or magnetic resonance imaging (MRI) is critical for a radiographic evaluation of the severity and characteristics of the deformity as well as any other intrathoracic pathology (18,49). Since the deformity may significantly worsen when a patient exhales, performing the imaging during both inspiratory and expiratory phases is recommended (31). Thoracic imaging also helps show the degree of cardiac compression, cardiac displacement, and pulmonary atelectasis if present. The deformity of the bony and cartilaginous skeleton can be visualized in a three-dimensional view which also helps patients visualize the extent of their deformity and the impact it has on the mediastinal structures (50). The measurement and calculation of the Haller Index is performed from imaging, which is the ratio of the lateral diameter of the chest to the distance between the sternum and spine, at the point of maximal depression). This is often requested by insurance companies for qualification of repair even though its relevance in the adult patient is undocumented. Despite the general acceptance of HI 3.2 as the cutoff for defining severe pectus, indexes of less than 3.2 do still represent significant variants from normal and may be associated with significant cardiopulmonary disability and symptoms as well as body image problems (18,26,31,49,51,52). A Haller index (HI) <3.2 should lead to evaluation by correction index which is a much better estimation of the severity of deformity with >10% being significant enough to consider surgery if symptomatic and compression is present (53).
Adult patients commonly present with several cardiovascular conditions such as hypertension or coronary diseases. Depending on the patients’ cardiovascular risk profile, a 12-lead electrocardiogram (ECG) or more complex tests could be important to rule out the presence of such conditions. Right bundle branch block and other conduction abnormalities are commonly associated with the adult Pex patient (10,23,26) thus highlighting the importance of an ECG to exclude intracardiac conduction disorders and dysrhythmias.
An echocardiogram is performed to document the effects of the depressed sternum on the right heart chambers (as they are the most anteriorly located) as well as associated interference with diastolic or systolic function (22,26,39,41,51,54). It is recommended to be performed preoperatively; however, getting accurate transthoracic views can be difficult due to the displacement of the heart from the deformity (55,56). Compression by the chest wall is mainly in the anteroposterior plane and best seen in a transverse axis (39). The apical four-chamber view can also visualize any extrinsic compression to the right ventricle. A positional echo with imaging of the right ventricle and right outflow tract to look for significant decreases in cardiac output and right-sided hemodynamics due to increased compression in position change from supine to sitting or leaning forward is available at some institutions and we find it of great value in assessing low deformities (57). In adult patients with history of connective tissue disorders (which are commonly associated with Pex deformity), an echocardiogram is also critical to assess valvular abnormalities, including mitral valve prolapse, and evaluation of the aortic root size and valvular competency. In patients requiring cardiac surgery, concurrent repair of pectus is recommended and should be considered as subsequent repair may require a redo sternotomy with a higher risk of complications (58,59). In some patients, a transesophageal view may be necessary to better evaluate the right chambers of the heart and any outflow obstruction present, as well as to exclude some potential concomitant cardiac abnormalities (39).
Although not available in all institutions, CPET is used to help quantify how Pex affects a patient’s cardiopulmonary function and ability to exercise. Several studies implementing CPET measurement showed O2 pulse (a surrogate for stroke volume) and oxygen consumption (VO2max) significantly below predicted values in patients with Pex (7,31,34,44,45,52). More recent investigations demonstrated that almost 70% of adult Pex patients showed preoperative abnormal oxygen consumption as assessed by CPET. The statistically significant improvement seen in patients CPET after surgical correction, makes this test an important indicator for strongly supporting the need for surgery in this population (44). A normal VO2max should not preclude repair as this study also noted significant improvement in patients that had normal (>80%) preoperative VO2 max values.
Pulmonary function tests (PFT) could help detect restrictive respiratory impairment due to the reduction of internal thoracic volume caused by the deformity (1). In general, PFTs should be obtained in adult patients with symptoms of significant dyspnea or history of asthma or smoking to rule out underlying emphysema (50). Most Pex patients are expected to have normal or low normal PFTs (26,51).
Indications for surgery
The indications for intervention are not fully standardized in the adult population, yet from our experience and from other experts in the field, corrective surgery should be considered in patients with two or more of the following criteria. Individual insurance companies have different criteria for coverage and should be reviewed:
- Haller index of 3.2 or greater or Correction index of 10% or greater (53,60);
- Cardiac compression, displacement, mitral valve prolapse, or conduction abnormalities (16);
- Pulmonary function testing showing restrictive respiratory disease (61);
- Cardiopulmonary deficits assessed by CPET;
- Symptoms, especially progression;
- Psychosocial effects (12,14,62).
In summary, consideration of surgical treatment of the adult Pex patient should be given for severe anatomic deformities and patients with symptoms or cardiopulmonary deficits attributable to the deformity. As more experience with adult pectus repair accumulates, criteria for repair are likely to evolve.
Preparation for surgery
For our patient cohort, posture training exercises are highly recommended preoperatively. These exercises aim to strengthen the upper back muscle groups including latissimus dorsi, trapezius, and rhomboids. As for the front of the chest, we encourage patients to stretch using a foam thoracic spine roller which helps to open and loosen the chest as much as possible. After pectus surgery, back spasms and pain are common and these measurements can be helpful when done in the weeks to months prior to surgery. In addition, selective testing for metal allergy could be performed preoperatively based on personal or family history. In our cohort, adults have better history as to their known allergies and exposure to metal and jewelry. Implementing this method in our practice has been sufficient and we had no problems with allergy thus far. Other institutions have started utilizing selective testing with the aim of using titanium bars in patients with allergies (63). We also utilize titanium bars selectively in patients that weigh >85–90 kgs or have a rigid chest with a diameter wider than 14.5–15 inches to allow for greater support.
Pectus excavatum repair techniques
Over the years, a variety of techniques for surgical repair of Pex have been used on patients of all ages. The two most common methods used today for repair of the adult include modifications of the open Ravitch approach and the MIRPE.
The original open procedure was described by Ravitch in the 1940’s. The modified open technique has been used for several decades and is still in use to this day. It involves resection of the deformed costal cartilage to allow the return of the sternum to its normal position with or without sternal osteotomy (23,64-66). There are a variety of modifications reported including the use of mesh, others include placement of a metal strut, and plating to support the sternum which may be left in place for 6 months to 1 year (67,68). The open technique may be for some surgeons better suited for patients who have a combination of Pex with pectus carinatum, significant asymmetry, or extensive defects involving the uppermost ribs and cartilage. Recurrence rates after repair of Pex using the open technique have been reported in 2–10% of patients (23,64,69).
The MIRPE was published in 1998 by Nuss and has since been implemented by most pediatric surgeons treating the pectus deformity. In the MIRPE technique, a plane is created behind the sternum with the help of thoracoscopy, and curved bars are inserted and rotated such that its curve elevates the area of deformity. The bars are secured to the chest wall and removed in 2–3 years after the chest wall cartilage have remodeled. This technique has gained widespread popularity because of its minimally invasive approach, as evidenced by small skin incisions, no need for cartilage resection, short operative time, minimal blood loss (70) and comparable postoperative morbidity (71) with respect to the modified Ravitch procedure (18). The advantages of MIRPE include avoidance of rib cartilage resections, sternal osteotomies, conspicuous mid-chest scars, less blood loss, and an overall shorter operative time. Even adult patients with significant asymmetry associated with their deformity may benefit from MIRPE correction.
Since the original publication of the Nuss procedure, several modifications have been made to the MIRPE technique (72-80) (Table 2). Modifications involved various aspects of the surgery including use of thoracoscopy, patient positioning and location of incisions, methods of passing the bars across the chest and their guidance, details of the bars placed (size, shape, positioning, and number), and methods of bar fixation and stabilization (80). In our practice we have utilized several modifications which have allowed for successful extension of the procedure into even advanced aged adult patients. These modifications include (but are not limited to) use of multiple support bars, forced sternal elevation, reinforcement of intercostal spaces, and multi point fixation to secure the bars. With these technique modifications, successful MIRPE has been reported in adults up to the age of 72 years old (32), with surgery resulting in resolution of symptoms, improved quality of life, and satisfying results (59,80,103).
Table 2
| Surgical modification | Study | Description |
|---|---|---|
| Forced sternal elevation | ||
| Crane technique | Park et al. (81), 2008, Kelly et al. (63), 2022 | A percutaneous wire is passed through the bony tissue of the sternum and the wire is connected to a table-mounted crane system. This alleviates pressure on the hinge points which prevents tearing/stripping of intercostal muscles. The bar displacement rate, major complication rates and reoperation rates all decreased after implementing this new approach |
| Two Langenbeck handheld retractors | Tedde et al. (82), 2012 | Incision made in the intercostal space adjacent to the sternum at the deepest portion of the defect. Enters the hemithorax from the left side first, position 1st retractor at the hinge point, followed by entering the right hemithorax to introduce a camera and 2nd retractor allowing safe retrosternal instrumentation |
| Horseshoe-shaped sternal elevator | Takagi et al. (83), 2012 | No extra skin incision is needed for the elevator, its usage enlarges the retrosternal space for safer passage of thoracoscopically guided introducer and allows visualization of substernal tunneling |
| Vacuum bell | Haecker et al. (84), 2012 | Vacuum Bell is placed on the defect and suction is initiated to reduce defect. Applicable only to young and elastic chest wall defects |
| Subxiphoid incision/sternal lift + anchor | Johnson et al. (85), 2013 | A subxiphoid incision is made and a retractor is placed under the sternum to assist with elevation of sternum |
| Subxiphoid incision allows access to create a plane between the posterior sternum and pericardium. Lift is inserted beneath the sternum, allowing sternal elevation and locked in place by anchor | ||
| Bone clamp and Rultract retractor | Jaroszewski et al. (79), 2014 | Incisions made either parallel or perpendicular to sternum, tips of bone clamp placed into anterior table of the sternum and attached to the cable coming off a table mounted Rultract retractor. This reduces risk of intercostal muscles stripping and reduces stress on intercostal spaces during bars insertion and rotation |
| T-fastener suture technique | Kim et al. (86), 2014 | Chest incisions are made lateral to the sternum, sutures are delivered outside the lateral chest and tied to a metal plate with 3 holes. T-fastener sutures are used to elevate the anterior chest from both sides of the sternum by tying them to a crank attached to a cross bar. No specialized equipment is required (metal plate commonly used in orthopedic practice), no incisions need to be made (needle holes created require no suture closures), and no fracture or tear occur to anterior chest. Metal plate must be retrieved at the end which presents difficulty after bars are positioned |
| Bar stabilization technique | ||
| Five-point fixation (multipoint suture fixation MPF), stabilizers, CFT and HP, and bridge technique | Park et al. (78), 2004, (81), 2008, (87), 2011, (88,89), 2015 | MPF offered bar fixation to upper and lower ribs at the ends of the bar and a fifth wire at the hinge point medially with all sutures done through a single incision on each side. Attachment of stabilizers to both ends of the pectus bar to prevent bar flipping. CFT holds the bar to a rib by hooking it with a metal blade. HP reinforced the hinge points (the entrance points of the bar to the thoracic cavity) with a metal plate. Combining HP and CFT in adults: after bars insertion lifts the chest wall depression, both bar ends were fixed together by bridge plates and screws which enhances stability of the bars and eliminates need for suturing |
| Third point of fixation | Hebra et al. (73), 2006 | Alongside crossbars/lateral stabilizers, a third point of fixation consisting of absorbable suture is placed around the bar and around an anterior rib next to the right side of the sternum |
| Medial stabilizers and multiple PDS sutures | Pilegaard et al. (90), 2008, Pio et al. (91), 2016 | Stabilizer placed closer to the entrance of the bar into the thoracic cavity, thereby decreasing the risk of rotation/displacement as the stabilizer functions as a hinge |
| Unilateral stabilizer and multiple PDS sutures | Kelly et al. (92), 2010 | Attaching a metal stabilizer on the left and placing multiple pericostal PDS sutures around the bar and underlying ribs |
| FiberWire multiple points fixations of bars circumferentially and bilaterally | McMahon et al. (75), 2014 | Multipoint fixation with FiberWire that are secured around ribs (lateral and medial), fixation on both sides of the bar, and utilizes the bar’s lateral holes to minimize lateral dislocation |
| Figure-of-eight FiberWire reinforcement, FiberWire multipoint fixation and sternal fixation | Jaroszewski et al. (72), 2016 | “Hammock” Figure-of-Eight FiberWire tie that incorporates the rib above and below the interspace that the bar will be placed in to reinforce and prevent lateral posterior bar migration and intercostal muscle stripping. FiberWire multipoint fixation around ribs and bars medial and lateral, sternal holes drilled for suture that incorporates bar and sternal bone for medial fixation |
| Stabilizer secured with wire/FiberWire + several pericostal PDS sutures | Nuss et al. (17), 1998 | Stabilizer is attached and secured to the bar with non-absorbable sutures on the left side + multiple PDS sutures placed around the bar and the underlying rib on the right side |
| Bars configuration | ||
| Compound | Park et al. (78), 2004, Yoon et al. (93), 2010 | The compound bar presents a concept of exaggerated convexity in the center of the bar, with a smaller central arc between the hinge point and adjoining at either side by two larger arcs. The smaller central arc makes the bar convex enough to elevate the depression and the larger lateral arcs can adjust the width of the bar easily to fit the size of the chest |
| Multiple bars | Nuss (94), 2008, Pilegaard et al. (90), 2008, Nagaso et al. (95), 2010, Stanfill et al. (96), 2012 | Using 2 bars have been implemented by surgeons treating adults with Pex to fully correct the deformity and decrease rate of recurrence/requirement of revision. Multiple bars allow for better distribution of pressure (decreasing risk of bar migration) and may also decrease the pain |
| Jaroszewski et al. (72), 2016 | >40% of patients required 3 bars to fully correct the deformity | |
| New steel bar | Li et al. (97), 2015 | Introduced new steel bar through bilateral thoracic minimally invasive incisions using a thoracoscope for guidance. The bar was installed or removed by pushing and pulling without flipping it |
| Cross bar technique | Park et al. (98), 2016, Sayan et al. (99), 2021 | Cross bars cover the promontory of the depression and the whole anterior chest wall (including lateral parts) by avoiding hinge compressions and residual depressions |
| Introducer bar complex | Wang et al. (100), 2021 | New kind of steel bar curved according to normal structure of the human anterior chest wall and includes 15 different specifications. One end designed to connect to introducer/stabilizer. Connecting the bar to the stabilizer creates the introducer-bar complex which doesn’t require rotation or turning, it is pushed in our pulled out |
| Thoracoscopy | ||
| Unilateral thoracoscopy | Croitoru et al. (101), 2002 | Direct visualization of the mediastinal structures using right thoracoscopy via an additional small incision for thoracoscopic observation in the right pleural cavity under insufflation of CO2 made the procedure much safer |
| Bilateral thoracoscopy | Cheng et al. (102), 2008 | The modified bilateral thoracoscopy is utilized via the wound made for bar insertion without extra incisions for the thoracoscope. It allows for excellent visualization over each pleural cavity. It could eliminate the risk of cardiopulmonary injuries as it allows direct bilateral inspection of mediastinum and facilitate mediastinal dissection |
| Pectoscopy | Park et al. (74), 2010 | A specially designed video-scope approach to guide the introducer or the pectus clamp as it is passed through the mediastinum |
MIRPE, minimally invasive repair for pectus excavatum; MPF, multipoint fixation; CFT, claw fixator; HP, hinge plate; PDS, polydioxanone; Pex, pectus excavatum.
MIRPE technique for adult cases
In the adult patient, critical issues with MIRPE include safe dissection across the anterior mediastinum without inducing pericardial inflammation. Thoracoscopy via the right chest should be used in all cases. As discussed in Table 2, forced sternal elevation allows better visualization, which increases the safety of the procedure and decreases the force required to enter and rotate bars in the less flexible adult chest wall (80). We utilize a Rultract retractor (Rultract Inc., Cleveland, OH, USA) with an extension arm attached to the left side of the operating table at the level of the clavicle. Stab incisions are placed on the sternal defect a few cm apart and the tips of a perforating clamp (we utilize the Lewin Spinal Perforating Forceps, V. Mueller NL6960; CareFusion, Inc., San Diego, CA, USA) are manipulated into the anterior table of the sternum. The cable connector is then attached to the clamp and the sternum elevated (Figure 3). Other methods of sternal elevation have been described in Table 2.

It is critical that the bars enter and exit the thorax medially otherwise the defect and sternum will not be pushed anteriorly (Figure 4A,4B). The bar should be positioned approximately 1–1.5 cm lateral to the internal mammary artery (IMA). If it is not, the bar will not push the defect anteriorly, and the deformity will not be corrected. We routinely reinforce the intercostal spaces that will hold the bar with a figure-of-eight FiberWire “hammock” (Figure 5) which incorporates the rib above and below the interspace. The hammock is placed slightly lateral to the bar exit site to prevent the intercostal muscle stripping and the ribs and interspace from widening out and it allows the weight of the bar to rest on the suture versus the intercostal muscles as it exits the chest. A stabilizer could also be utilized medially (medial stabilizer) if deemed necessary. It is usually used in the low bars which are at a higher risk of displacement (Figure 6).

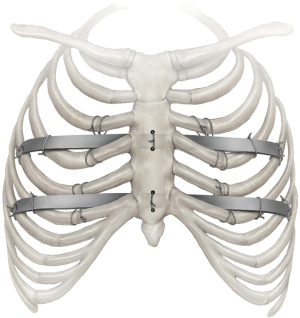
We place 2–3 bars to achieve complete correction. Bars should not be too long as to cross beyond the mid axillary line. Bars can be placed in multiple configurations. Cross bars are used by some surgeons; however, they have been reported to have an increased risk of pleural effusions (99). Over time and experience, we have increased the frequency of placing 3 bars (Figure 6). We have also noted an increase in the incidence of pleural effusions as our percentage of 3 bar cases has grown to >55% and routinely leave a chest tube thru the camera port site. Others have reported higher effusions in adult patients and there may be an increased inflammatory response (104). Multiple bars distribute the sternal pressure over a broader area and allow more complete correction in the adult patient. Using multiple bars may also decrease the risk of displacement (21,30,80). Placement of a second or even third bar is often necessary and desirable to achieve optimal cosmetic results and to fully elevate the chest in the adult patient (11,24,25,28,30,69,70,80). In our experience, indications for two or more bars in the adult included a depressed area >3 intercostal spaces and a HI greater than 3.5.
Both IMA may be occluded out by the Nuss bars especially on an asymmetric side. This is a risk factor that should be explained to the patient and is part of the consent process. In patients with strong family history of coronary artery disease, we stress this and the potential that the vessel will likely not be usable in the future should they require a coronary artery bypass graft. Robotic takedown of a section of the IMA could be utilized to diminish the risk of injuring the artery (105) which was reported in 40% of patients by Yüksel et al. (106).
Premature removal of bars has shown a high incidence of recurrence in the adult population and a time frame between 2–3 years has been recommended for the adult patient by at least two publications; however, we have increased our recommended time for bars in the adults to 3–3.5 years due to the more rigid chest wall and significant pressure that is on the bars (11,24). Recurrence rates as low as 2–5% have been reported for the MIRPE adult patients. However, follow-up is on a small number of patients for limited time periods (11,25,30,69,80).
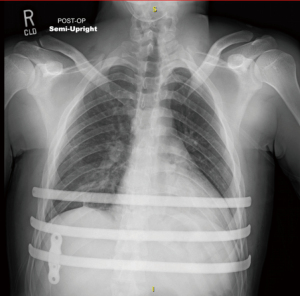
Postoperatively, stretching is paramount in the early period starting on the second week. Initially, this can be accomplished by patients raising their arms above their heads and climbing the wall with full arm extension. Patients can anticipate return to full activity around 6–8 weeks. There should be no heavy lifting for at least 6 weeks (nothing greater than 10 pounds) and only after our team reviews the patients 6 weeks postoperative chest X-ray.
MIRPE outcomes
Concerns have been raised regarding a higher incidence of complications following the more complex MIRPE procedure in adults. Data regarding a direct comparison of complications between adult and pediatric population are not abundant and many of these reports are from early in the learning period of the procedure (72,107,108). Kim et al. documented a difference in their post operative complications with MIRPE in adults versus children of 58.3% vs. 11.1% with bar displacement in up to 1/3rd of adult patients (109). These complications include an increased risk of bleeding, cardiac perforation, longer hospitalizations, greater postoperative pain, significant rates of bar migration, and a higher overall recurrence rate. The modifications of the procedure by different surgeons over the years (Table 2) have helped to reduce the rate of complications both in pediatrics and in adult cases. Complications and improvement in outcomes have been a large driving force for implementation of these modifications.
Understandably, experience weighs heavily on surgeon’s complication rates and much of the early reports on the Nuss procedures reported high rates of complications, including bar rotation. These outcomes have subsequently improved significantly in experienced hands; therefore, it is important when reviewing data to note the date range and the center the publication includes. There is a significant learned curve with the MIRPE (at least 25 pediatric cases and likely double for adults) (110) and with surgeon experience increased, the MIRPE has become a common procedure with life threatening complications occurring in less than 0.1% of cases (21,111). Table 3 reviews the outcomes in recent studies from 2010 onwards after MIRPE in larger studies of adult Pex patients.
Table 3
| Study | N | Age, year | Haller index | Complications | Results |
|---|---|---|---|---|---|
| Viggiano et al. (112), 2022 | 93 patients | Median 23 (range, 18–42) | Mean 5.1 (range, 2.3–12.6) | Overall 12.9% | • All 93 patients were evaluated in follow-up at 3, 12, and 24 months after MIRPE and 6 months after bar removal |
| • Seroma/hematoma (2.1%) | • No operative/perioperative mortalities, no life-threatening complications | ||||
| • Wound infection (2.1%) | • Better or much better quality of life after operation was reported in 94.7% of patients after 6 months of bar removal | ||||
| • Hemothorax (1.1%) | • 97.8% of patients were satisfied or very/extremely satisfied with cosmetic appearance 3 months after MIRPE and 6 months after bar removal | ||||
| • Pneumothorax requiring chest tube (4.3%) | |||||
| • Bar displacement (4.3%) | |||||
| De Loos et al. (18), 2021 | 327 patients (272 in young group range, 11–24 years and 55 in adults group range, 25–47 years) | Young group median 16 | Young group median 3.7 | • Young group vs. adult group respectively: | • Median follow-up was 34 months for the young group and 36 months for the adults |
| Adult group median 32 | Adult group median 3.6 | • Bar displacement requiring intervention 3% vs. 4% | • Operation in adults was longer than in young group (35 vs. 30 minutes respectively, P=0.004) | ||
| • Bar removal within 3 years for chronic pain 2% vs. 7% | • Minor complications occurred more often in the adults group (young 4% vs. adult 11%, P=0.002), yet both groups had the same length of hospital stay duration | ||||
| • Pneumothorax (requiring chest tube) 0.4% vs. 0% | |||||
| • Reoperation for bleeding, 0.4% vs. 0% | |||||
| • Empyema 0.4% vs. 0% | |||||
| • Pneumonia 1% vs. 4% | |||||
| • Wound infection 2% vs. 0% | |||||
| • Chronic pain without bar removal 1% vs. 7% | |||||
| Pilegaard (107), 2016 | 1,713 patients (604 >18 years) | Median 16 (range, 7–58) | NA | Overall 7.6% | • During the study period, an increase in the number of bars used and an increase in proportion of older patients undergoing MIRPE. A decrease in bar length and a decrease in length of hospital stay were also noted |
| • Bar rotation (1.2%) and dislocation (0.8%) | • No mortalities took place | ||||
| • Pneumothorax (1.1%) | |||||
| • Deep infection (0.9%) | |||||
| • Pneumonia (0.6%) | |||||
| • Seroma (0.4%) | |||||
| • Pleural effusion (0.3%) | |||||
| • Sternotomy (0.1%) | |||||
| • Sternal fracture (0.06%) | |||||
| • Removal of bar before time (0.4%) | |||||
| • Removal of stabilizer due to pain (1.4%) | |||||
| • Bar end dropped into chest cavity (0.2%) | |||||
| • Bleeding requiring intervention (0.1%) | |||||
| Pilegaard (113), 2011 | 52 patients | Median 37 (range, 30–53) | • Pneumothorax (48%) all resolved spontaneously except for 1 patient | • 70% needed two bars or more for satisfactory corrective results | |
| • Bar removal prematurely (3.8%) | • No operative deaths | ||||
| • Bar migration requiring intervention (1.9%) | |||||
| • Deep infection (1.9%) | |||||
| Jaroszewski et al. (72), 2016 |
266 patients (compared 18–29 years cohort vs. 30–72 years cohort) | 115 patients mean 23.7 (range, 18–29) | Mean 5.6 (range, 2.5–26.7, mean 5.8 (range, 2.5–24.9) | 18–29 y cohort vs. 30–72 respectively: | • MIRPE was successfully performed in 96.5% of patients 18–29 years and in 88.7% of patients 30–72 years |
| 151 patients mean 40.4 (range, 30–72) | • Bar rotation 1.7% vs. 6.6% | • For 30–72 years patients, open cartilage resection or sternal osteotomy were more commonly performed | |||
| • Infection 0.9% vs. 1.3% | • Three bars were required in >40% of patients for complete correction | ||||
| • Pleural effusion 2.6% vs. 6% | • Frequency of bar rotation requiring reoperation was not statistically significant between the two groups (P=0.74) | ||||
| • Pneumothorax (requiring chest tube) 0.9% vs. 0.7% | • No recurrences reported | ||||
| • Pulmonary embolism 0% vs. 1.3% | |||||
| • Bleeding requiring transfusion 0% vs. 0.7% | |||||
| • Reoperation for bleeding, 1.7% vs. 0.7% | |||||
| • Pneumonia 0% vs. 4% | |||||
| • Urinary tract infection 0% vs. 4% | |||||
| • Urinary retention requiring catheterization 7.8% vs. 8.6% | |||||
| • Readmission for pain control 0% vs. 2% | |||||
| Pawlak et al. (114), 2016 | 680 patients (group A range, 7–14 years =156), (group B range, 15–20 years =328), (group C range, 21–49 years =196) | Mean 18.2±5.4 (range, 7–49) | Group A: 3.83 (2.44–10.7) | Overall 35% | • From all age groups, good and very good cosmetic effects were achieved in 97.7% |
| Group A: mean 12.2±2.0 | Group B: 3.72 (2.20–6.69) | • Pneumothorax (A =14.7%, B =27.1%, C =22.4%). Requiring drainage (A =26.1%, B =27%, C =50%) | • Recurrence was observed more often in younger patients although they had the lowest surgical morbidity | ||
| Group B: 17.2±1.6 | Group C: 3.59 (1.94–5.16) | • Pleural effusion (A =5.7%, B =6.4%, C =11.2%). | • Although complications rate was high, it didn’t interfere with satisfactory outcome of the surgical repair | ||
| Group C: 25.2±4.8 | • Pleural hematoma (A =0.6%, B =0.9%, C =0.5%) | ||||
| • Bar rotation (A =0.6%, B =3.6%, C =2%) | |||||
| • Fever (A =1.9%, B =3.6%, C =4.6%) | |||||
| Recurrence (A =3.2%, B =1.2%, C =1.5%) | |||||
| Erşen et al. (115), 2016 | 836 patients (236 >18 years) | Mean 16.8 (range, 2–45) | 4.4 (3.3–11) | • Bar displacement (5%) | • Complications overall rates among the adults and younger patients were 26.2% and 11.8% respectively (P=0.007). Although complications rate was higher, adults did not have a longer length of hospital stay when compared to younger patients |
| Adults mean 23.2 (range, 18–45) | • Pneumothorax (2%) | • Patients with >1 bar had less pain in the adult group | |||
| • Cardiac injury—small ventricular defect (0.4%) | |||||
| • Wound infection (1%) | |||||
| • Prolonged pain (1%) | |||||
| • Pleural effusion (0.8%) | |||||
| • Pneumonia (0.4%) | |||||
| • Sternotomy (0.4%) | |||||
| • Thoracic outlet syndrome (0.4%) | |||||
| Sacco Casamassima et al. (116), 2016 |
98 patients (39 participated in the survey) | Median 30.9 (range, 21.8–55.1) | Mean 4.3±1.3 | • Prolonged chest pain (12.2%) | • 1 bar was placed in 89.7% of patients |
| • Pneumothorax (11.2%) | • 2 patients required reoperation for recurrence | ||||
| • Wound infection (10.2%) | • 94.4% of patients reported satisfactory overall results | ||||
| • Pleural effusion (8.2%), pleural effusion requiring chest tube (1%) | • 79.5% of patients would have the operation again | ||||
| • Reoperation for bar displacement (4.1%) | |||||
| • Seroma (3.1%) | |||||
| • Hemothorax (2%) | |||||
| • Pneumonia (2%) | |||||
| • Pulmonary embolism (1%) | |||||
| Hoksch et al. (117), 2016 | 129 patients (19 patients followed >10 years) | Median 21 (range, 13–56) | Median 4.8 (range, 2.4–11.7) | Overall 14.7% | • There was no mortality and no severe injury of any anatomical structure during MIRPE or during bar removal |
| • Bar displacement (7%) | • Patients were followed up at 3, 12, and 36 months | ||||
| • Dressler syndrome (2.3%) | • After long-term observation, >90% of patients reported their quality of life after the operation as better or much better | ||||
| • Hematothorax (1.6%) | • >10 years follow-up patients reported 84.2% of very high satisfaction with cosmetic results and 94.7% of patients would have the operation again | ||||
| • Seroma/hematoma (1.6%) | |||||
| • Pneumothorax (0.8%) | |||||
| • Wound infection (0.8%) | |||||
| • Dislocation of stabilizer (0.8%) | |||||
| Hanna et al. (118), 2013 | 73 patients (51 participated in survey) | Median 20 (range, 16–51) | NA | Overall 15.1% | • 81% of patients had 1 bar placed |
| • Pneumothorax (4.1%) | • 4 reoperations took place, 2 for bar displacement and 2 for poor cosmesis (all operations done thoracoscopically) | ||||
| • Bar displacement (2.7%) | • Majority of respondents believe their overall health and exercise tolerance improved after MIRPE | ||||
| • Poor cosmesis (2.7%) | • 92.1% reported subjective improvement in their chest wall appearance | ||||
| • Bar infection (1.4%) | • 80% of patients were satisfied with the cosmetic result | ||||
| • Bruising (1.4%) | • 96% of patients would have the operation again | ||||
| • Ileus (1.4%) | |||||
| • Pericarditis (1.4%) | |||||
| Park et al. (87), 2011 | 102 patients (H group used hinge plate =27, N group did not use hinge plate =75) | Mean 19.4 [15–35] | NA | Overall 20% | • Reoperation were done in four patients, three patients due to bar dislocation and one patient for hemothorax |
| • Pleural effusion (9%) | No bar displacement occurred in the H group patients (those with hinge plate) | ||||
| • Bar dislocation (3%) | |||||
| • Pneumothorax (3%) | |||||
| • Seroma (3%) | |||||
| • Hemothorax (2%) |
MIRPE, minimally invasive repair for pectus excavatum; NA, not available.
Most recent publications by experienced centers have shown that MIRPE is safely performed in adult patients with minimal blood loss, shorter operating times, and relatively few postoperative complications (11,22,24,25,28,30,69,70,92). Pleural effusions were a common complication in most adult series (3–17%) with a number requiring thoracentesis or chest tube placement postoperatively (11,24,25,66,69,70,92).
Quality of life and patient satisfaction
The exercise limitations caused by the deformity along with the cosmetic disfigurement may cause a decrease in quality of life and alteration of patients’ social behavior (40,118-122). Lack of self-confidence, poor body-image, avoidance of social activities, and emotional difficulties are noted in Pex patients. Feelings of anxiety, depression, sadness, and frustration have also been reported (119). The importance of corrective surgery for improvement in psychological distress, quality of life, and exercise tolerance has been documented in the literature. The majority of these studies report a mixed population of children and adolescents with few adults; therefore, it is difficult to make broad based assumptions as to their application to the adult population (117,121,123,124).
Krasopoulos et al. (121) proposed the two-step Nuss Questionnaire modified for Adults (NQ-mA) and a Single Step Questionnaire (SSQ). These questionnaires measured the disease-specific quality-of-life changes after surgery and assessed the impact of surgery on the physical and psychological well-being of postoperative patients. They noted a significant improvement in self-esteem, social functioning, and a high level of satisfaction following surgery. Their questionnaire also included the impact of surgical wounds/scars on the overall cosmetic result, consciousness of the presence of metallic bar, the decision to have the operation again and questions about postoperative pain which may have limited the patient satisfaction after surgery. Most of the patients were very satisfied with their scars, almost all of them were conscious of the presence of bar, but none of them considered that to be a major inconvenience. Pain was a significant concern in the immediate postoperative period which decreased significantly after several weeks and none of their patients were on analgesics 4–5 months after surgery. Other surgeons have subsequently utilized this modified survey for postoperative assessment (116,117,123) (Table 3).
Pain control methods
Postoperative pain remains an ongoing concern for adults undergoing MIRPE (125). Many analgesic approaches have been implemented to minimize postoperative pain and decrease use of opioids. These approaches include thoracic epidural, patient-controlled analgesia (PCA), subcutaneous catheters and most recently cryoablation therapy, all which reported successful pain control in the immediate postoperative period (126-130). Current techniques are successfully managing perioperative pain with the majority of patients reporting resolution of significant pain one month after surgery (49). In our experience (Figure 7), a standardized protocol achieved good pain control and included gabapentin, ibuprofen, acetaminophen, and narcotics with local anesthesia through the use of cryoablation in the most recent years (21,119,123). Over the course of 11 years at our institution, we utilized epidural (90 patients), subcutaneous catheter (428 patients), and cryoablation (211 patients). All three groups compared together, patients who underwent cryoablation had the shortest length of hospital stay (1.9±1.5 days) and utilized the least amount of morphine equivalents. Neuropathic pain following cryoablation has been reported and is expected to emerge around 8 weeks postoperatively. In our experience, it would be difficult to distinguish the source of neuropathic pain whether it is from the cryoablation or secondary to the bars placed (131). There were minimal patients that experienced pain during this time frame and the cryoablation group was lower than other cohorts. At occasions, adjuvant medications for postoperative pain management have been deployed to help control the pain if continued to bother our patients, including the use of ketorolac, diazepam, and gabapentin (127,128). A randomized clinical trial including adult Pex patients (mean age 20.9 years) showed a decrease in hospital length of stay and opioids requirement in the Cryoablation group versus thoracic epidural analgesia, while offering equivalent pain control (132).
Conclusions
Minimally invasive repair of pectus excavatum in adult patients showed increased difficulty and higher complication rates when compared to pediatric and adolescent populations. Skilled surgeons with experience can perform the procedure safely and successfully using technique modifications to the original Nuss procedure. The published data supports the cardiopulmonary benefits of MIRPE repair in adults with Pex. Patients who have undergone surgery show good satisfaction, a significant improvement in self-image, and report surgery to have had a positive impact on their wellbeing and ability to exercise.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Erik R. de Loos, Jean H. T. Daemen and Frank-Martin Haecker) for the series “Minimally Invasive Treatment of Pectus Deformities” published in Journal of Thoracic Disease. The article has undergone external peer review.
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-87/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-87/coif). The series “Minimally Invasive Treatment of Pectus Deformities” was commissioned by the editorial office without any funding or sponsorship. DEJ is involved in development projects and intellectual property with ZimmerBiomet thru Mayo Clinic Ventures. The authors have no other conflicts of interest to declare.
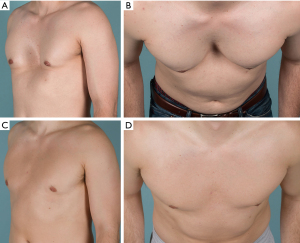
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All images are published with the participant’s consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fokin AA, Steuerwald NM, Ahrens WA, et al. Anatomical, histologic, and genetic characteristics of congenital chest wall deformities. Semin Thorac Cardiovasc Surg 2009;21:44-57. [Crossref] [PubMed]
- Cobben JM, Oostra RJ, van Dijk FS. Pectus excavatum and carinatum. Eur J Med Genet 2014;57:414-7. [Crossref] [PubMed]
- Beier JP, Weber PG, Reingruber B, et al. Aesthetic and functional correction of female, asymmetric funnel chest - a combined approach. Breast 2009;18:60-5. [Crossref] [PubMed]
- Fonkalsrud EW. Management of pectus chest deformities in female patients. Am J Surg 2004;187:192-7. [Crossref] [PubMed]
- Ma IT, Rebecca AM, Notrica DM, et al. Pectus excavatum in adult women: repair and the impact of prior or concurrent breast augmentation. Plast Reconstr Surg 2015;135:303e-12e. [Crossref] [PubMed]
- Park HJ, Gu JH, Jang JC, et al. Correction of pectus excavatum with breast hypoplasia using simultaneous pectus bar procedure and augmentation mammoplasty. Ann Plast Surg 2014;73:190-5. [Crossref] [PubMed]
- Biavati M, Kozlitina J, Alder AC, et al. Prevalence of pectus excavatum in an adult population-based cohort estimated from radiographic indices of chest wall shape. PLoS One 2020;15:e0232575. [Crossref] [PubMed]
- Sigalet DL, Montgomery M, Harder J, et al. Long term cardiopulmonary effects of closed repair of pectus excavatum. Pediatr Surg Int 2007;23:493-7. [Crossref] [PubMed]
- Chao CJ, Jaroszewski DE, Kumar PN, et al. Surgical repair of pectus excavatum relieves right heart chamber compression and improves cardiac output in adult patients--an intraoperative transesophageal echocardiographic study. Am J Surg 2015;210:1118-24; discussion 1124-5. [Crossref] [PubMed]
- Udholm S, Maagaard M, Pilegaard H, et al. Cardiac function in adults following minimally invasive repair of pectus excavatum. Interact Cardiovasc Thorac Surg 2016;22:525-9. [Crossref] [PubMed]
- Del Frari B, Blank C, Sigl S, et al. The questionable benefit of pectus excavatum repair on cardiopulmonary function: a prospective study. Eur J Cardiothorac Surg 2021;61:75-82. [Crossref] [PubMed]
- Kelly RE Jr, Cash TF, Shamberger RC, et al. Surgical repair of pectus excavatum markedly improves body image and perceived ability for physical activity: multicenter study. Pediatrics 2008;122:1218-22. [Crossref] [PubMed]
- Jaroszewski DE, Fonkalsrud EW. Repair of pectus chest deformities in 320 adult patients: 21 year experience. Ann Thorac Surg 2007;84:429-33. [Crossref] [PubMed]
- Kragten HA, Siebenga J, Höppener PF, et al. Symptomatic pectus excavatum in seniors (SPES): a cardiovascular problem?: A prospective cardiological study of 42 senior patients with a symptomatic pectus excavatum. Neth Heart J 2011;19:73-8. [Crossref] [PubMed]
- Jaroszewski D, Steidley E, Galindo A, et al. Treating heart failure and dyspnea in a 78-year-old man with surgical correction of pectus excavatum. Ann Thorac Surg 2009;88:1008-10. [Crossref] [PubMed]
- Nuss D, Obermeyer RJ, Kelly RE. Nuss bar procedure: past, present and future. Ann Cardiothorac Surg 2016;5:422-33. [Crossref] [PubMed]
- Nuss D, Kelly RE Jr, Croitoru DP, et al. A 10-year review of a minimally invasive technique for the correction of pectus excavatum. J Pediatr Surg 1998;33:545-52. [Crossref] [PubMed]
- de Loos ER, Pennings AJ, van Roozendaal LM, et al. Nuss Procedure for Pectus Excavatum: A Comparison of Complications Between Young and Adult Patients. Ann Thorac Surg 2021;112:905-11. [Crossref] [PubMed]
- Johnson WR, Fedor D, Singhal S. Systematic review of surgical treatment techniques for adult and pediatric patients with pectus excavatum. J Cardiothorac Surg 2014;9:25. [Crossref] [PubMed]
- Hebra A, Swoveland B, Egbert M, et al. Outcome analysis of minimally invasive repair of pectus excavatum: review of 251 cases. J Pediatr Surg 2000;35:252-7; discussion 257-8. [Crossref] [PubMed]
- Kanagaratnam A, Phan S, Tchantchaleishvili V, et al. Ravitch versus Nuss procedure for pectus excavatum: systematic review and meta-analysis. Ann Cardiothorac Surg 2016;5:409-21. [Crossref] [PubMed]
- Casar Berazaluce AM, Jenkins TM, Garrison AP, et al. The chest wall gender divide: females have better cardiopulmonary function and exercise tolerance despite worse deformity in pectus excavatum. Pediatr Surg Int 2020;36:1281-6. [Crossref] [PubMed]
- Shamberger RC, Welch KJ, Castaneda AR, et al. Anterior chest wall deformities and congenital heart disease. J Thorac Cardiovasc Surg 1988;96:427-32. [Crossref] [PubMed]
- Li S, Tang ST, Tong Q, et al. Nuss repair of pectus excavatum after surgery for congenital heart disease: experience from a single institution. J Thorac Cardiovasc Surg 2014;148:657-61. [Crossref] [PubMed]
- Gürkan U, Aydemir B, Aksoy S, et al. Echocardiographic assessment of right ventricular function before and after surgery in patients with pectus excavatum and right ventricular compression. Thorac Cardiovasc Surg 2014;62:231-5. [PubMed]
- Das BB, Recto MR, Yeh T. Improvement of cardiopulmonary function after minimally invasive surgical repair of pectus excavatum (Nuss procedure) in children. Ann Pediatr Cardiol 2019;12:77-82. [Crossref] [PubMed]
- Cartoski MJ, Nuss D, Goretsky MJ, et al. Classification of the dysmorphology of pectus excavatum. J Pediatr Surg 2006;41:1573-81. [Crossref] [PubMed]
- Maagaard M, Tang M, Ringgaard S, et al. Normalized cardiopulmonary exercise function in patients with pectus excavatum three years after operation. Ann Thorac Surg 2013;96:272-8. [Crossref] [PubMed]
- Hu T, Feng J, Liu W, et al. Modified sternal elevation for children with pectus excavatum. Chin Med J (Engl) 2000;113:451-4. [PubMed]
- Castellani C, Windhaber J, Schober PH, et al. Exercise performance testing in patients with pectus excavatum before and after Nuss procedure. Pediatr Surg Int 2010;26:659-63. [Crossref] [PubMed]
- Coln E, Carrasco J, Coln D. Demonstrating relief of cardiac compression with the Nuss minimally invasive repair for pectus excavatum. J Pediatr Surg 2006;41:683-6; discussion 683-6. [Crossref] [PubMed]
- Velazco CS, Arsanjani R, Jaroszewski DE. Nuss procedure in the adult population for correction of pectus excavatum. Semin Pediatr Surg 2018;27:161-9. [Crossref] [PubMed]
- Kaguraoka H, Ohnuki T, Itaoka T, et al. Degree of severity of pectus excavatum and pulmonary function in preoperative and postoperative periods. J Thorac Cardiovasc Surg 1992;104:1483-8. [Crossref] [PubMed]
- Morshuis WJ, Folgering HT, Barentsz JO, et al. Exercise cardiorespiratory function before and one year after operation for pectus excavatum. J Thorac Cardiovasc Surg 1994;107:1403-9. [Crossref] [PubMed]
- Haller JA Jr, Loughlin GM. Cardiorespiratory function is significantly improved following corrective surgery for severe pectus excavatum. Proposed treatment guidelines. J Cardiovasc Surg (Torino) 2000;41:125-30. [PubMed]
- Neviere R, Montaigne D, Benhamed L, et al. Cardiopulmonary response following surgical repair of pectus excavatum in adult patients. Eur J Cardiothorac Surg 2011;40:e77-82. [Crossref] [PubMed]
- Malek MH, Fonkalsrud EW, Cooper CB. Ventilatory and cardiovascular responses to exercise in patients with pectus excavatum. Chest 2003;124:870-82. [Crossref] [PubMed]
- Chao CJ, Jaroszewski D, Gotway M, et al. Effects of Pectus Excavatum Repair on Right and Left Ventricular Strain. Ann Thorac Surg 2018;105:294-301. [Crossref] [PubMed]
- Ravanbakhsh S, Farina JM, Bostoros P, et al. Sex Differences in Objective Measures of Adult Patients Presenting for Pectus Excavatum Repair. Ann Thorac Surg 2022;114:1159-67. [Crossref] [PubMed]
- O'Keefe J, Byrne R, Montgomery M, et al. Longer term effects of closed repair of pectus excavatum on cardiopulmonary status. J Pediatr Surg 2013;48:1049-54. [Crossref] [PubMed]
- Willekes CL, Backer CL, Mavroudis C. A 26-year review of pectus deformity repairs, including simultaneous intracardiac repair. Ann Thorac Surg 1999;67:511-8. [Crossref] [PubMed]
- Mocchegiani R, Badano L, Lestuzzi C, et al. Relation of right ventricular morphology and function in pectus excavatum to the severity of the chest wall deformity. Am J Cardiol 1995;76:941-6. [Crossref] [PubMed]
- Töpper A, Polleichtner S, Zagrosek A, et al. Impact of surgical correction of pectus excavatum on cardiac function: insights on the right ventricle. A cardiovascular magnetic resonance study†. Interact Cardiovasc Thorac Surg 2016;22:38-46. [Crossref] [PubMed]
- Jaroszewski DE, Farina JM, Gotway MB, et al. Cardiopulmonary Outcomes After the Nuss Procedure in Pectus Excavatum. J Am Heart Assoc 2022;11:e022149. [Crossref] [PubMed]
- Kelly RE Jr, Mellins RB, Shamberger RC, et al. Multicenter study of pectus excavatum, final report: complications, static/exercise pulmonary function, and anatomic outcomes. J Am Coll Surg 2013;217:1080-9. [Crossref] [PubMed]
- Farina JM, Jaroszewski DE, Arsanjani R, et al. Improved Right Ventricular Diastolic Function Assessed by Hepatic Vein Flow After Pectus Excavatum Repair. Annals of Thoracic Surgery Short Reports. 2023; In press. [Crossref]
- Skoczyński S, Kudela G, Brożek G, et al. Pulmonary function, exercise capacity and dyspnea in patients 7 years after Nuss surgery. Adv Med Sci 2022;67:179-86. [Crossref] [PubMed]
- Szydlik S, Jankowska-Szydlik J, Zwaruń D, et al. An effect of Nuss Procedure on lung function among patients with pectus excavatum. Pol Przegl Chir 2013;85:1-5. [Crossref] [PubMed]
- Kelly RE Jr, Shamberger RC, Mellins RB, et al. Prospective multicenter study of surgical correction of pectus excavatum: design, perioperative complications, pain, and baseline pulmonary function facilitated by internet-based data collection. J Am Coll Surg 2007;205:205-16. [Crossref] [PubMed]
- Kelly RE Jr. Pectus excavatum: historical background, clinical picture, preoperative evaluation and criteria for operation. Semin Pediatr Surg 2008;17:181-93. [Crossref] [PubMed]
- Westphal FL, Lima LC, Lima Neto JC, et al. Prevalence of pectus carinatum and pectus excavatum in students in the city of Manaus, Brazil. J Bras Pneumol 2009;35:221-6. [Crossref] [PubMed]
- Pyeritz RE, McKusick VA. The Marfan syndrome: diagnosis and management. N Engl J Med 1979;300:772-7. [Crossref] [PubMed]
- St Peter SD, Juang D, Garey CL, et al. A novel measure for pectus excavatum: the correction index. J Pediatr Surg 2011;46:2270-3. [Crossref] [PubMed]
- Obermeyer RJ, Cohen NS, Jaroszewski DE. The physiologic impact of pectus excavatum repair. Semin Pediatr Surg 2018;27:127-32. [Crossref] [PubMed]
- Raggio IM, Martínez-Ferro M, Bellía-Munzón G, et al. Diastolic and Systolic Cardiac Dysfunction in Pectus Excavatum: Relationship to Exercise and Malformation Severity. Radiol Cardiothorac Imaging 2020;2:e200011. [Crossref] [PubMed]
- Rodriguez-Granillo GA, Toselli L, Farina J, et al. Usefulness of strain cardiac magnetic resonance for the exposure of mild left ventricular systolic abnormalities in pectus excavatum. J Pediatr Surg 2022;57:319-24. [Crossref] [PubMed]
- 57. Pulivarthi V, Jaroszewski D, Arsanjani R, Abstract 16999: Effect of Positional Echocardiogram on Right Sided Hemodynamics in Pectus Excavatum Patients vs. Healthy Adults. [online] Circulation. Available online: https://www.ahajournals.org/doi/10.1161/circ.138.suppl_1.16999
- Stephens EH, Dearani JA, Jaroszewski DE. Pectus Excavatum in Cardiac Surgery Patients. Ann Thorac Surg 2023;115:1312-21. [Crossref] [PubMed]
- Jaroszewski DE, Gustin PJ, Haecker FM, et al. Pectus excavatum repair after sternotomy: the Chest Wall International Group experience with substernal Nuss bars. Eur J Cardiothorac Surg 2017;52:710-7. [Crossref] [PubMed]
- Poston PM, Patel SS, Rajput M, et al. The correction index: setting the standard for recommending operative repair of pectus excavatum. Ann Thorac Surg 2014;97:1176-9; discussion 1179-80. [Crossref] [PubMed]
- Goretsky MJ, Kelly RE Jr, Croitoru D, et al. Chest wall anomalies: pectus excavatum and pectus carinatum. Adolesc Med Clin 2004;15:455-71. [Crossref] [PubMed]
- Frantz FW. Indications and guidelines for pectus excavatum repair. Curr Opin Pediatr 2011;23:486-91. [Crossref] [PubMed]
- Kelly RE Jr, Obermeyer RJ, Goretsky MJ, et al. Recent Modifications of the Nuss Procedure: The Pursuit of Safety During the Minimally Invasive Repair of Pectus Excavatum. Ann Surg 2022;275:e496-502. [Crossref] [PubMed]
- Dzielicki J, Korlacki W, Janicka I, et al. Difficulties and limitations in minimally invasive repair of pectus excavatum--6 years experiences with Nuss technique. Eur J Cardiothorac Surg 2006;30:801-4. [Crossref] [PubMed]
- Chen Z, Amos EB, Luo H, et al. Comparative pulmonary functional recovery after Nuss and Ravitch procedures for pectus excavatum repair: a meta-analysis. J Cardiothorac Surg 2012;7:101. [Crossref] [PubMed]
- Johnson JN, Hartman TK, Pianosi PT, et al. Cardiorespiratory function after operation for pectus excavatum. J Pediatr 2008;153:359-64. [Crossref] [PubMed]
- Fonkalsrud EW, Salman T, Guo W, et al. Repair of pectus deformities with sternal support. J Thorac Cardiovasc Surg 1994;107:37-42. [Crossref] [PubMed]
- Buchwald J, Ligarski D, Polewczyk T. Long-term results after the modified Ravitch procedure performed in children and adolescents - a one-time procedure without the need to use additional support of the sternum. A retrospective study. Kardiochir Torakochirurgia Pol 2020;17:173-7. [Crossref] [PubMed]
- Tikka T, Kalkat MS, Bishay E, et al. A 20-year review of pectus surgery: an analysis of factors predictive of recurrence and outcomes. Interact Cardiovasc Thorac Surg 2016;23:908-13. [Crossref] [PubMed]
- Mao YZ, Tang S, Li S. Comparison of the Nuss versus Ravitch procedure for pectus excavatum repair: an updated meta-analysis. J Pediatr Surg 2017;52:1545-52. [Crossref] [PubMed]
- Lam MW, Klassen AF, Montgomery CJ, et al. Quality-of-life outcomes after surgical correction of pectus excavatum: a comparison of the Ravitch and Nuss procedures. J Pediatr Surg 2008;43:819-25. [Crossref] [PubMed]
- Jaroszewski DE, Ewais MM, Chao CJ, et al. Success of Minimally Invasive Pectus Excavatum Procedures (Modified Nuss) in Adult Patients (≥30 Years). Ann Thorac Surg 2016;102:993-1003. [Crossref] [PubMed]
- Hebra A, Jacobs JP, Feliz A, et al. Minimally invasive repair of pectus excavatum in adult patients. Am Surg 2006;72:837-42. [Crossref] [PubMed]
- Park HJ, Jeong JY, Jo WM, et al. Minimally invasive repair of pectus excavatum: a novel morphology-tailored, patient-specific approach. J Thorac Cardiovasc Surg 2010;139:379-86. [Crossref] [PubMed]
- McMahon LE, Johnson KN, Jaroszewski DE, et al. Experience with FiberWire for pectus bar attachment. J Pediatr Surg 2014;49:1259-63. [Crossref] [PubMed]
- Pilegaard HK. Nuss technique in pectus excavatum: a mono-institutional experience. J Thorac Dis 2015;7:S172-6. [PubMed]
- Molik KA, Engum SA, Rescorla FJ, et al. Pectus excavatum repair: experience with standard and minimal invasive techniques. J Pediatr Surg 2001;36:324-8. [Crossref] [PubMed]
- Park HJ, Lee SY, Lee CS, et al. The Nuss procedure for pectus excavatum: evolution of techniques and early results on 322 patients. Ann Thorac Surg 2004;77:289-95. [Crossref] [PubMed]
- Jaroszewski DE, Johnson K, McMahon L, et al. Sternal elevation before passing bars: a technique for improving visualization and facilitating minimally invasive pectus excavatum repair in adult patients. J Thorac Cardiovasc Surg 2014;147:1093-5. [Crossref] [PubMed]
- Notrica DM. Modifications to the Nuss procedure for pectus excavatum repair: A 20-year review. Semin Pediatr Surg 2018;27:133-50. [Crossref] [PubMed]
- Park HJ, Chung WJ, Lee IS, et al. Mechanism of bar displacement and corresponding bar fixation techniques in minimally invasive repair of pectus excavatum. J Pediatr Surg 2008;43:74-8. [Crossref] [PubMed]
- Tedde ML, de Campos JR, Wihlm JM, et al. The Nuss procedure made safer: an effective and simple sternal elevation manoeuvre. Eur J Cardiothorac Surg 2012;42:890-1. [Crossref] [PubMed]
- Takagi S, Oyama T, Tomokazu N, et al. A new sternum elevator reduces severe complications during minimally invasive repair of the pectus excavatum. Pediatr Surg Int 2012;28:623-6. [Crossref] [PubMed]
- Haecker FM, Sesia SB. Intraoperative use of the vacuum bell for elevating the sternum during the Nuss procedure. J Laparoendosc Adv Surg Tech A 2012;22:934-6. [Crossref] [PubMed]
- Johnson WR, Fedor D, Singhal S. A novel approach to eliminate cardiac perforation in the nuss procedure. Ann Thorac Surg 2013;95:1109-11. [Crossref] [PubMed]
- Kim D, Idowu O, Palmer B, et al. Anterior chest wall elevation using a T-fastener suture technique during a Nuss procedure. Ann Thorac Surg 2014;98:734-6. [Crossref] [PubMed]
- Park HJ, Jeong JY, Kim KT, et al. Hinge reinforcement plate for adult pectus excavatum repair: a novel tool for the prevention of intercostal muscle strip. Interact Cardiovasc Thorac Surg 2011;12:687-91. [Crossref] [PubMed]
- Park HJ, Kim KS, Lee S, et al. A next-generation pectus excavatum repair technique: new devices make a difference. Ann Thorac Surg 2015;99:455-61. [Crossref] [PubMed]
- Park HJ, Kim KS, Moon YK, et al. The bridge technique for pectus bar fixation: a method to make the bar un-rotatable. J Pediatr Surg 2015;50:1320-2. [Crossref] [PubMed]
- Pilegaard HK, Licht PB. Routine use of minimally invasive surgery for pectus excavatum in adults. Ann Thorac Surg 2008;86:952-6. [Crossref] [PubMed]
- Pio L, Carlucci M, Leonelli L, et al. Minimally Invasive Repair of Pectus Excavatum Without Bar Stabilizers Using Endo Close. J Laparoendosc Adv Surg Tech A 2016;26:148-52. [Crossref] [PubMed]
- Kelly RE, Goretsky MJ, Obermeyer R, et al. Twenty-one years of experience with minimally invasive repair of pectus excavatum by the Nuss procedure in 1215 patients. Ann Surg 2010;252:1072-81. [Crossref] [PubMed]
- Yoon YS, Kim HK, Choi YS, et al. A modified Nuss procedure for late adolescent and adult pectus excavatum. World J Surg 2010;34:1475-80. [Crossref] [PubMed]
- Nuss D. Minimally invasive surgical repair of pectus excavatum. Semin Pediatr Surg 2008;17:209-17. [Crossref] [PubMed]
- Nagaso T, Miyamoto J, Kokaji K, et al. Double-bar application decreases postoperative pain after the Nuss procedure. J Thorac Cardiovasc Surg 2010;140:39-44, 44.e1-2.
- Stanfill AB, DiSomma N, Henriques SM, et al. Nuss procedure: decrease in bar movement requiring reoperation with primary placement of two bars. J Laparoendosc Adv Surg Tech A 2012;22:412-5. [Crossref] [PubMed]
- Li G, Jiang Z, Xiao H, et al. A novel modified Nuss procedure for pectus excavatum: a new steel bar. Ann Thorac Surg 2015;99:1788-92. [Crossref] [PubMed]
- Park HJ. A technique for complex pectus excavatum repair: the cross-bar technique for grand canyon type deformity (Park classification). Ann Cardiothorac Surg 2016;5:526-7. [Crossref] [PubMed]
- Sayan B, Bekiroglu N, Yuksel M. Pectus cross bars increase hospital readmission rates due to serous pleural effusion. Gen Thorac Cardiovasc Surg 2022;70:352-8. [Crossref] [PubMed]
- Wang L, Liu J, Li Y, et al. Modified Nuss operation using introducer-bar complex for pectus excavatum in adults: a retrospective study. J Cardiothorac Surg 2021;16:267. [Crossref] [PubMed]
- Croitoru DP, Kelly RE Jr, Goretsky MJ, et al. Experience and modification update for the minimally invasive Nuss technique for pectus excavatum repair in 303 patients. J Pediatr Surg 2002;37:437-45. [Crossref] [PubMed]
- Cheng YL, Lee SC, Huang TW, et al. Efficacy and safety of modified bilateral thoracoscopy-assisted Nuss procedure in adult patients with pectus excavatum. Eur J Cardiothorac Surg 2008;34:1057-61. [Crossref] [PubMed]
- Ewais MM, Chaparala S, Uhl R, et al. Outcomes in adult pectus excavatum patients undergoing Nuss repair. Patient Relat Outcome Meas 2018;9:65-90. [Crossref] [PubMed]
- Cheng YL, Lin CT, Wang HB, et al. Pleural effusion complicating after Nuss procedure for pectus excavatum. Ann Thorac Cardiovasc Surg 2014;20:6-11. [Crossref] [PubMed]
- Cabrera A, Pulivarthi VSKK, Lacky J, et al. Robotic Takedown of Internal Mammary Artery to Prevent Occlusion From Bars During Nuss Pectus Repair. Ann Thorac Surg 2020;109:e423-4. [Crossref] [PubMed]
- Yüksel M, Özalper MH, Bostanci K, et al. Do Nuss bars compromise the blood flow of the internal mammary arteries? Interact Cardiovasc Thorac Surg 2013;17:571-5. [Crossref] [PubMed]
- Pilegaard HK. Single centre experience on short bar technique for pectus excavatum. Ann Cardiothorac Surg 2016;5:450-5. [Crossref] [PubMed]
- Aronson DC, Bosgraaf RP, van der Horst C, et al. Nuss procedure: pediatric surgical solution for adults with pectus excavatum. World J Surg 2007;31:26-9; discussion 30. [Crossref] [PubMed]
- Kim DH, Hwang JJ, Lee MK, et al. Analysis of the Nuss procedure for pectus excavatum in different age groups. Ann Thorac Surg 2005;80:1073-7. [Crossref] [PubMed]
- Ong CC, Choo K, Morreau P, et al. The learning curve in learning the curve: a review of Nuss procedure in teenagers. ANZ J Surg 2005;75:421-4. [Crossref] [PubMed]
- Hebra A, Kelly RE, Ferro MM, et al. Life-threatening complications and mortality of minimally invasive pectus surgery. J Pediatr Surg 2018;53:728-32. [Crossref] [PubMed]
- Viggiano D, Bongiolatti S, Borgianni S, et al. Nuss Technique for Pectus Excavatum in Adult Patients: Cosmetic Satisfaction and Improvement of Quality of Life in a Single-Center Experience. Front Surg 2022;9:903791. [Crossref] [PubMed]
- Pilegaard HK. Extending the use of Nuss procedure in patients older than 30 years. Eur J Cardiothorac Surg 2011;40:334-7. [Crossref] [PubMed]
- Pawlak K, Gąsiorowski Ł, Gabryel P, et al. Early and Late Results of the Nuss Procedure in Surgical Treatment of Pectus Excavatum in Different Age Groups. Ann Thorac Surg 2016;102:1711-6. [Crossref] [PubMed]
- Erşen E, Demirkaya A, Kılıç B, et al. Minimally invasive repair of pectus excavatum (MIRPE) in adults: is it a proper choice? Wideochir Inne Tech Maloinwazyjne 2016;11:98-104. [Crossref] [PubMed]
- Sacco Casamassima MG, Gause C, Goldstein SD, et al. Patient Satisfaction After Minimally Invasive Repair of Pectus Excavatum in Adults: Long-Term Results of Nuss Procedure in Adults. Ann Thorac Surg 2016;101:1338-45. [Crossref] [PubMed]
- Hoksch B, Kocher G, Vollmar P, et al. Nuss procedure for pectus excavatum in adults: long-term results in a prospective observational study. Eur J Cardiothorac Surg 2016;50:934-9. [Crossref] [PubMed]
- Hanna WC, Ko MA, Blitz M, et al. Thoracoscopic Nuss procedure for young adults with pectus excavatum: excellent midterm results and patient satisfaction. Ann Thorac Surg 2013;96:1033-6; discussion 1037-8. [Crossref] [PubMed]
- Jaroszewski D, Notrica D, McMahon L, et al. Current management of pectus excavatum: a review and update of therapy and treatment recommendations. J Am Board Fam Med 2010;23:230-9. [Crossref] [PubMed]
- Jacobsen EB, Thastum M, Jeppesen JH, et al. Health-related quality of life in children and adolescents undergoing surgery for pectus excavatum. Eur J Pediatr Surg 2010;20:85-91. [Crossref] [PubMed]
- Krasopoulos G, Dusmet M, Ladas G, et al. Nuss procedure improves the quality of life in young male adults with pectus excavatum deformity. Eur J Cardiothorac Surg 2006;29:1-5. [Crossref] [PubMed]
- Kuru P, Bostanci K, Ermerak NO, et al. Quality of life improves after minimally invasive repair of pectus excavatum. Asian Cardiovasc Thorac Ann 2015;23:302-7. [Crossref] [PubMed]
- Metzelder ML, Kuebler JF, Leonhardt J, et al. Self and parental assessment after minimally invasive repair of pectus excavatum: lasting satisfaction after bar removal. Ann Thorac Surg 2007;83:1844-9. [Crossref] [PubMed]
- Lomholt JJ, Jacobsen EB, Thastum M, et al. A prospective study on quality of life in youths after pectus excavatum correction. Ann Cardiothorac Surg 2016;5:456-65. [Crossref] [PubMed]
- Papic JC, Finnell SM, Howenstein AM, et al. Postoperative opioid analgesic use after Nuss versus Ravitch pectus excavatum repair. J Pediatr Surg 2014;49:919-23; discussion 923. [Crossref] [PubMed]
- Jaroszewski DE, Temkit M, Ewais MM, et al. Randomized trial of epidural vs. subcutaneous catheters for managing pain after modified Nuss in adults. J Thorac Dis 2016;8:2102-10. [Crossref] [PubMed]
- Gebhardt R, Mehran RJ, Soliz J, et al. Epidural versus ON-Q local anesthetic-infiltrating catheter for post-thoracotomy pain control. J Cardiothorac Vasc Anesth 2013;27:423-6. [Crossref] [PubMed]
- Ried M, Schilling C, Potzger T, et al. Prospective, comparative study of the On-Q® PainBuster® postoperative pain relief system and thoracic epidural analgesia after thoracic surgery. J Cardiothorac Vasc Anesth 2014;28:973-8. [Crossref] [PubMed]
- Weber T, Mätzl J, Rokitansky A, et al. Superior postoperative pain relief with thoracic epidural analgesia versus intravenous patient-controlled analgesia after minimally invasive pectus excavatum repair. J Thorac Cardiovasc Surg 2007;134:865-70. [Crossref] [PubMed]
- Futagawa K, Suwa I, Okuda T, et al. Anesthetic management for the minimally invasive Nuss procedure in 21 patients with pectus excavatum. J Anesth 2006;20:48-50. [Crossref] [PubMed]
- Jaroszewski D, Bostoros P, Farina JM, et al. Evolution of Pain Control for Adult Pectus Excavatum Repair. Annals of thoracic surgery. 2023; - accepted for publication). [Crossref] [PubMed]
- Graves CE, Moyer J, Zobel MJ, et al. Intraoperative intercostal nerve cryoablation During the Nuss procedure reduces length of stay and opioid requirement: A randomized clinical trial. J Pediatr Surg 2019;54:2250-6. [Crossref] [PubMed]



