
Utility of multimodal sampling and testing during advanced bronchoscopy for diagnosing atypical respiratory infections in a Coccidioides-endemic region
Highlight box
Key findings
• For atypical respiratory infections manifesting as focal thoracic lesions, a multimodal approach to guided bronchoscopic sampling and testing enhances diagnostic yield, while maintaining a favorable procedure safety profile.
• Cytohistology testing and nodal sampling are beneficial for pulmonary coccidioidomycosis, and culture for mycobacterial disease.
What is known and what is new?
• Traditional bronchoscopic techniques such as bronchoalveolar lavage, and tests such as culture and microscopy, have variable value for diagnosing lower respiratory infections.
• Tissue sampling and cytohistology testing, guided by advanced bronchoscopic modalities, enhance the diagnosis for atypical respiratory infections.
What is the implication, and what should change now?
• The approach to bronchoscopic sampling of a focal thoracic lesion suspected due to atypical infection should utilize advanced guidance modalities, employ multimodal techniques and testing, and be adjusted according to clinical context and regional infection patterns.
Introduction
Background
Respiratory infections caused by fungi, mycobacteria, and other atypical organisms can present in a variety of clinical circumstances and result in severe disease (1-6). Therefore, efficient and specific diagnosis is essential. Several non-invasive diagnostic methods are available, but perform inconsistently in practice (7-12). For many of these infections, culture and/or cytohistologic identification through direct sampling remains the gold standard.
Rationale and knowledge gap
Collecting lower respiratory secretions using bronchoscopic bronchoalveolar lavage (BAL) or bronchial washings (BW) for cytologic and/or culture analysis is a well-established means for evaluating infection. However, the value of these techniques depends on microbial factors, clinical context, and the testing methods utilized (6,13,14). For example, Pneumocystis is better identified by cytologic microscopy (14-16), while diagnostic yield for both culture and cytology testing is inconsistent for mycobacterial, fungal, and viral infections (16-26). BAL/BW performance also varies considerably in the intensive care unit (ICU) setting and for immunosuppressed patients (27-31).
Bronchoscopic tissue sampling using forceps biopsy or needle aspiration may complement BAL/BW by directly confirming infection and hastening the diagnosis (14,16,32). Tissue can also be cultured for a comparatively more specific analysis. However, studies evaluating the utility of transbronchial lung biopsy for diagnosing infection are limited. They demonstrate conflicting results, infrequently incorporate tissue culture testing, and mostly pre-date the advanced bronchoscopy era (18,25,26,33-38).
Advances in guidance technologies, such as endobronchial ultrasound (EBUS) and electromagnetic navigation (EMN), have augmented the breadth and accuracy of bronchoscopy for assessing focal thoracic disease. Collectively termed ‘advanced diagnostic bronchoscopy’ (ADB), it is widely used and considered standard of care for the tissue evaluation of lung cancer (39-45). Infections such as those caused by fungi and mycobacteria may manifest similarly, and some are endemic in a large part of the Americas (46-51). Consequently, they can represent a sizeable proportion of the case-mix evaluated by bronchoscopists. The available literature offers promise on ADB’s performance in this context, but is mostly limited to small case series, studies focusing on convex-probe EBUS, or tuberculosis-prevalent populations (32,52-56). Therefore, the utility of modern bronchoscopic tissue sampling for these infections remains unclear.
Objective
Our goal for this study was 2-fold. The first was to ascertain by what magnitude tissue sampling improved ADB diagnostic performance over BAL/BW in patients with focal thoracic lesions due to infections not caused by routine bacteria. Second, we comprehensively analyzed the performance characteristics of commonly employed techniques and tests to help guide the bronchoscopic approach for such patients in a Coccidioides-endemic region. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-83/rc).
Methods
Design
This was a retrospective observational cohort study analyzing registry data of all bronchoscopy procedures performed between January 2012 and December 2021, supplemented by electronic medical record. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). It was approved by the institutional review board of Loma Linda University Medical Center (No. 5190131) and individual consent for this retrospective analysis was waived.
Patients eligible were those who (Figure 1): (I) received ADB for focal thoracic lesions; (II) had both lower respiratory tract secretion and tissue samples procured by ADB; (III) had both cytohistology and culture results available; (IV) were ultimately diagnosed with a non-malignant disorder. Subjects that had coincident infection and malignancy diagnosed were also included.
All study variables were pre-defined and a data extraction form was created a priori. Each subject’s medical record was reviewed and compared to the existing database to ensure accuracy. Data relevant to the study but not part of the existing database was collected as needed. Two separate investigators independently performed same-subject data extraction to ensure consistency. Discrepancies were adjudicated by a third investigator.
Outcomes
The primary outcome was the increase in ADB diagnostic yield for atypical respiratory infections with the addition of tissue sampling over BAL/BW alone using testing methods of culture and cytohistology among subjects with non-malignant disease. As secondary outcomes we analyzed the individual and synergistic efficacy of commonly used bronchoscopic techniques and tests, per infection type.
Definitions
We considered an infection ‘atypical’ if it was not caused by routine extracellular bacteria. A focal thoracic lesion was any lesion within the thorax with clearly identifiable borders.
ADB diagnostic yield for the primary and secondary outcomes was based on final culture, cytology, or cytohistology results. For atypical infections, ADB was diagnostic if an organism was identified in the proper clinical context. Typical bacterial infection was diagnosed if organisms were seen within tissue samples or if cultures met accepted yield thresholds for a given testing method (57,58). Time to infection diagnosis was from specimen collection to direct organism identification (cytohistology) or first speciation (culture).
The final clinical diagnosis was determined by combination of ADB-obtained data, other invasive and non-invasive testing, and clinical course. This assessment was recorded and then compared to the subject’s final clinical diagnosis within the medical record. A lesion without a specific etiology after initial evaluation that had remained stable or decreased in size after one year of imaging surveillance and did not have an explanatory clinical diagnosis was classified as ‘non-specific’.
Bronchoscopy procedures
After a routine pre-bronchoscopy risk assessment, the decision to proceed was at the discretion of the attending bronchoscopist. Per our protocol, target coagulation parameters for bronchoscopic biopsy included a platelet count of over 50,000/µL and prothrombin time and international normalized ratio levels below two times the upper limit of normal.
During the study period, four interventional pulmonologists performed ADB consistent with established technique (59,60) and using one or a combination of the following advanced-guidance modalities: convex-probe endobronchial ultrasound (cEBUS; BF-UC-180/190 F, Olympus America Inc., Cypress, CA, USA), radial-probe endobronchial ultrasound (rEBUS; UM-S20-17S, Olympus America Inc.), and electromagnetic navigation (EMN; Veran Medical Technologies, St. Louis, MO, USA).
Routine sampling techniques included bronchoalveolar lavage (BAL), bronchial washings (BW), transbronchial needle aspiration (TBNA), and transbronchial forceps biopsy (TBFB). While sampling order and number of specimens procured were individualized per case, our approach followed commonly used methods (18,60-62). If an infection was suspected, 2–3 additional tissue samples from the target lesion were obtained using TBNA and/or TBFB and sent for culture. All lung lesions received directed BAL. For cases with only nodal sampling, BW collected during the procedure was analyzed. Other accessory tools were infrequently utilized in practice, and not included in our primary evaluation.
Specimen processing
We considered testing of a specimen as ‘cytohistology’ if it was processed in formalin fixative. These were either centrifuged cells obtained by TBNA (‘cell block’) or biopsied tissue using TBFB. BAL/BW fluid collected for cytology was preserved in CytoLyt solution (Hologic, Inc., Marlborough, MA, USA). Any specimen obtained for culture (tissue, cells, fluid) was placed in non-bacteriostatic saline. Results of rapid on-site cytologic evaluation (ROSE) were not incorporated into the analysis.
Statistical analysis
Diagnostic yield for a test, technique, or combination thereof was the proportion of a given diagnosis achieved among the sample being analyzed. T-tests and chi-square tests were used to evaluate for differences between continuous and categorical variables of independent samples, respectively. Comparisons of diagnostic performance between two different techniques or tests (e.g., culture vs. cytohistology) were conducted using McNemar’s tests. Logistic regression was used to ascertain independent associations with diagnosis by either cytohistology or culture testing in both the overall cohort and in subjects with a final diagnosis of atypical infection. Variables for this model were chosen by clinical relevance.
When assessing the increase in diagnosis from using a single technique (e.g., BAL/BW) to a combination of techniques (e.g., BAL/BW and TBNA/TBFB), confidence intervals are provided. McNemar’s tests are not appropriate since for a given subject a combined diagnosis also implies a diagnosis with at least one of the two methods. Confidence intervals for the probability of diagnosis using both methods for individuals who were undiagnosed using the first were also calculated. Statistics were performed using the R software package (R Core Team, 2021).
Results
A total of 403 subjects met inclusion criteria, with characteristics summarized in Table 1. A mix of ADB modalities was used to sample a total 1,054 thoracic lesions (2.6±1.4 per bronchoscopy). All subjects had received BAL/BW-culture and TBNA/TBFB-cytohistology testing. BAL/BW-cytology and TBNA/TBFB-culture were also each performed in 319 (79%). Acuity was high, with 184 bronchoscopies (46%) performed in the inpatient setting—59 (15%) in the ICU.
Table 1
| Characteristics | Overall (N=403) | Atypical infection (N=136) | All other non-malignant diagnoses†‡ (N=267) | P value |
|---|---|---|---|---|
| Patient | ||||
| Age, years, mean ± SD | 57.8±16.3 | 57.4 ±16.8 | 57.9 ±16.1 | 0.764 |
| Female gender, n (%) | 181 (44.9) | 61 (44.9) | 120 (44.9) | 1.000 |
| Immunosuppressed status, n (%)§ | 126 (31.3) | 57 (41.9) | 69 (25.8) | 0.002 |
| Inpatient status, n (%) | 184 (45.7) | 66 (48.5) | 118 (44.2) | 0.471 |
| ICU status, n (%) | 59 (14.6) | 21 (15.4) | 38 (14.2) | 0.767 |
| Lung lesions | ||||
| Number of patients with lung lesion | 308 | 120 | 188 | |
| Number sampled per patient, mean ± SD | 0.97±0.73 | 1.12±0.64 | 0.90±0.76 | 0.003 |
| Size of primary lesion, mm, mean ± SD | 44.0±24.0 | 38.1±20.3 | 47.7±25.4 | <0.001 |
| Upper lobe location, n (%) | 184 (59.7) | 75 (62.5) | 109 (58.0) | 0.503 |
| Nodule/mass (vs. consolidation/infiltrate), n (%) | 117 (38.0) | 62 (51.7) | 55 (29.3) | <0.001 |
| Solid attenuation, n (%) | 103 (33.4) | 39 (32.5) | 64 (34.0) | 0.691 |
| Cavitation present, n (%) | 70 (22.7) | 42 (35.0) | 28 (14.9) | <0.001 |
| Lymph nodes | ||||
| Number of patients with lymph node | 263 | 83 | 180 | |
| Number sampled per patient, mean ± SD | 1.6±1.5 | 1.4±1.3 | 1.7±1.5 | 0.033 |
| Size of largest node, mm, mean ± SD | 17.3±8.0 | 16.9±8.3 | 17.4±7.9 | 0.631 |
| Bronchoscopy procedure | ||||
| cEBUS only, n (%) | 138 (34.2) | 22 (16.2) | 116 (43.5) | <0.001 |
| rEBUS or EMN only, n (%) | 136 (33.8) | 47 (34.6)¶ | 89 (33.3) | 0.893 |
| Combined modalities, n (%) | 129 (32.0) | 67 (49.3)¶ | 62 (23.2) | <0.001 |
| Moderate sedation (vs. GA), n (%) | 280 (69.5) | 90 (66.2) | 190 (71.2) | 0.361 |
| Trainee involved, n (%) | 249 (61.8) | 74 (54.4) | 175 (65.5) | 0.039 |
| Duration, min, mean ± SD | 63.1±27.0 | 65.7±29.7 | 61.8±25.4 | 0.191 |
| Bronchoscopy complications, n (%) | ||||
| Any | 55 (13.7) | 15 (11.0) | 40 (15.0) | 0.348 |
| Minor | 44 (10.9) | 14 (10.3) | 30 (11.2) | 0.896 |
| Major†† | 14 (3.5) | 2 (1.5) | 12 (4.5) | 0.199 |
†, includes typical respiratory bacterial infections =57, specific non-infectious disorders =106, and cases with a non-specific final clinical diagnosis =104. ‡, non-infectious diagnoses include: sarcoidosis =62; pneumonia/pneumonitis unspecified =62; granulomatous disease (nodule, pneumonitis or adenopathy) =18; non-specific benign nodule =14; reactive adenopathy =10; rheumatologic-associated lung disease =9; cryptogenic organizing pneumonia =7; drug-induced lung disease =4; hypersensitivity pneumonitis =3; radiation-pneumonitis =3; eosinophilic pneumonia =2; pneumoconiosis =2; IgG-4 related disease =2; Castleman disease =2; hemophagocytic lymphohistiocytosis =2; other =8. §, ‘Immunosuppressed’ was defined by presence of at least one of the following at time of initial evaluation: acquired immunodeficiency syndrome; neutropenia; post-transplantation immunosuppressive therapy of any type; at least 2 weeks therapy with greater than 20 mg prednisone-equivalent per day for any reason; any cytotoxic therapy within the month prior to evaluation; primary immunodeficiency of any type; active lympho-hematogenous malignancy; poorly controlled diabetes mellitus (hemoglobin A1c >9.0%). ¶, among the atypical infection cohort, radial probe EBUS and electromagnetic navigation were used during 80 and 34 procedures, respectively, and individually each provided a specific cytohistologic diagnosis in 50% of cases. ††, major complications overall: pneumothorax requiring a chest tube =9 (2.2%), respiratory failure requiring endotracheal intubation =6 (1.5%), bleeding requiring intervention beyond routine measures =2 (0.5%), escalation of care =6 (1.5%). No cardiac arrest or death occurred. All other complications were considered minor. SD, standard deviation; ICU, intensive care unit; cEBUS, convex-probe endobronchial ultrasound; rEBUS, radial-probe endobronchial ultrasound; EMN, electromagnetic navigation; GA, general anesthesia; IgG, immunoglobulin G.
Overall, 140 atypical infections were discovered in 136 subjects using all methods, with ADB contributing a diagnosis in 119 (87.5%) of these. Subjects with atypical infection were more likely to receive simultaneous lung and nodal sampling (Table 1). Their lung lesions were smaller and more likely to be nodules, masses, and cavitary. Immediate bronchoscopy-related major complications occurred in 2 (1.5%): 1 pneumothorax requiring chest tube (0.7%) and 1 respiratory failure requiring endotracheal intubation (0.7%). Bleeding requiring intervention beyond routine bronchoscopic measures did not occur in any with atypical infection.
Coccidioidomycosis was the most frequently identified infection (n=49) and was the only atypical infection more likely to be discovered in immunocompetent subjects [odds ratio (OR) =4.57, 95% confidence interval (CI): 1.77–11.83; P=0.002]. All other atypical infections except mycobacteria were more frequently diagnosed in the immunosuppressed. A specific etiology explaining thoracic lesions could not be identified in 104 subjects, despite comprehensive diagnostics for underlying infection (Table 2).
Table 2
| Evaluation | Pneumonia, unspecified (N=62) | Granulomatous disease (N=18) |
Other benign nodule and/or adenopathy (N=24) | Total (N=104) |
|---|---|---|---|---|
| ADB cytohistology result, n (%) | ||||
| Non-specific inflammatory tissue | 43 (69.4) | 1 (5.6) | 21 (87.5) | 65 (62.5) |
| Supportive cytohistology† | 16 (25.8) | 15 (83.3) | 0 | 31 (29.8) |
| Fibrous tissue | 1 (1.6) | 1 (5.6) | 1 (4.2) | 3 (2.9) |
| Non-lesional | 3 (4.8) | 1 (5.6) | 1 (4.2) | 5 (4.8) |
| ADB-ancillary testing performed (PCR or antigen), n (%) | ||||
| At least one test | 39 (62.9) | 9 (50.0) | 6 (25.0) | 54 (51.9) |
| Mycobacterium tuberculosis PCR | 29 (46.8) | 7 (38.9) | 2 (8.3) | 38 (36.5) |
| Coccidioides PCR | 13 (21.0) | 4 (22.2) | 2 (8.3) | 19 (18.3) |
| Other | 17 (27.4) | 5 (27.8) | 5 (20.8) | 27 (26.0) |
| Serum or urine testing performed (serology or antigen), n (%) | ||||
| At least one test | 54 (87.1) | 17 (94.4) | 14 (58.3) | 85 (81.7) |
| Coccidioides serology | 51 (82.2) | 17 (94.4) | 14 (58.3) | 82 (78.8) |
| Histoplasma serology or antigen | 37 (59.7) | 11 (61.1) | 8 (33.3) | 56 (53.8) |
| Cryptococcus serology | 28 (45.2) | 11 (61.1) | 5 (20.8) | 44 (42.3) |
| Aspergillus antigen | 30 (48.4) | 2 (11.1) | 2 (8.3) | 36 (34.6) |
| Other | 37 (59.7) | 11 (61.1) | 8 (33.3) | 56 (53.8) |
| Additional tissue sampling, n (%) | ||||
| Percutaneous biopsy | 4 (6.5) | 3 (16.7) | 1 (4.2) | 8 (7.7) |
| Surgery | 1 (1.6) | 0 | 1 (4.2) | 2 (1.9) |
| Autopsy | 1 (1.6) | 0 | 0 | 1 (1.0) |
| Received only imaging surveillance after bronchoscopy | 9 (14.5) | 0 | 7 (29.1) | 16 (15.4) |
†, granulomas, necrosis, organizing pneumonia, acute lung injury, or cell-specific inflammation. ADB, advanced diagnostic bronchoscopy; PCR, polymerase chain reaction.
Performance of ADB testing methods
Among the entire cohort (N=403), ADB yielded a specific diagnosis of atypical infection in 119 subjects (29.5%; Table 3). Mycobacteria was the only subgroup more frequently identified by culture than cytohistology (8.4% vs. 2.7%; P<0.001).
Table 3
| Atypical infection | Culture¶¶, yield n (%) | Specific cytohistology, yield n (%) | Culture or specific cytohistology, yield n (%) | Increase in diagnostic yield when adding specific cytohistology to culture (95% CI) |
|---|---|---|---|---|
| Any atypical infection† | 96 (23.8)* | 75 (18.6) | 119 (29.5) | 5.7 (3.8–8.4) |
| Dimorphic fungi‡ | 28 (6.9) | 34 (8.4) | 43 (10.7) | 3.8 (2.3–6.1) |
| Opportunistic fungi§ | 29 (7.2) | 23 (5.7) | 33 (8.2) | 1.0 (0.4–2.5) |
| Mycobacteria¶ | 34 (8.4)** | 11 (2.7) | 34 (8.4) | 0 (0–0.9) |
| Other extracellular organisms†† | 5 (1.2) | 6 (1.5) | 8 (2.0) | 0.8 (0.2–2.2) |
| Intracellular organisms‡‡ | 0 | 1 (0.2) | 1 (0.2) | 0.2 (0–0.7) |
†, 140 total atypical infections diagnosed in 136 subjects using all methods; ‡, 51 total infections: Coccidioides =49, Histoplasma =2; §, 36 total infections: Aspergillus =18, Cryptococcus =10, Mucor/Rhizopus sp. =6, Candida =2; ¶, 34 total infections: non-tuberculous =21, M. tuberculosis =13; ††, 10 total infections: Nocardia =8, Actinomyces = 1, Pneumocystis =1; ‡‡, 9 total infections: Legionella =2, metapneumovirus =2, SARS-CoV-2 =2, Coxiella =1, herpes simplex virus =1, Mycoplasma =1; ¶¶, within each group, culture yield was compared to cytohistology yield using McNemar’s tests; *, P<0.05; **, P<0.001. CI, confidence interval.
To determine independent associations with either cytohistology or culture diagnosis, we performed multivariate analysis controlling for patient, lesional and procedure-related factors (Tables 4,5). Among the entire cohort, immunosuppressed status was associated with diagnosis by both cytohistology (OR =3.46, 95% CI: 1.77–6.78; P<0.001) and culture (OR =2.12, 95% CI: 1.20–3.74; P=0.010). However, in those with an ultimate diagnosis of atypical infection, this association persisted only with cytohistology (OR =5.71, 95% CI: 1.64–19.82; P=0.006). In addition, Coccidioidal disease was strongly associated with diagnosis by cytohistology (OR =7.64, 95% CI: 2.51–23.26; P<0.001) and less likely to be diagnosed by culture (OR =0.23, 95% CI: 0.07–0.78; P=0.018). Lung cavitation predicted yield with culture in both models.
Table 4
| Independent variables | Cytohistology | Culture | |||
|---|---|---|---|---|---|
| Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | ||
| Male gender | 0.98 (0.56–1.71) | 0.937 | 0.78 (0.48–1.25) | 0.302 | |
| Age | 1.01 (0.99–1.02) | 0.398 | 1.02 (1.00–1.04) | 0.011 | |
| Immunosuppressed state | 3.46 (1.77–6.78) | <0.001 | 2.12 (1.20–3.74) | 0.010 | |
| Hospitalized | 1.30 (0.64–2.62) | 0.470 | 0.91 (0.50–1.66) | 0.758 | |
| ICU location | 0.94 (0.41–2.15) | 0.884 | 1.33 (0.64–2.76) | 0.448 | |
| General anesthesia | 1.67 (0.90–3.11) | 0.105 | 0.51 (0.29–0.91) | 0.022 | |
| Trainee assisting procedure | 0.67 (0.38–1.17) | 0.158 | 0.77 (0.47–1.25) | 0.291 | |
| Number of overall samples obtained | 1.04 (0.79–1.36) | 0.783 | 0.87 (0.68–1.11) | 0.273 | |
| Lung lesion sampled | 0.89 (0.22–3.55) | 0.864 | 1.65 (0.53–5.16) | 0.391 | |
| Lung lesion size | 1.00 (0.99–1.02) | 0.744 | 1.00 (0.99–1.02) | 0.603 | |
| Upper lobe location | 1.18 (0.64–2.17) | 0.593 | 0.69 (0.23–2.05) | 0.543 | |
| Nodule or mass (vs. consolidation or infiltrate) | 1.36 (0.61–3.03) | 0.457 | 0.86 (0.43–1.73) | 0.681 | |
| Solid lung lesion (vs. part-solid or ground glass) | 1.00 (0.47–2.14) | 0.993 | 1.98 (1.04–3.79) | 0.039 | |
| Cavitation present | 1.85 (0.83–4.13) | 0.130 | 7.56 (3.63–15.74) | <0.001 | |
| Lymph node sampled | 1.69 (0.59–4.82) | 0.324 | 2.08 (0.82–5.29) | 0.125 | |
| Lymph node size | 1.05 (1.01–1.09) | 0.015 | 1.02 (0.98–1.06) | 0.355 | |
CI, confidence interval; ICU, intensive care unit.
Table 5
| Independent variables | Cytohistology | Culture | |||
|---|---|---|---|---|---|
| Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | ||
| Male gender | 0.40 (0.16–1.01) | 0.053 | 0.34 (0.11–0.98) | 0.046 | |
| Age | 1.01 (0.99–1.04) | 0.322 | 1.05 (1.02–1.09) | 0.004 | |
| Immunosuppressed state | 5.71 (1.64–19.82) | 0.006 | 1.92 (0.52–7.14) | 0.33 | |
| Hospitalized | 1.65 (0.56–4.87) | 0.366 | 0.44 (0.13–1.55) | 0.203 | |
| ICU location | 1.16 (0.32–4.18) | 0.823 | 0.63 (0.16–2.45) | 0.509 | |
| General anesthesia | 2.45 (0.94–6.37) | 0.067 | 0.53 (0.19–1.49) | 0.228 | |
| Trainee assisting procedure | 0.81 (0.34–1.91) | 0.630 | 0.65 (0.25–1.73) | 0.39 | |
| Number of overall samples obtained | 1.11 (0.68–1.82) | 0.668 | 1.08 (0.63–1.86) | 0.774 | |
| Lung lesion sampled | 0.26 (0.02–3.74) | 0.320 | 5.98 (0.18–198.39) | 0.317 | |
| Lung lesion size | 1.05 (1.02–1.09) | 0.004 | 1.01 (0.97–1.05) | 0.577 | |
| Upper lobe location | 1.23 (0.47–3.22) | 0.678 | 0.69 (0.23–2.05) | 0.505 | |
| Nodule or mass (vs. consolidation or infiltrate) | 1.08 (0.47–3.22) | 0.903 | 0.93 (0.22–3.87) | 0.925 | |
| Solid lung lesion (vs. part-solid or ground glass) | 0.93 (0.26–3.31) | 0.917 | 1.48 (0.37–5.89) | 0.58 | |
| Cavitation present | 0.9 (0.24–3.33) | 0.875 | 5.68 (1.16–27.81) | 0.032 | |
| Lymph node sampled | 0.71 (0.12–4.21) | 0.709 | 0.08 (0.01–0.65) | 0.018 | |
| Lymph node size | 1.07 (0.99–1.16) | 0.069 | 1.16 (1.05–1.28) | 0.003 | |
| Coccidioidomycosis | 7.64 (2.51–23.26) | <0.001 | 0.23 (0.07–0.78) | 0.018 | |
CI, confidence interval; ICU, intensive care unit.
Overall average time to organism identification by cytology/cytohistology was 3.4±1.7 days, compared to 19.2±16.7 days with culture (P<0.001). The diagnostic interval difference was largest for mycobacteria (4.1 vs. 33.2 days; P<0.01) and smallest for opportunistic fungi (2.9 vs. 11.1 days; P<0.01).
Performance of ADB techniques
Individual and combined performance of techniques and tests were analyzed in the 252 subjects for which both respiratory secretion and tissue sampling were subjected to dual culture and cytology/cytohistology testing. Specific diagnostic yields for atypical infection of individual technique-test combinations are summarized in Figure 2. Overall, BAL/BW-cytology was inferior to all others (P<0.001), providing a diagnosis in only 15 subjects (6.0%).
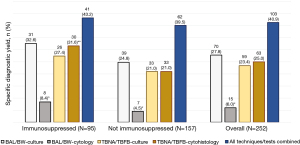
Figure 3 summarizes the diagnostic yields of bronchoscopic techniques among dual-tested samples, separately and combined, for atypical infection subgroups (N=252). Overall, adding TBNA/TBFB to BAL/BW diagnosed an additional 30 subjects with atypical infection (increase in proportion =11.9%, 95% CI: 8.5–16.5%). Multimodal sampling was most beneficial for dimorphic fungi: adding TBNA/TBFB to BAL/BW more than doubled the yield, from 7.1% to 15.1% (increase =8.0%, 95% CI: 5.2–11.9%).
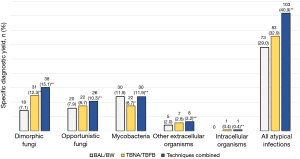
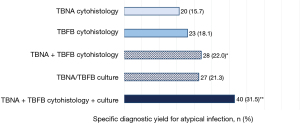
Of the 127 subjects for which both TBNA and TBFB were used to sample the same lung lesion (Figure 4), TBNA provided a specific cytohistologic diagnosis of atypical infection in 20 (16%). Adding TBFB diagnosed an additional 8 subjects (6.3% increase, 95% CI: 3.2–11.9%). Adding tissue culture to dual TBNA/TBFB cytohistology-tested lung samples further enhanced the overall yield to 40 subjects (32%), doubling the proportion diagnosed over using TBNA cytohistology alone (increase of 15.8%, 95% CI: 10.4–23.1%).
Among the 114 subjects which received simultaneous lung and lymph node sampling (Table 6), lung and nodal tissue provided a diagnosis of atypical infection in 26 and 24 subjects, respectively (22.8% vs. 21.2%; P=0.72). The techniques combined for a yield in 40 subjects (35.1%). Adding nodal to lung sampling was most beneficial for coccidioidal infections, increasing the proportion diagnosed from 10% to 18%, and also identifying 11% of cases undiagnosed by lung sampling (95% CI: 5.4–17%).
Table 6
| Infection | Lung sampling‡§, yield n (%) | Nodal sampling¶, yield n (%) | Lung or nodal sampling, yield n (%) |
Increase in yield proportion (%) by adding lung to nodal sampling [95% CI] | Increase in yield proportion (%) by adding nodal to lung sampling [95% CI] | Proportion (%) diagnosed with nodal sampling that were negative with lung sampling [95% CI] |
|---|---|---|---|---|---|---|
| Any atypical infection | 26 (22.8) | 24 (21.2) | 40 (35.1) | 14 [8.8–22] | 12 [7.5–20] | 17 [10–25] |
| Dimorphic fungi†† | 11 (9.7) | 17 (14.9) | 21 (18.4) | 3.5 [1.4–8.7] | 8.8 [4.8–15] | 11 [5.4–17] |
| Mycobacteria | 7 (6.1) | 4 (3.5) | 10 (8.8) | 5.3 [2.4–11] | 2.7 [0.9–7.5] | 4.4 [1.0–7.9] |
| Opportunistic fungi | 5 (4.4) | 2 (1.8)‡‡ | 7 (6.1) | 4.3 [1.9–9.9] | 1.7 [0.4–6.2] | 3.5 [0.5–6.4] |
| Other atypical infections | 3 (2.6) | 1 (0.9) | 3 (2.6) | 1.7 [0.5–6.2] | 0 [0–3.2] | – |
†, at least one cytohistology or culture result available from both sampling modalities in a given subject. ‡, transbronchial needle aspiration and/or transbronchial forceps biopsy of lung lesions guided by radial-probe EBUS and/or electromagnetic navigational bronchoscopy. §, no significant differences comparing lung sampling to nodal sampling using McNemar’s tests. ¶, transbronchial needle aspiration and/or intranodal forceps biopsy guided by convex-probe endobronchial ultrasound bronchoscopy. ††, all coccidioidomycosis. ‡‡, both diagnostic nodal samples were from subjects with cryptococcal infection. CI, confidence interval; EBUS, endobronchial ultrasound.
Ancillary testing
A heterogeneous complement of serum and ADB-ancillary testing data was available. The diagnostic contribution to atypical infection is summarized and compared to ADB-culture and cytohistology testing in Tables 7,8.
Table 7
| Infection being evaluated (No. subjects for which both tests available) | No. diagnosed in cohort | No. diagnosed by either test | Only ADB-culture/ cytohistology diagnostic |
Only non-invasive testing‡ diagnostic | Both tests diagnostic | Neither test diagnostic |
|---|---|---|---|---|---|---|
| Coccidioides (N=335) | 48 (14.3)§ | 47 (14.0) | 7 (2.1) | 4 (1.2) | 36 (10.7) | 288 (86.0) |
| Aspergillus (N=141) | 16 (11.3)¶ | 15 (10.6) | 5 (3.5) | 1 (0.7) | 9 (6.4) | 126 (89.4) |
| Cryptococcus (N=159) | 8 (5.0)§ | 7 (4.4) | 3 (1.9) | 0 | 4 (2.5) | 152 (95.6) |
| Histoplasma (N=47) | 1 (2.1) | 1 (2.1) | 0 | 0 | 1 (100.0) | 46 (97.9) |
Data are presented as n (% of total dual tests). †, ADB-culture/cytohistology compared to ancillary testing for all infection groups using McNemar’s tests; no significant differences found. ‡, Coccidioides and Cryptococcus serology, Aspergillus serum antigen, Histoplasma urine antigen. §, additional infection diagnosed by tissue sampling via transthoracic approach. ¶, additional infection diagnosed by antigen testing of bronchoalveolar lavage fluid. ADB, advanced diagnostic bronchoscopy.
Table 8
| Infection being evaluated (subjects for which both tests available) | Diagnosed in cohort | Diagnosed by either test | Only ADB-culture/ cytohistology diagnostic |
Only ADB-ancillary testing‡ diagnostic | Both tests diagnostic | Neither test diagnostic |
|---|---|---|---|---|---|---|
| Coccidioides (N=61) | 13 (21.3)§ | 12 (19.7) | 4 (6.6) | 0 | 8 (13.1) | 49 (80.3) |
| Tuberculosis (N=116) | 10 (8.6) | 10 (8.6) | 3 (2.6) | 0 | 7 (6.0) | 106 (91.4) |
| Aspergillus (N=43) | 3 (7.0) | 3 (7.0) | 0 | 0 | 3 (100.0) | 40 (93.0) |
| Pneumocystis (N=24) | 1 (4.2) | 1 (4.2) | 0 | 0 | 1 (100.0) | 23 (95.8) |
Data are presented as n (% of total dual tests). †, ADB-culture/cytohistology compared to ancillary testing for all infection groups using McNemar’s tests; no significant differences found. ‡, all ancillary tests performed on bronchoalveolar lavage/bronchial washings fluid; all tests polymerase chain reaction expect Aspergillus antigen. §, additional infection diagnosed by high Coccidioides antibody titer. ADB, advanced diagnostic bronchoscopy.
Discussion
Herein, we found that a multimodal approach to both sampling and testing during advanced-guidance bronchoscopy augmented the yield for atypical infection over the use of individual techniques, while maintaining a favorable procedure safety profile. The magnitude of gain varied across infection types, and patients with coccidioidomycosis benefitted most from cytohistology testing and nodal sampling.
To our knowledge, to date this is the most comprehensive assessment of the utility of ADB for assessing atypical respiratory infections in a Coccidioides-endemic region. Our study also adds value to the existing literature by assessing only focal disease, for which conventional bronchoscopy is less reliable. We examined both the individual contribution and synergy of common bronchoscopic techniques, only included subjects that had dual culture and cytohistology testing, and used definitive identification of an atypical organism as the diagnostic criterium since this most decisively directs therapy. Within this context we address several common questions encountered during the bronchoscopic evaluation of atypical respiratory infection.
When should guided bronchoscopic tissue sampling be performed in addition to BAL/BW?
Existing guidelines favor BAL as the primary bronchoscopic technique for evaluating most suspected respiratory infections, especially those manifesting with diffuse disease (9,16,63,64). Advice regarding tissue sampling is more restrained due to variability of reported yields, differences in clinical circumstance, concerns over procedure-related risks, the emergence of ancillary tests, and a source data pool mostly pre-dating the ADB era.
The enhanced scope and accuracy of ADB-tissue sampling would be most complementary for infections presenting as lung nodules and lymphadenopathy, to which BAL/BW may have imprecise access. Dimorphic fungi fit this profile, as they often manifest with focal thoracic lesions. Furthermore, BAL/BW and other traditional bronchoscopic methods have a comparatively low diagnostic sensitivity for histoplasmosis and coccidiodomycosis (25,26). Our results concurred, showing that the addition of ADB-guided tissue sampling most benefited this subgroup by more than doubling the yield over BAL/BW. Adjusted analysis also revealed that coccidioidal disease was independently associated with a cytohistologic diagnosis. Tissue examination may be particularly useful in cases of locally contained infection which may limit organism shedding and thus suppress the yield of BAL/BW culture and/or polymerase chain reaction (PCR) testing (Figure 5A).
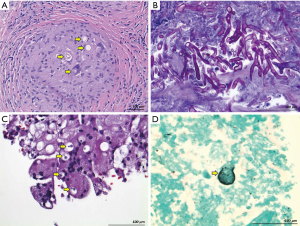
The added diagnostic value of ADB-tissue sampling over BAL/BW was more modest for opportunistic fungi. Nevertheless, TBNA/TBFB may also help distinguish invasive disease from simple colonization by directly demonstrating fungal elements within tissue (Figure 5B). This clinically vital distinction, not easily made with less-specific BAL/BW-culture, PCR, or antigen tests, is commonly required with Aspergillus species and has therapeutic ramifications (20).
Based on these findings, when performing bronchoscopy for clinically suspected fungal disease, we consider BAL/BW to be suboptimal as the only investigative technique.
We also found tissue cytohistology established diagnosis by an average of 16 days earlier than culture testing. Those with mycobacterial infection benefited most, with final diagnosis accelerated by 29 days. This can have important clinical implications, particularly for tuberculosis, since delays may postpone treatment or prolong exposure to potentially toxic empiric therapies (2,9,65). Furthermore, while molecular testing may be useful for guiding initial therapeutic decisions, culture-based analysis remains the gold standard for evaluating antimicrobial sensitivity (65,66). Therefore, we agree with current guidelines that tissue sampling with culture testing may be valuable as both an efficient and comprehensive approach for selected cases of suspected mycobacterial disease (66).
In summary, our results support that unless contraindicated, ADB-tissue sampling should supplement BAL/BW for evaluating most cases of focal thoracic lesions in the setting of suspected atypical infection. In a Coccidioides-endemic region, we suggest this approach be routine and not necessarily depend on a patient’s immune status, since we detected these infections more frequently in immunocompetent hosts.
When sampling a focal lung lesion suspected due to an atypical respiratory infection, should the bronchoscopist use TBNA, TBFB, or both? Should tissue culture also be tested?
The value of multimodal sampling during ADB is well-established for lung cancer patients (67-69). However, such data for infectious respiratory disease is limited and the utility of cytohistology testing ranges widely (70-73). This heterogeneity is likely due in part to organism-specific influences that dictate culture growth patterns and success of histologic identification (74-76). In support, we found both culture and cytohistology yield varied across infection types despite a fairly uniform bronchoscopic approach during the study period. Furthermore, in our practice the ADB-cytohistologic yield for malignancy is higher than that for infection, despite source lesions being comparatively smaller (77). These considerations highlight the importance of diversifying lung tissue procurement and testing methods when evaluating atypical respiratory infection.
We analyzed the utility of adding TBFB to TBNA, rather than in the reverse order, since in practice the typical approach is to first perform TBNA, particularly when ROSE is utilized (69). We found that by combining culture and cytohistology testing of TBNA/TBFB-obtained lung samples, diagnostic yield doubled compared to using TBNA-cytohistology alone. Cytohistologic synergy of the two sampling techniques most aided fungal diagnosis (Figure 5C), and tissue culture that of mycobacteria.
In summary, when sampling a lung lesion due to a suspected atypical infection, a reasonable approach is that if ROSE of the TBNA sample does not reveal malignant cells, extra specimens should be obtained using TBFB and tested for both cytohistology and culture. If ROSE is not available, both techniques should be utilized.
For patients suspected of atypical respiratory infection who have concomitant lung and lymph node lesions, should sampling of both be performed?
Nodal biopsy in the setting of non-malignant disease often yields only reactive, diagnostically non-contributory lymph tissue (78-80). However, sampling directly infected nodes may increase the likelihood of visualizing organisms or a characteristic inflammatory response (i.e., granuloma), and could also augment culture yield.
Dimorphic fungi and mycobacteria can infect lymph nodes and yield prominent, caseous, and/or partially calcified adenopathy (3-5,78). As these infections may manifest with small, difficult to access lung lesions, the coexisting nodal disease offers an alternative diagnostic target using cEBUS guidance (32,52,54-56,81-84). In support, our data demonstrated synergy between lung and nodal tissue assessment. Not surprisingly, this result was primarily driven by Coccidioides (and to a lesser extent mycobacteria and Cryptococcus), for which nodal sampling almost doubled the proportion diagnosed and identified infection in 11% of cases for which lung sampling was not specifically diagnostic (Figure 5D). Conversely, Aspergillus, Mucor, and Candida species were not identified in lymph tissue, suggesting their relative lack of proclivity for nodal spread.
In summary, based on our findings, when Coccidioides (and possibly mycobacteria and Cryptococcus) is suspected, cEBUS-guided sampling of thoracic adenopathy should be performed and tissue tested for both cytohistology and culture.
Limitations and other considerations
The main limitation of our study is its retrospective and single institution design, though the data originated from a prospectively maintained database.
Our study should not be interpreted as insight into the absolute diagnostic sensitivity of ADB for atypical infection. Even though we believe ADB diagnostic sensitivity is high in this setting, establishing a reliable reference standard necessary for such analysis can be problematic for non-malignant disease. Even with the thorough evaluation of our ‘non-specific’ cohort (Table 2), given the characteristics of disease and limitations of retrospective design, definitively excluding all self-limiting atypical infections such as dimorphic fungi—which are prevalent in our region—is not possible. Nevertheless, our assessment of the synergistic impact of ADB techniques on the diagnostic yield of atypical infection among patients with non-malignant disease provided similarly useful general conclusions.
We did not incorporate into our primary hypothesis the value of antigen and PCR analysis of respiratory samples, and we acknowledge the individualized benefit of these ancillary tests. However, despite advances in their applications, practical considerations may limit their availability and use, and concerns persist over clinical utility (9,11,85-94). Within our limited sample of histologically and/or culture-proven disease, these tests performed inconsistently (Table 8). Culture and cytohistology testing remain the cornerstone of routine bronchoscopy practice, especially when prospectively evaluating an undiagnosed focal thoracic lesion. Thus, we thought the generalizability of our analysis was maximized by focusing on these methods.
We also did not study the impact of bronchial brushings (BB) as this technique is not routine in our practice. A previous internal quality review found BB adds little to the overall bronchoscopic yield for infection, consistent with existing guidelines and other reports (18,36).
Finally, our findings are most relevant for practice in a Coccidioides-endemic region. However, because other dimorphic fungi such as Histoplasma and Blastomyces have similar clinical characteristics, our results could be reasonably applied in corresponding endemic areas.
Conclusions
We found that multimodal evaluation using commonly utilized techniques and tests during advanced-guidance bronchoscopy enhances specific diagnostic yield for patients with atypical respiratory infections. Cytohistology testing and nodal tissue sampling are beneficial for pulmonary coccidiodomycosis, and culture for mycobacterial disease. The value of emerging advanced bronchoscopic modalities, such as robotics, augmented real-time guidance, and transbronchial cryobiopsy, are yet unexplored in this setting. Our findings could provide a basis for future investigation with these approaches.
Acknowledgments
We thank Drs. Camilla J. Cobb, MD, Jeremy K. Deisch, MD, and Chelsea Heimbaugh, MD (Loma Linda University Department of Pathology and Human Anatomy) for providing clinical and technical assistance.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-83/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-83/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-83/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-83/coif). AAC has received consulting and speaker fees from Intuitive Surgical, Inc. BF has received speaker fees from Insmed, Inc. and consulting fees from Intuitive Surgical, Inc., and STERIS Life Sciences. EH has received consulting fees from Biodesix Inc., Intuitive Surgical, Inc., and Olympus Corp. The other authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Guimarães MD, Marchiori E, Meirelles GS, et al. Fungal infection mimicking pulmonary malignancy: clinical and radiological characteristics. Lung 2013;191:655-62. [Crossref] [PubMed]
- Pal R, Singh B, Bhadada SK, et al. COVID-19-associated mucormycosis: An updated systematic review of literature. Mycoses 2021;64:1452-9. [Crossref] [PubMed]
- Jeong YJ, Lee KS. Pulmonary tuberculosis: up-to-date imaging and management. AJR Am J Roentgenol 2008;191:834-44. [Crossref] [PubMed]
- Capone D, Marchiori E, Wanke B, et al. Acute pulmonary coccidioidomycosis: CT findings from 15 patients. Br J Radiol 2008;81:721-4. [Crossref] [PubMed]
- Conces DJ Jr. Histoplasmosis. Semin Roentgenol 1996;31:14-27. [Crossref] [PubMed]
- Skalski JH, Limper AH. Fungal, Viral, and Parasitic Pneumonias Associated with Human Immunodeficiency Virus. Semin Respir Crit Care Med 2016;37:257-66. [Crossref] [PubMed]
- Crum NF, Lederman ER, Stafford CM, et al. Coccidioidomycosis: a descriptive survey of a reemerging disease. Clinical characteristics and current controversies. Medicine (Baltimore) 2004;83:149-75. [Crossref] [PubMed]
- Pfeiffer CD, Fine JP, Safdar N. Diagnosis of invasive aspergillosis using a galactomannan assay: a meta-analysis. Clin Infect Dis 2006;42:1417-27. [Crossref] [PubMed]
- Patterson TF, Thompson GR 3rd, Denning DW, et al. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis 2016;63:e1-60. [Crossref] [PubMed]
- Richer SM, Smedema ML, Durkin MM, et al. Improved Diagnosis of Acute Pulmonary Histoplasmosis by Combining Antigen and Antibody Detection. Clin Infect Dis 2016;62:896-902. [Crossref] [PubMed]
- Zhou W, Li H, Zhang Y, et al. Diagnostic Value of Galactomannan Antigen Test in Serum and Bronchoalveolar Lavage Fluid Samples from Patients with Nonneutropenic Invasive Pulmonary Aspergillosis. J Clin Microbiol 2017;55:2153-61. [Crossref] [PubMed]
- Kassis C, Durkin M, Holbrook E, et al. Advances in Diagnosis of Progressive Pulmonary and Disseminated Coccidioidomycosis. Clin Infect Dis 2021;72:968-75. [Crossref] [PubMed]
- Walsh TJ, Dixon DM. Spectrum of Mycoses. In: Baron S, editor. Medical Microbiology. 4th ed. Galveston (TX): University of Texas Medical Branch at Galveston; 1996. Chapter 75.
- Weldon-Linne CM, Rhone DP, Bourassa R. Bronchoscopy specimens in adults with AIDS. Comparative yields of cytology, histology and culture for diagnosis of infectious agents. Chest 1990;98:24-8. [Crossref] [PubMed]
- Golden JA, Hollander H, Stulbarg MS, et al. Bronchoalveolar lavage as the exclusive diagnostic modality for Pneumocystis carinii pneumonia. A prospective study among patients with acquired immunodeficiency syndrome. Chest 1986;90:18-22. [Crossref] [PubMed]
- Du Rand IA, Blaikley J, Booton R, et al. British Thoracic Society guideline for diagnostic flexible bronchoscopy in adults: accredited by NICE. Thorax 2013;68:i1-i44. [Crossref] [PubMed]
- Baughman RP, Dohn MN, Loudon RG, et al. Bronchoscopy with bronchoalveolar lavage in tuberculosis and fungal infections. Chest 1991;99:92-7. [Crossref] [PubMed]
- Kennedy DJ, Lewis WP, Barnes PF. Yield of bronchoscopy for the diagnosis of tuberculosis in patients with human immunodeficiency virus infection. Chest 1992;102:1040-4. [Crossref] [PubMed]
- Kahn FW, Jones JM, England DM. The role of bronchoalveolar lavage in the diagnosis of invasive pulmonary aspergillosis. Am J Clin Pathol 1986;86:518-23. [Crossref] [PubMed]
- Levy H, Horak DA, Tegtmeier BR, et al. The value of bronchoalveolar lavage and bronchial washings in the diagnosis of invasive pulmonary aspergillosis. Respir Med 1992;86:243-8. [Crossref] [PubMed]
- Glazer M, Nusair S, Breuer R, et al. The role of BAL in the diagnosis of pulmonary mucormycosis. Chest 2000;117:279-82. [Crossref] [PubMed]
- Lodding IP, Schultz HH, Jensen JU, et al. Cytomegalovirus Viral Load in Bronchoalveolar Lavage to Diagnose Lung Transplant Associated CMV Pneumonia. Transplantation 2018;102:326-32. [Crossref] [PubMed]
- Patrucco F, Albera C, Bellocchia M, et al. SARS-CoV-2 Detection on Bronchoalveolar Lavage: An Italian Multicenter experience. Respiration 2020;99:970-8. [Crossref] [PubMed]
- Muthu V, Gandra RR, Dhooria S, et al. Role of flexible bronchoscopy in the diagnosis of invasive fungal infections. Mycoses 2021;64:668-77. [Crossref] [PubMed]
- Wallace JM, Catanzaro A, Moser KM, et al. Flexible fiberoptic bronchoscopy for diagnosing pulmonary coccidioidomycosis. Am Rev Respir Dis 1981;123:286-90. [PubMed]
- Prechter GC, Prakash UB. Bronchoscopy in the diagnosis of pulmonary histoplasmosis. Chest 1989;95:1033-6. [Crossref] [PubMed]
- Luna CM, Vujacich P, Niederman MS, et al. Impact of BAL data on the therapy and outcome of ventilator-associated pneumonia. Chest 1997;111:676-85. [Crossref] [PubMed]
- Vélez L, Correa LT, Maya MA, et al. Diagnostic accuracy of bronchoalveolar lavage samples in immunosuppressed patients with suspected pneumonia: analysis of a protocol. Respir Med 2007;101:2160-7. [Crossref] [PubMed]
- Al-Qadi MO, Cartin-Ceba R, Kashyap R, et al. The Diagnostic Yield, Safety, and Impact of Flexible Bronchoscopy in Non-HIV Immunocompromised Critically Ill Patients in the Intensive Care Unit. Lung 2018;196:729-36. [Crossref] [PubMed]
- Choo R, Anantham D. Role of bronchoalveolar lavage in the management of immunocompromised patients with pulmonary infiltrates. Ann Transl Med 2019;7:49. [Crossref] [PubMed]
- Shah AS, O'Horo JC, Tang S, et al. Fungal Diagnostic Stewardship in Bronchoscopy Specimens for Immunocompetent Patients in the Intensive Care Unit. Mayo Clin Proc 2019;94:1781-5. [Crossref] [PubMed]
- Shah RA, Vempilly JJ, Noor Ul Husnain SM, et al. Combined Endosonography Reduces Time to Diagnose Pulmonary Coccidioidomycosis. J Bronchology Interv Pulmonol 2018;25:152-5. [Crossref] [PubMed]
- Cazzadori A, Di Perri G, Todeschini G, et al. Transbronchial biopsy in the diagnosis of pulmonary infiltrates in immunocompromised patients. Chest 1995;107:101-6. [Crossref] [PubMed]
- Bulpa PA, Dive AM, Mertens L, et al. Combined bronchoalveolar lavage and transbronchial lung biopsy: safety and yield in ventilated patients. Eur Respir J 2003;21:489-94. [Crossref] [PubMed]
- Jain P, Sandur S, Meli Y, et al. Role of flexible bronchoscopy in immunocompromised patients with lung infiltrates. Chest 2004;125:712-22. [Crossref] [PubMed]
- Patel NR, Lee PS, Kim JH, et al. The influence of diagnostic bronchoscopy on clinical outcomes comparing adult autologous and allogeneic bone marrow transplant patients. Chest 2005;127:1388-96. [Crossref] [PubMed]
- Chellapandian D, Lehrnbecher T, Phillips B, et al. Bronchoalveolar lavage and lung biopsy in patients with cancer and hematopoietic stem-cell transplantation recipients: a systematic review and meta-analysis. J Clin Oncol 2015;33:501-9. [Crossref] [PubMed]
- Bourne MH Jr, Norton MS, Midthun DE, et al. Utility of Transbronchial Biopsy in the Immunocompromised Host With New Pulmonary Radiographic Abnormalities. Mayo Clin Proc 2021;96:1500-9. [Crossref] [PubMed]
- Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e211S-50S.
- El-Osta H, Jani P, Mansour A, et al. Endobronchial Ultrasound for Nodal Staging of Patients with Non-Small-Cell Lung Cancer with Radiologically Normal Mediastinum. A Meta-Analysis. Ann Am Thorac Soc 2018;15:864-74. [Crossref] [PubMed]
- Labarca G, Folch E, Jantz M, et al. Adequacy of Samples Obtained by Endobronchial Ultrasound with Transbronchial Needle Aspiration for Molecular Analysis in Patients with Non-Small Cell Lung Cancer. Systematic Review and Meta-Analysis. Ann Am Thorac Soc 2018;15:1205-16. [Crossref] [PubMed]
- Kuijvenhoven JC, Leoncini F, Crombag LC, et al. Endobronchial Ultrasound for the Diagnosis of Centrally Located Lung Tumors: A Systematic Review and Meta-Analysis. Respiration 2020;99:441-50. [Crossref] [PubMed]
- Folch EE, Labarca G, Ospina-Delgado D, et al. Sensitivity and Safety of Electromagnetic Navigation Bronchoscopy for Lung Cancer Diagnosis: Systematic Review and Meta-analysis. Chest 2020;158:1753-69. [Crossref] [PubMed]
- Sainz Zuñiga PV, Vakil E, Molina S, et al. Sensitivity of Radial Endobronchial Ultrasound-Guided Bronchoscopy for Lung Cancer in Patients With Peripheral Pulmonary Lesions: An Updated Meta-analysis. Chest 2020;157:994-1011. [Crossref] [PubMed]
- Desai NR, Gildea TR, Kessler E, et al. Advanced Diagnostic and Therapeutic Bronchoscopy: Technology and Reimbursement. Chest 2021;160:259-67. [Crossref] [PubMed]
- Gazzoni FF, Severo LC, Marchiori E, et al. Fungal diseases mimicking primary lung cancer: radiologic-pathologic correlation. Mycoses 2014;57:197-208. [Crossref] [PubMed]
- Petrini B, Sköld CM, Bronner U, et al. Coccidioidomycosis mimicking lung cancer. Respiration 2003;70:651-4. [Crossref] [PubMed]
- Ross P Jr, Magro CM, King MA. Endobronchial histoplasmosis: a masquerade of primary endobronchial neoplasia--a clinical study of four cases. Ann Thorac Surg 2004;78:277-81. [Crossref] [PubMed]
- Chaddha U, Patil PD, English R, et al. The Imitation Game: A 55-Year-Old Man With a Lung and Adrenal Mass. J Bronchology Interv Pulmonol 2017;24:e52-4. [Crossref] [PubMed]
- Hussaini SMQ, Madut D, Tong BC, et al. Pulmonary blastomycosis presenting as primary lung cancer. BMC Infect Dis 2018;18:336. [Crossref] [PubMed]
- Kooblall M, Keane B, Murray G, et al. Histoplasmosis mimicking primary lung neoplasm. BMJ Case Rep 2014;2014:bcr2013203335. [Crossref] [PubMed]
- Hassan T, McLaughlin AM, O'Connell F, et al. EBUS-TBNA performs well in the diagnosis of isolated thoracic tuberculous lymphadenopathy. Am J Respir Crit Care Med 2011;183:136-7. [Crossref] [PubMed]
- Navani N, Molyneaux PL, Breen RA, et al. Utility of endobronchial ultrasound-guided transbronchial needle aspiration in patients with tuberculous intrathoracic lymphadenopathy: a multicentre study. Thorax 2011;66:889-93. [Crossref] [PubMed]
- Li W, Zhang T, Chen Y, et al. Diagnostic Value of Convex Probe Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration in Mediastinal Tuberculous Lymphadenitis: A Systematic Review and Meta-Analysis. Med Sci Monit 2015;21:2064-72. [Crossref] [PubMed]
- Sodhi A, Supakul R, Williams GW, et al. Role of Transbronchial Needle Aspiration (Conventional and EBUS Guided) in the Diagnosis of Histoplasmosis in Patients Presenting with Mediastinal Lymphadenopathy. South Med J 2017;110:33-6. [Crossref] [PubMed]
- Mirrakhimov AE, Hnatiuk O, Grant T, et al. Pulmonary Coccidioidomycosis Diagnosed by Endobronchial Ultrasound With Fine Needle Aspiration Biopsy of a Paratracheal Pulmonary Nodule. J Bronchology Interv Pulmonol 2019;26:e63-5. [Crossref] [PubMed]
- Meduri GU, Chastre J. The standardization of bronchoscopic techniques for ventilator-associated pneumonia. Chest 1992;102:557S-64S. [Crossref] [PubMed]
- Berton DC, Kalil AC, Teixeira PJ. Quantitative versus qualitative cultures of respiratory secretions for clinical outcomes in patients with ventilator-associated pneumonia. Cochrane Database Syst Rev 2014;CD006482. [Crossref] [PubMed]
- Mudambi L, Ost DE. Advanced bronchoscopic techniques for the diagnosis of peripheral pulmonary lesions. Curr Opin Pulm Med 2016;22:309-18. [Crossref] [PubMed]
- Wahidi MM, Herth F, Yasufuku K, et al. Technical Aspects of Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration: CHEST Guideline and Expert Panel Report. Chest 2016;149:816-35. [Crossref] [PubMed]
- Chrissian A, Misselhorn D, Chen A. Endobronchial-ultrasound guided miniforceps biopsy of mediastinal and hilar lesions. Ann Thorac Surg 2011;92:284-8. [Crossref] [PubMed]
- Ninan N, Wahidi MM. Basic Bronchoscopy: Technology, Techniques, and Professional Fees. Chest 2019;155:1067-74. [Crossref] [PubMed]
- Haydour Q, Hage CA, Carmona EM, et al. Diagnosis of Fungal Infections. A Systematic Review and Meta-Analysis Supporting American Thoracic Society Practice Guideline. Ann Am Thorac Soc 2019;16:1179-88. [Crossref] [PubMed]
- Hage CA, Carmona EM, Epelbaum O, et al. Microbiological Laboratory Testing in the Diagnosis of Fungal Infections in Pulmonary and Critical Care Practice. An Official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med 2019;200:535-50. Erratum in: Am J Respir Crit Care Med 2019;200:1326. [Crossref] [PubMed]
- Nahid P, Dorman SE, Alipanah N, et al. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America Clinical Practice Guidelines: Treatment of Drug-Susceptible Tuberculosis. Clin Infect Dis 2016;63:e147-95. [Crossref] [PubMed]
- Lewinsohn DM, Leonard MK, LoBue PA, et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of Tuberculosis in Adults and Children. Clin Infect Dis 2017;64:111-5. [Crossref] [PubMed]
- Chao TY, Chien MT, Lie CH, et al. Endobronchial ultrasonography-guided transbronchial needle aspiration increases the diagnostic yield of peripheral pulmonary lesions: a randomized trial. Chest 2009;136:229-36. [Crossref] [PubMed]
- Ost DE, Ernst A, Lei X, et al. Diagnostic Yield and Complications of Bronchoscopy for Peripheral Lung Lesions. Results of the AQuIRE Registry. Am J Respir Crit Care Med 2016;193:68-77. [Crossref] [PubMed]
- Gildea TR, Folch EE, Khandhar SJ, et al. The Impact of Biopsy Tool Choice and Rapid On-Site Evaluation on Diagnostic Accuracy for Malignant Lesions in the Prospective: Multicenter NAVIGATE Study. J Bronchology Interv Pulmonol 2021;28:174-83. [Crossref] [PubMed]
- Hayama M, Okamoto N, Suzuki H, et al. Radial endobronchial ultrasound with a guide sheath for diagnosis of peripheral cavitary lung lesions: a retrospective study. BMC Pulm Med 2016;16:76. [Crossref] [PubMed]
- Gu Y, Wu C, Yu F, et al. Application of endobronchial ultrasonography using a guide sheath and electromagnetic navigation bronchoscopy in the diagnosis of atypical bacteriologically-negative pulmonary tuberculosis. Ann Transl Med 2019;7:567. [Crossref] [PubMed]
- Zheng X, Wang L, Chen J, et al. Diagnostic value of radial endobronchial ultrasonographic features in predominant solid peripheral pulmonary lesions. J Thorac Dis 2020;12:7656-65. [Crossref] [PubMed]
- Hong KS, Lee KH, Chung JH, et al. Utility of Radial Probe Endobronchial Ultrasound Guided Transbronchial Lung Biopsy in Bronchus Sign Negative Peripheral Pulmonary Lesions. J Korean Med Sci 2021;36:e176. [Crossref] [PubMed]
- Cordeiro RA, Brilhante RS, Rocha MF, et al. Phenotypic characterization and ecological features of Coccidioides spp. from Northeast Brazil. Med Mycol 2006;44:631-9. [Crossref] [PubMed]
- Roden AC, Schuetz AN. Histopathology of fungal diseases of the lung. Semin Diagn Pathol 2017;34:530-49. [Crossref] [PubMed]
- Willinger B. Culture-Based Techniques. Methods Mol Biol 2017;1508:195-207. [Crossref] [PubMed]
- Shahangian S, Furukawa BS, Hsia DW, Chrissian AA. Electromagnetic navigation transthoracic needle aspiration improves diagnostic yield of bronchoscopic biopsy for peripheral lung lesions. Poster Presentation at International Meeting of the American Thoracic Society, 2022.
- Nin CS, de Souza VV, do Amaral RH, et al. Thoracic lymphadenopathy in benign diseases: A state of the art review. Respir Med 2016;112:10-7. [Crossref] [PubMed]
- Eickhoff L, Golpon H, Zardo P, et al. Endobronchial Ultrasound in Suspected Non-Malignant Mediastinal Lymphadenopathy. Pneumologie 2018;72:559-67. [Crossref] [PubMed]
- Santos LM, Figueiredo VR, Demarzo SE, et al. The role of endobronchial ultrasound-guided transbronchial needle aspiration in isolated intrathoracic lymphadenopathy in non-neoplastic patients: a common dilemma in clinical practice. J Bras Pneumol 2020;46:e20180183. [Crossref] [PubMed]
- Gailey MP, Klutts JS, Jensen CS. Fine-needle aspiration of histoplasmosis in the era of endoscopic ultrasound and endobronchial ultrasound: cytomorphologic features and correlation with clinical laboratory testing. Cancer Cytopathol 2013;121:508-17. [Crossref] [PubMed]
- Ye W, Zhang R, Xu X, et al. Diagnostic Efficacy and Safety of Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration in Intrathoracic Tuberculosis: A Meta-analysis. J Ultrasound Med 2015;34:1645-50. [Crossref] [PubMed]
- Cheng G, Mahajan A, Oh S, et al. Endobronchial ultrasound-guided intranodal forceps biopsy (EBUS-IFB)-technical review. J Thorac Dis 2019;11:4049-58. [Crossref] [PubMed]
- Martin-Deleon R, Llabrés de Prada M, Pérez FM, et al. Diagnosis of Pulmonary Cryptococcosis by EBUS-TBNA in a Healthy Young Man. J Bronchology Interv Pulmonol 2019;26:e32-4. [Crossref] [PubMed]
- Binnicker MJ, Buckwalter SP, Eisberner JJ, et al. Detection of Coccidioides species in clinical specimens by real-time PCR. J Clin Microbiol 2007;45:173-8. [Crossref] [PubMed]
- Brownback KR, Pitts LR, Simpson SQ. Utility of galactomannan antigen detection in bronchoalveolar lavage fluid in immunocompromised patients. Mycoses 2013;56:552-8. [Crossref] [PubMed]
- Theron G, Peter J, Meldau R, et al. Accuracy and impact of Xpert MTB/RIF for the diagnosis of smear-negative or sputum-scarce tuberculosis using bronchoalveolar lavage fluid. Thorax 2013;68:1043-51. [Crossref] [PubMed]
- Azadeh N, Sakata KK, Saeed A, et al. Comparison of Respiratory Pathogen Detection in Upper versus Lower Respiratory Tract Samples Using the BioFire FilmArray Respiratory Panel in the Immunocompromised Host. Can Respir J 2018;2018:2685723. [Crossref] [PubMed]
- Drick N, Seeliger B, Greer M, et al. DNA-based testing in lung transplant recipients with suspected non-viral lower respiratory tract infection: A prospective observational study. Transpl Infect Dis 2018;20:e12811. [Crossref] [PubMed]
- Lachant DJ, Croft DP, McGrane Minton H, et al. The clinical impact of pneumocystis and viral PCR testing on bronchoalveolar lavage in immunosuppressed patients. Respir Med 2018;145:35-40. [Crossref] [PubMed]
- Dizon D, Mitchell M, Dizon B, et al. The utility of real-time polymerase chain reaction in detecting Coccidioides immitis among clinical specimens in the Central California San Joaquin Valley. Med Mycol 2019;57:688-93. [Crossref] [PubMed]
- Hardak E, Fuchs E, Leskes H, et al. Diagnostic role of polymerase chain reaction in bronchoalveolar lavage fluid for invasive pulmonary aspergillosis in immunocompromised patients - A retrospective cohort study. Int J Infect Dis 2019;83:20-25. [Crossref] [PubMed]
- Ko RE, Jeong BH, Chon HR, et al. Clinical usefulness of routine AFB culture and MTB PCR of EBUS-TBNA needle rinse fluid. Respirology 2019;24:667-74. [Crossref] [PubMed]
- Li G, Huang J, Li Y, et al. The Value of Combined Radial Endobronchial Ultrasound-Guided Transbronchial Lung Biopsy and Metagenomic Next-Generation Sequencing for Peripheral Pulmonary Infectious Lesions. Can Respir J 2020;2020:2367505. [Crossref] [PubMed]



