
Extracorporeal membrane oxygenation in the surgical management of large mediastinal masses: a narrative review
Introduction
Background
The surgical management of asymptomatic mediastinal tumors such as thymoma in the anterior mediastinal space or neurogenic tumor in the posterior mediastinal compartment is typically straightforward. Preoperative imaging modalities such as computed tomography (CT) scan or magnetic resonance imaging (MRI) are essential for preoperative planning. Occasionally, cardiothoracic surgeons are called upon to manage massive complex tumors of various etiologies located in the mediastinal compartments, some of which may require surgical intervention. The perioperative management of such mediastinal masses may be challenging given the potential for decompensation with general anesthesia and supine positioning. In the setting of hypoxemia or hemodynamic collapse on induction of general anesthesia, conventional measures are often unsuccessful and there is a high risk of neurologic insult, cardiac arrest, and death (1). This has led to the study of risk stratification, anesthetic management, and circulatory support to enable management for mass diagnosis and resection. Our review will focus on the role of mechanical circulatory support in the setting of high-risk patients with mediastinal masses.
Extracorporeal circulation has long been a valuable tool for operations within the thoracic cavity. After initial experience with cross circulation by Lillihei and others, the cardiopulmonary bypass (CPB) circuit was successfully used in cardiac surgery by Dr. Kirklin in 1955 (2). Through the use of reservoirs, pumps, oxygenators and heat exchangers, blood flow and gas exchange are managed. Further advances include cardioplegic arrest for myocardial protection and complex operations requiring a still operative field. Extracorporeal membrane oxygenation (ECMO) serves as an alternative to CPB in that no reservoir is used, providing the ability for prolonged circuit runs and lower anticoagulation requirements at the expense of being less able to rapidly adjust volume status of the patient. ECMO may be operated in several configurations, largely veno-venous (V-V) and veno-arterial (V-A) for those patients requiring pulmonary and cardiopulmonary support respectively.
Rationale and knowledge gap
It may be surmised that the use of circulatory support via ECMO could prevent instability in the management of patients with large mediastinal masses. Among the first reports of ECMO utilization in the setting of a mediastinal mass was from Hall in 1975 (3). They describe rapid deterioration of a 14-year-old patient upon induction of anesthesia from compression of the pulmonary artery (PA) by the mass. The patient was expediently awakened and V-A ECMO was established through the femoral vessels under local anesthesia prior to proceeding with the operation uneventfully. This case is particularly notable due to their observation of general anesthesia inducing profound hypoxemia despite lack of symptoms preoperatively. Utilization has spread in a variety of circumstances to facilitate treatment of mediastinal masses in clinical practice. A number of articles have provided case reports and series of ECMO use in the management of mediastinal masses, as well as several excellent studies evaluating the anesthetic risk of such patients. As teams plan and implement mechanical circulatory support in such cases, it is valuable to have a resource summarizing pertinent considerations and concerns.
Objective
We seek to provide a narrative review to help guide clinicians in patient selection and implementation of ECMO support. We will present the physiology of the disease process, risks assessment methods and practical implementation of ECMO in this subset of patients. We present this article in accordance with the Narrative Review reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1391/rc).
Methods
An extensive search for literature on the topic of ECMO in the management of mediastinal masses was performed. Table 1 demonstrates our approach to the search based on the guidelines of this journal. MeSH search terms of ‘extracorporeal circulation’ OR ‘cardiopulmonary bypass’ OR ‘anesthesia’ in conjunction with ‘mediastinal disease’ OR ‘mediastinal cancer’ formed the basis of the literature review. Following identification of articles based on these methods, references listed in each article were reviewed and examined for suitability for inclusion in this review. Each article was reviewed by one or more authors. We provide a narrative description based on the results of this search.
Table 1
| Items | Specification |
|---|---|
| Date of search | August 1, 2022 |
| Databases and other sources searched | PubMed |
| Search terms used (MeSH) | [‘extracorporeal circulation’ OR ‘cardiopulmonary bypass’ Or ‘anesthesia’] AND [‘mediastinal disease’ OR ‘mediastinal cancer’] |
| Timeframe | 1950–2022 |
| Inclusion criteria | Inclusion: English language only |
| Selection Process | EMW performed initial literature search with subsequent help from all authors |
| Additional comments | References of each individual article also considered for inclusion |
Key findings
Physiology
The adverse hemodynamic effects of mediastinal masses are largely driven by the presence of the mass within a cavity of fixed volume. Major airways and vascular structures may both be compromised by mass effect. The trachea is particularly vulnerable to compression by large mediastinal masses. Complete distal airway obstruction creates a ventilation-perfusion mismatch unable to be corrected by positive pressure ventilation. In a similar manner compression of the main or branch PA leads to ventilated tissue without perfusion, leading to hypoxemia compounded by obstructive shock. Mass effect may also contribute to a restrictive pattern on cardiac function, in effect creating tamponade. The right atrium and ventricle are particularly susceptible. Obstruction of the superior vena cava (SVC) may also create an SVC syndrome presenting with facial swelling, plethora, headache and altered mental status (4).
Anatomic obstruction by a mediastinal mass is greatly worsened in the operating room. The supine positioning typically utilized for biopsy or resection increases compression due to gravity effects and cephalad displacement of the diaphragm, particularly with anterior mediastinal masses (5,6). Induction of anesthesia and paralytics result in loss of muscle tone that provides support within the thoracic cavity and neck; loss of smooth muscle tone also makes airways more susceptible to compression (7). Finally, positive pressure ventilation increases intrathoracic pressure, worsening compression of pulmonary vessels and right heart failure (8). Spontaneous ventilation creates negative intrathoracic pressure that may help reduce this compression effect and is an important pillar of the anesthetic management of these patients (6).
Patient selection
Preoperative selection of patients at risk of requiring ECMO support for mass resection is critical. While definitive criteria have not been standardized, several approaches for patient evaluation have been suggested. A detailed history of patient symptoms may elucidate potential airway or vascular compression with supine position. Patients may describe inability to sleep supine at night and describe use of pillows or a recliner. Symptoms experienced may include dyspnea, stridor, and lightheadedness with supine positioning. Controlled provocative testing in the exam room by slowly reclining the patient’s exam table to elucidate symptoms is useful. Identification of a comfortable position as a ‘rescue position’ has also been described to identify potential temporizing position in the event of impending hemodynamic collapse in the operating room (5).
Several objective measures have also been evaluated for identification of at-risk patients. Cross sectional imaging, typically a CT of the thorax with intravenous contrast, is invaluable for anatomic evaluation. Tumor size, location, and relation to surrounding structures is reviewed. Encasement or direct invasion may significantly alter surgical planning. Direct compression and mass effect has been demonstrated to be a strong predictive factor for requiring hemodynamic support (9). Compression of the tracheal lumen greater than 50% of cross-sectional area has been associated with total airway obstruction during general anesthesia in multiple studies (10,11). Compression of main or branch PAs also may demonstrate concern for development of profound ventilation-perfusion mismatch. Mass size also demonstrates some predictive value with 130 cc volume suggested (11). Classically, anterior mediastinal masses are at higher risk of obstruction, though large posterior mediastinal masses have also been described as sources of hemodynamic compromise from left atrial compression (12-14).
Pulmonary function testing (PFT) may also provide useful clues to aid in risk stratification. A mixed obstructive and restrictive defect is classically seen with mediastinal masses. Peak expiratory flow rate (PEFR) is a useful indicator and validated to have increased risk with general anesthesia in pediatric patients with PEFR <40% predicted (11). Postural PFTs comparing seated to supine readings have been described, though a study by Hnatiuk challenges their utility (15).
Overall risk stratification is performed with clinician judgment. Blank et al. report their approach to risk stratification based on postural symptoms, degree of tracheal compression, bronchial compression, pericardial effusion and SVC syndrome (5). Similar systems have also been proposed by Li focusing on symptoms and tracheal compression (16). Ng et al. confirm that these factors are the most reliable indicators of complications with general anesthesia through retrospective review in pediatric patients (17). By delineating those patients at risk for complications a management plan can be created; this is best performed in close communication between the thoracic surgery and anesthesiology teams. Figures 1,2 demonstrate examples of high and intermediate risk patients respectively.
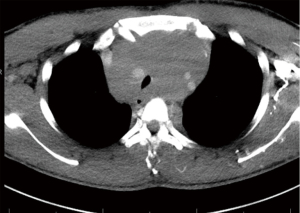
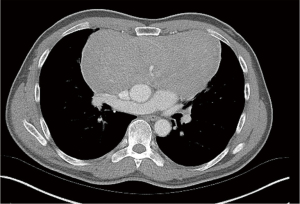
Practical consideration in extracorporeal circulation
The first consideration in establishing a surgical plan for extracorporeal circulation is the type of circuit to be used. Direct invasion of cardiac structures or anticipated vascular involvement requiring extensive resection and reconstruction are best managed using a formal CPB circuit. This would permit management of volume shifts and blood loss as well as potential utilization of cardioplegic arrest for myocardial protection and ability to resect and repair structures (18-22). The techniques and arrangement of CPB is beyond the scope of this article but is a valuable tool to consider.
ECMO is primarily utilized in the setting of concern for cardiovascular collapse or inability to perform gas exchange due to mass effect of the tumor on induction of anesthesia. Various configurations of ECMO may be considered. V-V ECMO may be utilized in circumstances in which pure airway compression is anticipated. Access may be configured via femoral-internal jugular vein (IJ), femoral-femoral or double lumen cannulas via the internal jugular vein. In the setting of a mediastinal mass, we prefer bilateral femoral venous access as this has the advantage of being able to be performed with the head of the bed elevated or in reverse Trendelenburg positioning. This avoids precipitation of respiratory compromise most prominent in the supine position. This comes at an increased risk of recirculation that may be addressed through careful positioning of the cannulas under fluoroscopy. Advantages of the V-V approach include avoidance of arterial cannulation, which is the primary source of morbidity following ECMO support though risk of limb malperfusion via cannula size or dissection. V-A ECMO allows for both support of gas exchange and hemodynamic function. The most common access site is via the common femoral artery and femoral vein. Central cannulation via the aorta and right atrium may be a consideration in the resection of a mediastinal mass invading cardiac structures without any concern for hemodynamic collapse on induction. Preoperative imaging must be carefully reviewed in this circumstance, as the presence of a large mediastinal mass may make exposure of the aorta and right atrium for central cannulation exceedingly difficult. The femoral vessels may be accessed via either an open cutdown or percutaneous access with ultrasound guidance depending on surgeon and institution preference. An appropriately sized arterial cannula and three stage venous cannula can provide full hemodynamic support during the operation. In the setting of diminutive femoral arteries or significant peripheral arterial disease, direct axillary artery cutdown can be performed under a local anesthetic with an 8–10 mm synthetic graft sewn in an end to side fashion as arterial access.
Special considerations with V-A support include maintaining limb perfusion to prevent ischemia or postoperative compartment syndrome, particularly of concern in younger patients without collateral circulation. In patients with small arterial vessels, prolonged anticipated operative time, or with need for ongoing support postoperatively a reperfusion cannula placed in the superficial femoral artery and connected via the side port of the arterial cannula provides ongoing leg perfusion. Differential hypoxemia would be uncommon in this patient population but may be encountered if ventilation held during ECMO support. This may be alleviated by the addition of a internal jugular cannula to the arterial outflow of the circuit.
The extent of preinduction preparation for ECMO is also a point to consider. A number of approaches are possible depending on the anticipated risk of requiring ECMO support. The first option is to complete full cannulation and connect to circuit under a local anesthetic prior to induction of anesthesia. This is the safest manner in which to prepare for support as flow can be established prophylactically or instantaneously in the event of instability. This does however carry the risk of vascular complications of ECMO access in circumstances in which support may not ultimately be utilized. These complications may include bleeding, pseudoaneurysm formation, and ischemia distally in the limb from arterial obstruction or dissection. A second option in those patients in whom need for support is deemed possible but not certain is to obtain vascular access, via guidewire placement or cut down technique, without cannula insertion prior to induction. With a surgeon scrubbed and perfusionist in room with circuit primed, cannula insertion may be performed expediently and ECMO support initiated in the event of any hemodynamic compromise. However, cannulation may not take place as quickly as envisioned preoperatively, particularly in the setting of an arresting patient with chest compressions ongoing. Anderson et al. describe this very scenario with delays in bypass initiation despite guidewire access and suggest that the 5–20 minute time to initiate bypass may not prevent neurologic insult or mortality (12). The final option is to have a surgical and perfusion team on standby in the room but without cannula or guidewire insertion. This approach suffers to a greater degree with concern for inability to cannulate in a safe period of time and is thus suboptimal for management of patients in which circulatory support is considered a possibility. It is our approach to have guidewires placed in patients deemed intermediate risk for ECMO requirement and for full cannulation in those deemed high risk.
The decision to initiate flow via preestablished cannulas or to transition from guidewires to cannula is driven by close and constant communication between the anesthesiology and surgical teams. Hypotension not responsive to repositioning or vasopressor infusion is a strong indication to initiate support. If the appropriate position for surgical exposure is unable to be achieved, support should be instituted. The second consideration is airway. Respiratory distress during an awake intubation or inability to either mask ventilate or pass a endotracheal tube past the compressed area should result in immediate initiation of ECMO support. Again, it is prudent to have a low threshold for initiation of ECMO support to avoid extremis and end organ damage.
Prior to initiation of ECMO support our institution administers an initial heparin bolus off 100 µ/kg with goal activated coagulation time (ACT) greater than 200. Unless concern for ongoing bleeding during the case, we maintain anticoagulation at this level throughout the surgical case. Patients are maintained at full flow for V-A ECMO while the mass is exposed. This support is maintained until either the mass is removed or until sufficient dissection has been performed that the mass effect of the lesion no longer has hemodynamic effect. At this point, the ECMO circuit is weaned as per institution preference. Once ECMO support has been able to be discontinued, the patient is decannulated during the index operation with percutaneous or open repair of the vessels per institution preference. Protamine to reverse anticoagulation may be administered and the operation concluded in a standard fashion. It would be a rare circumstance that continued support be required postoperatively.
Discussion
Utilization of the above information provides a framework for clinical application of circulatory support in these patients. We present herein a case of a 62-year-old male with biopsy proven atypical neuroendocrine tumor of the anterior mediastinum 18 cm × 12 cm × 9 cm in size (Figure 2). Positron emitting tomography (PET) imaging demonstrated bone uptake consistent with metastatic disease and chemotherapy was given with minimal response in tumor size. The patient was able to lie supine without difficulty though did endorse pain lying on the right side. Chest CT scan with intravenous contrast demonstrated no compression of the trachea and cardiovascular structures. Pulmonary function test was performed with forced expiratory volume in 1 second (FEV1) 75% predicted, diffusing capacity of carbon monoxide (DLCO) 63% predicted, PEFR 82% predicted. After extensive discussion between the surgical and anesthesiology teams, the patient was deemed intermediate risk of hemodynamic compromise with general anesthesia due to his postural symptoms without compression on CT. With this in mind, prior to induction in the operating room sheaths were placed in the right common femoral artery and vein. The cardiothoracic surgeon and perfusionist were both ready for initiation of bypass with circuit primed and lines prepared. The patient was induced fully prepped and draped with no hemodynamic compromise. This operation was able to be performed via sternotomy with bilateral anterior thoracotomy with no requirement for ECMO. The sheaths were removed postoperatively, and the patient recovered well from his operation. This particular case illustrates the utility of thorough workup and planning in carrying out these operations. While resource intensive, the conduct of this operation demonstrates a method of ensuring safety during the surgery.
A second representative case involves the management of a 29-year-old female presenting acutely with a symptomatic middle mediastinal mass, previously reported by our group in 2020 (23). This mass was positioned within the superior mediastinum and contributed to tracheal compression <25% of the normal tracheal lumen; no significant compression of vascular structures was noted (Figure 3). In this case, Seldinger technique was utilized to cannulate the bilateral femoral veins in V-V configuration under local anesthesia. Flow at 4 L/min was initiated prior to induction. Upon induction, a 28 Fr double lumen tube was placed with some difficulty and the patient was able to have flow reduced to 2 lpm for the duration of the operation performed through a right posterolateral thoracotomy. Decannulation was performed prior to extubation in the operating room. This case demonstrates an extreme in which loss of airway was nearly certain and initiation of ECMO prior to induction eliminated any concern regarding general anesthesia in this patient. It also demonstrates use of V-V configuration in select circumstances in which hemodynamic compromise is deemed of lower risk compared to airway compromise.

While the primary focus of this article is on the perioperative management of mediastinal masses, mechanical circulatory support may also be of benefit in nonsurgical scenarios. In patients with lymphoma and germ cell tumors with hemodynamic compromise, the use of ECMO while undergoing emergent chemotherapy has been well described with excellent outcomes (24-28). Its use has been limited to those patients with good prognosis to avoid futile support. ECMO support without mechanical ventilation may further aid in hemodynamic by preserving spontaneous respiratory function (27). Temporary ECMO support prior to placing tracheal stents for collapsed airways is reported (29). The support of patients with mediastinal masses undergoing unrelated surgeries is also described, as in cesarean section delivery (30,31).
Conclusion
The use of a multidisciplinary team consisting of surgeons, anesthesiologists, perfusionists and OR team is critical to the success in the use of ECMO in the resection of mediastinal masses. With diligent preparation, these high-risk patients may be optimally managed at the time of resection.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Ilhan Inci) for the series “Extracorporeal Life Support in Thoracic Surgery” published in Journal of Thoracic Disease. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1391/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1391/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1391/coif). The series “Extracorporeal Life Support in Thoracic Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Levin H, Bursztein S, Heifetz M. Cardiac arrest in a child with an anterior mediastinal mass. Anesth Analg 1985;64:1129-30. [Crossref] [PubMed]
- Hessel EA 2nd. A Brief History of Cardiopulmonary Bypass. Semin Cardiothorac Vasc Anesth 2014;18:87-100. [Crossref] [PubMed]
- Hall KD, Friedman M. Extracorporeal oxygenation for induction of anesthesia in a patient with an intrathoracic tumor. Anesthesiology 1975;42:493-5. [Crossref] [PubMed]
- Zhang S, Tan D, Wu W, et al. Extracorporeal membrane oxygenation (ECMO) assisted mediastinal tumor resection and superior vena cava replacement are safe and feasible. Thorac Cancer 2019;10:1846-51. [Crossref] [PubMed]
- Blank RS, de Souza DG. Anesthetic management of patients with an anterior mediastinal mass: continuing professional development. Can J Anaesth 2011;58:853-9, 860-7. [Crossref] [PubMed]
- Gardner JC, Royster RL. Airway collapse with an anterior mediastinal mass despite spontaneous ventilation in an adult. Anesth Analg 2011;113:239-42. [Crossref] [PubMed]
- Tempe DK, Arya R, Dubey S, et al. Mediastinal mass resection: Femorofemoral cardiopulmonary bypass before induction of anesthesia in the management of airway obstruction. J Cardiothorac Vasc Anesth 2001;15:233-6. [Crossref] [PubMed]
- Luckhaupt-Koch K. Mediastinal mass syndrome. Paediatr Anaesth 2005;15:437-8. [Crossref] [PubMed]
- Lam JC, Chui CH, Jacobsen AS, et al. When is a mediastinal mass critical in a child? An analysis of 29 patients. Pediatr Surg Int 2004;20:180-4. [Crossref] [PubMed]
- Azizkhan RG, Dudgeon DL, Buck JR, et al. Life-threatening airway obstruction as a complication to the management of mediastinal masses in children. J Pediatr Surg 1985;20:816-22. [Crossref] [PubMed]
- Béchard P, Létourneau L, Lacasse Y, et al. Perioperative cardiorespiratory complications in adults with mediastinal mass: incidence and risk factors. Anesthesiology 2004;100:826-34; discussion 5A. [Crossref] [PubMed]
- Anderson DM, Dimitrova GT, Awad H. Patient with posterior mediastinal mass requiring urgent cardiopulmonary bypass. Anesthesiology 2011;114:1488-93. [Crossref] [PubMed]
- Au V, Marsh B, Benkwitz C. Resection of a Posterior Mediastinal Mass in a 4-Year-Old Child Complicated by Difficult Airway Management and Emergent Use of Extracorporeal Membrane Oxygenation. Semin Cardiothorac Vasc Anesth 2020;24:349-54. [Crossref] [PubMed]
- Lalwani P, Chawla R, Kumar M, et al. Posterior mediastinal mass: do we need to worry much? Ann Card Anaesth 2013;16:289-92. [Crossref] [PubMed]
- Hnatiuk OW, Corcoran PC, Sierra A. Spirometry in surgery for anterior mediastinal masses. Chest 2001;120:1152-6. [Crossref] [PubMed]
- Li WW, van Boven WJ, Annema JT, et al. Management of large mediastinal masses: surgical and anesthesiological considerations. J Thorac Dis 2016;8:E175-84. [Crossref] [PubMed]
- Ng A, Bennett J, Bromley P, et al. Anaesthetic outcome and predictive risk factors in children with mediastinal tumours. Pediatr Blood Cancer 2007;48:160-4. [Crossref] [PubMed]
- Al-Jehani Y, Saleh W, Al Halees Z, et al. Successful resection of a huge paraganglioma utilizing cardiopulmonary bypass. Asian Cardiovasc Thorac Ann 2012;20:482-5. [Crossref] [PubMed]
- Pasic M, Potapov E. Neo-Left Atrium Construction on the Beating Heart After Extirpation of a Huge Mediastinal Tumor Invading Heart and Lung. Ann Thorac Surg 2015;100:2350-2. [Crossref] [PubMed]
- Szabados S, Varady E, Göbölös L. Cardiovascular flashlight. Paraganglioma of the aortopulmonary window. Eur Heart J 2009;30:1286. [Crossref] [PubMed]
- Taguchi S, Mori A, Suzuki R, et al. Mediastinal schwannoma diagnosed preoperatively as a cyst. Tex Heart Inst J 2014;41:76-9. [Crossref] [PubMed]
- Yoshioka M, Ichiguchi O, Hirayama T, et al. Radical excision of thymic adenocarcinoma with selective cerebral perfusion. Ann Thorac Surg 2008;85:1427-9. [Crossref] [PubMed]
- Kodia K, Liu Y, Ghodsizad A, et al. Use of venovenous extracorporeal membrane oxygenation for resection of a large paratracheal mass causing critical tracheal stenosis: A case report. J Card Surg 2021;36:367-70. [Crossref] [PubMed]
- Lueck C, Kuehn C, Hoeper MM, et al. Successful use of extracorporeal membrane oxygenation during induction chemotherapy in a patient with mediastinal tumor mass of a T lymphoblastic lymphoma. Ann Hematol 2016;95:1719-21. [Crossref] [PubMed]
- Rotz SJ, Almeida FA, Koyfman S, et al. Continuous infusion chemotherapy, radiotherapy, and FDG-PET are feasible during extracorporeal membrane oxygenation. Pediatr Blood Cancer 2020;67:e28429. [Crossref] [PubMed]
- Takeda S, Miyoshi S, Omori K, et al. Surgical rescue for life-threatening hypoxemia caused by a mediastinal tumor. Ann Thorac Surg 1999;68:2324-6. [Crossref] [PubMed]
- Wickiser JE, Thompson M, Leavey PJ, et al. Extracorporeal membrane oxygenation (ECMO) initiation without intubation in two children with mediastinal malignancy. Pediatr Blood Cancer 2007;49:751-4. [Crossref] [PubMed]
- Worku B, DeBois W, Sobol I, et al. Extracorporeal Membrane Oxygenation as a Bridge through Chemotherapy in B-Cell Lymphoma. J Extra Corpor Technol 2015;47:52-4. [Crossref] [PubMed]
- Huang YL, Yang MC, Huang CH, et al. Rescue of cardiopulmonary collapse in anterior mediastinal tumor: case presentation and review of literature. Pediatr Emerg Care 2010;26:296-8. [Crossref] [PubMed]
- Breda JR, Aljure O, Sfakianaki AK, et al. Cesarean section in patient with metastatic Ewing sarcoma requiring VA-ECMO support. J Card Surg 2021;36:4756-8. [Crossref] [PubMed]
- Roze des Ordons AL, Lee J, Bader E, et al. Cesarean delivery in a parturient with an anterior mediastinal mass. Can J Anaesth 2013;60:89-90. [Crossref] [PubMed]


