
Hyperthermic intrapleural chemotherapy: an update
Introduction
Background
The utilization of hyperthermic chemotherapy as an adjuvant to cytoreductive surgery (CRS) dates to the 1980s with initial applications in cases of peritoneal carcinomatosis (1). The advent of this “double-hit” therapy was based on the synergistic effects of a local cytotoxic agent with maximal penetration ensured by a hyperthermic environment (2). The promise of hyperthermic intraperitoneal chemotherapy (HIPEC) rapidly translated to applications in thoracic surgery. Hyperthermic intrathoracic chemotherapy (HITHOC), similar to HIPEC, employs a concentrated dose of chemotherapy infused in warmed normal saline that is circulated in the chest immediately following CRS. In establishing both a high local and low systemic concentration of chemotherapeutic agent toxicity is minimized in the setting of a highly targeted therapy (3).
Rationale and knowledge gap
The safety and efficacy of HITHOC in pleural malignancies has been well demonstrated, particularly for malignant pleural mesothelioma (MPM) and thymic tumors with pleural dissemination (4,5). Despite several studies demonstrating favorable outcomes associated with the utilization of HITHOC on both a national and international scale, there persists a wide variety in type, dose, and number of agents utilized, as well as temperature range, treatment duration, and operative setup (6). Marked improvement in outcomes and survival trends is variable among current data, however in the setting of a limited risk profile even a modest survival advantage is favorable. Current practice guidelines for the management of pleural malignancies remain ambiguous with minimal standardization in technique and approach regardless of indication (7,8).
Objective
The lack of consensus regarding optimal standardization and implementation of HITHOC in the setting of pleural malignancy demonstrates the need for succinct depiction of current trends and techniques. We aim to provide a concise review of the indications for HITHOC in addition to summarizing current trends in therapeutic technique utilizing both current literature and experiences at our institution.
Indications for HITHOC
In reviewing current literature, the most common indications for the application of HITHOC in CRS include MPM and thymic pleural malignancies. There are patients with select subtypes of pleural carcinomatosis who may benefit, however these cases remain infrequent and should be considered on a case-by-case basis.
Malignant pleural mesothelioma (MPM)
MPM is the most common primary pleural cancer but remains quite rare with an estimated incidence of 3,000 cases diagnosed annually in the US. Asbestos exposure is associated with approximately 85% of cases with a latency to disease presentation of up to 50 years. The prognosis remains quite poor with a median survival of less than 12 months when treated with chemotherapy alone, and 20 months following surgical intervention (9). Immune checkpoint inhibitors show promise in non-surgical candidates, extending median survival to 18 months, but have not been approved as neoadjuvant or adjuvant therapy for surgical candidates (10). An epithelioid histology is the most common subtype and is associated with improved prognosis when compared to sarcomatoid and biphasic (mixed) forms (11). Current tumor-node-metastasis (TNM) staging is based on the 8th edition American Joint Committee on Cancer (AJCC) guidelines. Resection is typically considered in surgical candidates with clinical stage I–IIIA disease.
Following initial evaluation and diagnostic workup, surgical evaluation includes mediastinal staging (EBUS or mediastinoscopy) and, at many centers, diagnostic laparoscopy to rule out subdiaphragmatic spread. As MPM covers all surfaces of the chest, successful R0 resection remains challenging. Instead, surgical intervention in MPM is undertaken with a goal of macroscopic complete resection (MCR), accomplished in either a lung-sparing or lung-sacrificing surgery. The more radical extrapleural pneumonectomy (EPP) entails en bloc resection of the entire lung, parietal pleura, and all involved hemidiaphragm and pericardium. Despite its success in achieving MCR, EPP is associated with significant perioperative morbidity and mortality, the extent of which was detailed in the MARS trial (12). Recent meta-analyses have indicated similar, if not superior, long-term survival in patients undergoing P/D with significantly reduced perioperative morbidity and mortality; the MARS2 trial is currently evaluating outcomes in patients with MPM that underwent pleurectomy and decortication (P/D) as opposed to those with non-operative management (13,14). Despite persistent ambiguity in the definition of maximum cytoreduction and thus what constitutes P/D, generally P/D involves resection of the entire visceral and parietal pleura often including resection of any involved diaphragm and/or pericardium.
Chemotherapy remains a mainstay of MPM treatment with a variety of protocols existing for the varying histologic subtypes and stages (15-17). Standard practice for patients with surgically treatable disease consists of four cycles of neoadjuvant or adjuvant cisplatin-pemetrexed therapy. With the CheckMate 743 trial demonstrating overall survival benefit in non-surgical candidates with MPM, current perioperative research efforts are investigating immunotherapy or chemoimmunotherapy in the neoadjuvant and maintenance settings. Results of these studies are incomplete but carry the potential to change the systemic therapy landscape for MPM (18). Despite multimodality treatment for MPM, studies have revealed an overall recurrence rate between 60–80% in patients treated with chemotherapy and P/D (19-21). A retrospective cohort study out of Japan demonstrated local recurrence in 68% of their population with another 20% presenting with both local and distant recurrence (19). Similarly, a Swiss study revealed local recurrence in 31% of their patients with simultaneous local and distant recurrence in 43% of patients; median freedom from recurrence was just 9 months from resection. They noted a reduction in local recurrence in those that received adjuvant radiotherapy, however this was not associated with any difference in outcomes—median post-recurrence survival was 7 months (20). The high prevalence of local recurrence is unsurprising as R0 resection is an unattainable goal in plurally disseminated disease, a notion that mirrors trends in peritoneal malignancy. The utilization of local cytotoxic therapy in the form of HIPEC and HITHOC provides a promising approach to address persistently high rates of local disease recurrence following CRS.
In recent decades, several clinical trials have been completed evaluating the efficacy of HITHOC when used in combination with CRS. These demonstrated increased median overall survival as well as 5-year survival rates (3). Despite such data, there still remains a lack of standard use of HITOC in MPM. As Migliore et al. recently noted, even latest task force guidelines neglect to discuss the applicability of HITHOC in MPM management (8). Zhou et al. completed a systematic review and meta-analysis in recent years that again demonstrated an increased median survival time in those receiving HITHOC in addition to surgical resection (both EPP and P/D) (22). Their conclusion is limited by an extremely heterogenous variety of techniques used to perform HITHOC. Additionally, Dawson et al. offer a systematic review in which median overall survival and disease-free survival were increased in those receiving HITHOC (4). Each of these studies note minimal associated complications, the majority of which are attributed to surgical resection rather than HITHOC use.
Pleural thymic malignancies
Originating from epithelial thymic cells, thymoma persists as the most common neoplasm of the anterior mediastinum. Despite a lack of known risk factors, thymoma is associated with paraneoplastic myasthenia gravis as well as other autoimmune conditions. Secondary to embryologic origins in the third and fourth branchial pouches, ectopic thymomas can occur—most often originating in the pleura, however this is exceedingly rare. Much more common is the instance of thymoma originating from the mediastinum and spreading along the pleura. A wide array in histologic subtype has led to development of the Masaoka staging system that is utilized to stage thymic tumors and select treatment (23).
Most institutions utilize a cisplatin-based neoadjuvant chemotherapy regimen for advanced thymoma, however as thymomas are generally indolent tumors they tend to be slower-growing and less responsive to chemoradiation. As such, surgical management is often the favorable intervention, even in stage IV disease (24). Mirroring MPM, thymic malignancies with pleural involvement do not enable an R0 resection; the goal remains MCR in such cases. As the utilization of HITHOC following MCR gained a wider, albeit heterogenous consensus as a component of multimodal therapy in the management of MPM, the strategy was also applied to management of stage IVa thymomas. Several studies have been conducted demonstrating improved overall and disease-free survival at 1 and 5 years, again with minimal change in complication rates. Aprile et al. offer a systematic review of the literature over the past two decades with inclusion of their own institutional experience (5). While such data remain limited by small sample size, they demonstrate similar postoperative morbidity with no procedure-related mortality in those undergoing HITHOC for relapsing thymoma as compared to those that only received CRS. Additionally, they noted a significant difference in disease-free interval from 57 to 88 months.
Other indications
While MPM and thymic pleural malignancies remain the primary indications for HITHOC, small studies have demonstrated application of HITHOC in other malignancies including pleural dissemination of lung adenocarcinoma, pleural metastases of colorectal carcinoma, and sarcoma with pleural spread (25,26). These are exceedingly rare indications with limited data available (27). Recent presentation at the 2022 International Thoracic Surgical Oncology Summit includes data for those undergoing P/D with HITHOC for pleural dissemination of metastatic lung, breast, gastrointestinal, gynecologic, and renal cancers. These data demonstrate favorable outcomes with minimal complications, from which Miller et al. concluded that additional long-term follow-up and refinement of inclusion criteria is warranted (28). Similarly, Migliore et al. demonstrate favorable outcomes with the utilization of HITHOC for malignant pleural effusion secondary to non-small cell lung cancer, however encourage further investigation in the form of a randomized controlled trial (29). Additionally, several retrospective studies have evaluated the utility of HITHOC in ovarian cancer with pleural involvement with promising results (30-32).
Technique
Our primary indication for HITHOC is stage I–IIIA epithelioid mesothelioma and stage IV thymoma with pleural dissemination. At our institution, all patients undergo extensive preoperative evaluation and those that are considered candidates for P/D are also considered for HITHOC. We consider excluding HITHOC in patients with chronic renal insufficiency or those with severe marrow suppression.
Our evaluation process for P/D and HITHOC therapy includes internal pathology review, clinical staging, multi-disciplinary tumor board discussion, and diagnostic testing to evaluate cardiac, pulmonary, and renal function. Clinical staging involves contrasted chest computed tomography (CT) and positron emission tomography (PET) scan. An magnetic resonance imaging (MRI) of the brain is only performed in select cases. Diagnostic tests include baseline labs, pulmonary function tests, and nuclear cardiac stress or stress echocardiogram. If concerns for pulmonary hypertension exist, a right heart catheterization is ordered in select patients. We perform endobronchial ultrasound (EBUS) or mediastinoscopy in conjunction with diagnostic laparoscopy in possible surgical candidates. Anesthesia considerations for HITHOC include placement of a double lumen endotracheal tube, arterial line, central venous line, and Foley catheter with bladder temperature monitoring. The utilization of a double lumen endotracheal tube enables single-lung ventilation, ensuring that the ipsilateral lung remains collapsed for the duration of the case. Thoracic epidurals can be used for pain control but have been linked with post-procedure hypotension; our pain management strategies include intraoperative intercostal nerve cryoablation after pleurectomy with a cryoICE cryoSPHERE cryoablation probe (Atricure Mason, OH, USA) in conjunction with long-acting intercostal nerve blocks (33). Procedure length can be extensive, so careful attention is paid to lateral decubitus positioning and intraoperative creatinine kinase monitoring is performed to monitor for rhabdomyolysis. CRS should be performed first via posterolateral thoracotomy through the sixth interspace. We use a similar P/D technique as is described by Ripley et al. (34). A summary of this technique is provided in conjunction with the modifications allowed for HITHOC in Table 1.
Table 1
| Summary of operative technique for pleurectomy/decortication (34) |
| Step 1: posterolateral thoracotomy, rib resection, and development of extrapleural dissection plane |
| Step 2: anterior extrapleural dissection |
| Step 3: apical extrapleural dissection |
| Step 4: posterior extrapleural dissection |
| Step 5: mediastinal and hilar lymph node dissection |
| Step 6: diaphragm resection |
| Step 7: pericardial resection |
| Step 8: visceral decortication |
| Step 9: diaphragm reconstruction |
| Step 10: pericardial reconstruction |
| Step 11: povidone-iodine scrub |
| Summary of institution operative technique for pleurectomy/decortication + HITHOC |
| Step 1: posterolateral thoracotomy, rib resection, and development of extrapleural dissection plane |
| Step 2: anterior extrapleural dissection |
| Step 3: apical extrapleural dissection |
| Step 4: posterior extrapleural dissection |
| Step 5: mediastinal and hilar lymph node dissection |
| Step 6: visceral decortication |
| Step 7: removal of visible bulky pericardial disease |
| Step 8: full thickness diaphragm resection with reconstruction |
| Step 9: HITHOC (single agent cisplatin 150–225 mg/m2, 42 ℃, 60 min) |
| Step 10: full thickness pericardial resection with reconstruction if needed |
HITHOC, hyperthermic intrathoracic chemotherapy.
Once CRS and reconstructive efforts are complete, a sterile cannula set (The Procedure Kit, Belmont Medical Technologies, Billerica MA, USA, product number 902-00045) is positioned within the thorax. Four small satellite incisions are made to accommodate two inflow cannulas and two outflow cannulas. The inflow cannulas are positioned and secured along the anterior and posterior mediastinum (Figure 1). Two temperature probes are placed for monitoring, one along the esophagus and the other along the pericardium (Figure 2). The outflow cannulas are secured to the inferior aspect of the lateral ribs, and the intercostal space is reapproximated to allow for temporary closure of the thoracotomy with a running monofilament locking stitch (Figure 3). Temporary closure of the chest enables HITHOC circulation with subsequent completion of diaphragmatic, pulmonary, or pericardial reconstructions. Additionally, this approach allows for confirmation of hemostasis following HITHOC and immediately prior to the conclusion of the case.


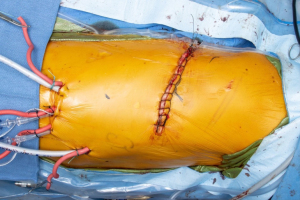
HITHOC is a highly concentrated dose of chemotherapy that is infused in 3–4 L of normal saline and warmed to 38–43 ℃. The rationale behind administration of intrapleural chemotherapy is centered around direct contact of cytotoxic drugs to an area of tumor, thus eliminating reliance on blood supply to the areas. The cellular effects of hyperthermia are well established; the granularity of which is beyond the scope of this review, however it is understood that local application of chemotherapy in a hyperthermic setting induces a synergistic effect in which the increased temperature increases penetration of chemotherapeutic agent, thus increasing the local cytotoxic effect (35). In recent meta-analyses, the most common cytotoxic drugs used in HITHOC are cisplatin, followed by doxorubicin and mitomycin C (22). Higher levels of pleural penetration have been demonstrated with cisplatin as compared to doxorubicin and doxorubicin has been associated with increased rates of direct cardiotoxicity (36). Additionally, dual therapy with cisplatin/mitomycin C is associated with increased toxicity, mainly by increased rates of marrow suppression (37). HITHOC agents, temperature, and treatment duration are institution specific and variable. A compilation of available options listed in the literature are available in Table 2. We regularly utilize cisplatin 150–225 mg/m2 of BSA for 60 min at 42 ℃ instilled through a roller pump, Hyperthermia Treatment Pump (Belmont Medical Technologies, Billerica MA, USA).
Table 2
| Chemotherapy agent | Temperature (℃) | Duration (min) |
|---|---|---|
| Cisplatin (50–225 mg/m2) | 41–43 | 60 |
| Cisplatin (75–150 mg/m2) + mitomycin C (8–15 mg/m2) | No heat | 60 |
| Cisplatin (80 mg/m2) + doxorubicin (20–30 mg/m2) | 40–41 | 90 |
| Cisplatin (200 mg) + doxorubicin (100 mg) | 42.5 | 60 |
| Cisplatin (80 mg/m2) + epirubicin (25 mg/m2) | 42.5 | 60 |
| Cisplatin (175–223 mg/m2) + gemcitabine (100–1,200 mg/m2) | 40–42 | 60 |
HITHOC, hyperthermic intrathoracic chemotherapy.
The HITHOC circuit consists of two drainage cannulas (outflow) and two return cannulas (inflow). We initially fill the circuit with approximately 2–3 L of normal saline fluid; following proper placement of the cannulas the heated fluid is infused to the patient to fill the thoracic cavity through the return cannulas. Once the thoracic cavity is adequately filled, the drainage line is opened to allow circulation and lavage. The drainage line sends the heated saline to an open, hard-shelled reservoir, then through the Belmont Procedure kit and device which heats the fluid and pumps it back to the patient through the return cannulas. Once target temperature (42 ℃) is achieved, the chemotherapeutic agent is added directly to the hard-shell reservoir to ensure full mixing with the heated, circulating saline. Circulation of the chemotherapy is then continued for 60 min. Administration immediately following CRS enables exposure of the entire pleural space to cytotoxic agent prior to formation of post-operative adhesions that would induce compartmentalization (35). The dose is fractionated, giving one third at initiation of HITHOC, an additional third at 20 min, and the final third at 40 min. Arterial blood gas monitoring is performed every 20 min during HITHOC infusion. At the end of the treatment period, the chemotherapeutic fluid is “chased” through the circuit with normal saline and re-routed into a waste bag, displacing the chemotherapeutic agent with approximately 2 L of normal saline. Once adequately “chased” into the waste bag, the remaining fluid in the chest is drained from the patient into the reservoir using vacuum assist prior to reopening the thoracotomy incision (5,8).
Hemostasis is assured and the operation is completed as planned. We generally perform any full thickness pericardial reconstruction at this point (Figure 4). This approach limits chemotherapy contact to the targeted pleural space as we fenestrate the pericardial patch to prevent post-procedure cardiac tamponade. Generally, one (or two) larger bore chest tubes are left in place due to the risk of bleeding from the pleurectomy. The patient is then reintubated with a single lumen endotracheal tube and completion bronchoscopy is performed. Patients who meet criteria are extubated in the operating room. If a patient does not meet appropriate respiratory or hemodynamic parameters, they are relocated to the ICU and remain intubated. Early extuabtion is favored to reduced positive ventilatory pressure effects on air leak and bronchopleural fistula. Careful attention to acidosis and renal function is practiced both intraoperatively and in the post-operative setting. Prophylactic antibiotics (Cefazolin) are continued when mesh is used for reconstruction until chest tubes are removed.

Discussion
While the potential benefit of HITHOC in cases of pleural malignancy has been well documented, its utilization remains highly variable in both indication and technique. For example, Ambrogi et al. describe the utilization of epirubicin and cisplatin at 42.5 degrees over a 60-min period in cases of recurrent thymoma with pleural involvement (38). Contrastingly, Kerscher et al. detail a single agent regimen utilizing cisplatin alone at 42 degrees in patients with MPM, while Sugarbaker et al. describe the use of either mitomycin C or doxorubicin (39,40). As previously detailed, our institution elects to utilize cisplatin monotherapy due to previously reported efficacy and decreased toxicity when compared to dual therapy (41,42). When combining a rare disease process (and thus limited patient population) with persistent ambiguity in technique the ability to draw strong conclusions regarding the efficacy and utility of HITHOC is severely limited. This lack of standardization remains one of the biggest hurdles in the advancement of HITHOC utilization in pleural malignancies of varied etiologies.
Zhou et al. report no associated mortality or high-grade complications in their meta-analysis (22). Ambrogi and colleagues report no observed intraoperative complications with completion of HITHOC perfusion in all patients; their most common associated complication was anemia in approximately one-third of patients (38). Outside of hematologic toxicities, renal impairment is a noted complication of HITHOC, as demonstrated by Richards et al.; Tilleman and colleagues discuss the potential benefit of pharmacologic cytoprotection utilizing amifostine and sodium thiosulfate to minimize renal toxicity. It is important to note that cisplatin is the main driver of renal toxicity. Prior studies demonstrate that application below 225 mg/m2 is a safe therapeutic dose, however baseline renal function should be evaluated when determining appropriate regimen on a case-by-case basis (43,44). As much of the currently existing data is limited to single institution case series, there is a paucity of comparison in outcomes between HITHOC for thymoma with pleural involvement as compared to MPM—these are commonly evaluated as one population. Additionally, it is important to note that many of the reported complications in prior studies are associated with surgical resection independent of HITHOC use. A summary of the morbidity and complications associated with P/D is provided in Table 3.
Table 3
| Hemothorax |
| Empyema |
| Bronchopleural fistula |
| Prolonged air leak |
| Respiratory failure |
| Pneumonia |
| Acute respiratory distress syndrome |
| Atrial fibrillation |
| Post-operative anemia requiring transfusion |
| Acute kidney injury/renal failure |
| Pulmonary embolism |
| Cerebrovascular event |
| Post-operative sepsis |
| Surgical site infection |
Current NCCN guidelines for pleural mesothelioma mention hyperthermic chemotherapy however do not provide a formal recommendation, stating that intrathoracic adjuvant therapies like HITHOC are of unclear benefit. To date, no standardization in surgical approach or administration of hyperthermic chemotherapy exist for primary or secondary pleural based malignancies, significantly hindering its broader application. This is likely in part due to the rarity of conditions for which HITHOC is indicated as well as individual surgeon and center preference. Such variety in application and approach continues to limit the potential advantages of HITHOC.
In contrast, the standardization of HIPEC therapy is assisted by the peritoneal cancer index (PCI), described by Sugarbaker et al. Briefly, during intraoperative evaluation the PCI score is used to quantify the extent of peritoneal disease. The abdominal cavity is divided into 13 regions and disease burden within each region is defined by a lesion size (LS) score (45-47). The PCI is understood to be an independent prognostic factor for morbidity and overall survival; within the various subsets of peritoneal disease, cutoffs for PCI exist to help determine when CRS with HIPEC should not be offered.
In addition to the PCI score, Sugarbaker reported a CC score as an objective description of the type of CRS performed. In short, a CC-0 indicates no remnant visible peritoneal seeding, CC-1 indicates tumor nodules post-reduction are <2.5 mm, CC-2 indicates nodules between 2.5 mm and 2.5 cm, and CC-3 is reserved for tumor nodules >2.5 cm or a confluence of unresectable nodules. Nodules <2.5 mm are thought to be penetrable by intracavity chemotherapy and therefore CC-1 would be considered a complete cytoreduction when HIPEC is utilized (47).
Such standardization in abdominal CRS begs the question—can a similar index be applied to pleural malignancies with considerations of cancer histology, thoracic disease burden, and detailed description of remnant pleural disease? Additionally, is there an increased role of CRS with HITHOC in secondary pleural malignancies in the setting of limited surgical morbidity and maintained patient quality of life? We believe that the current literature as well as experience at our own institution favors the implementation of HITHOC as an adjunct to CRS in pleural malignancies in appropriate candidates. The current ambiguity in guidelines substantially limits further evaluation of the potential benefits of HITHOC. We favor the creation of a dedicated task force to further examine the feasibility and applicability of HITHOC in this population with a goal of standardizing indications and technique. To do so, we feel the creation of a pleural cancer index to quantify pleural disease burden could offer invaluable standardization of surgical management, thus enabling utilization of HITHOC in a broader patient population that currently has limited alternative options.
Conclusions
In recent years, conversation regarding the utilization of HITHOC in malignant pleural disease has gained increasing momentum. The implementation of direct cytotoxic agents in a hyperthermic environment to optimize drug penetration offers immense potential as an adjunct to CRS, particularly in disease for which R0 resection remains challenging. Despite the promise demonstrated by current data, there remains a lack of standardization of HITHOC in both its indication and technique. We remain hopeful that continued discussion of successful HITHOC approaches will yield greater application of this adjunct therapy. The creation of a task force to further examine the demonstrated benefits and minimal risk profile of HITHOC could yield a pleural cancer index that standardizes the utilization of HITHOC in pleural malignancy of diverse etiologies may be timely.
Acknowledgments
We would like to sincerely thank our anesthesia colleagues, cardiopulmonary perfusion team, the OR team, nursing staff, and critical care teams that assist with and enable the utilization of HITHOC at our institution. Our advancements and patient successes are not possible without their tireless efforts.
Funding: None.
Footnote
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-454/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-454/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Neuwirth MG, Alexander HR, Karakousis GC. Then and now: cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (HIPEC), a historical perspective. J Gastrointest Oncol 2016;7:18-28. [Crossref] [PubMed]
- González-Moreno S, González-Bayón LA, Ortega-Pérez G. Hyperthermic intraperitoneal chemotherapy: Rationale and technique. World J Gastrointest Oncol 2010;2:68-75. [Crossref] [PubMed]
- Markowiak T, Larisch C, Hofmann HS, et al. Hyperthermic intrathoracic chemotherapy (HITHOC): narrative review of the current literature, recommendations and future studies. Ann Transl Med 2021;9:955. [Crossref] [PubMed]
- Dawson AG, Kutywayo K, Mohammed SB, et al. Cytoreductive surgery with hyperthermic intrathoracic chemotherapy for malignant pleural mesothelioma: a systematic review. Thorax 2023;78:409-17. [Crossref] [PubMed]
- Aprile V, Bacchin D, Korasidis S, et al. Hypertermic Intrathoracic Chemotherapy (HITHOC) for thymoma: a narrative review on indications and results. Ann Transl Med 2021;9:957. [Crossref] [PubMed]
- Rice D. Standardizing surgical treatment in malignant pleural mesothelioma. Ann Cardiothorac Surg 2012;1:497-501. [Crossref] [PubMed]
- Girard N, Ruffini E, Marx A, et al. Thymic epithelial tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015;26:v40-55. [Crossref] [PubMed]
- Migliore M, Ried M, Molins L, et al. Hyperthermic intrathoracic chemotherapy (HITHOC) should be included in the guidelines for malignant pleural mesothelioma. Ann Transl Med 2021;9:960. [Crossref] [PubMed]
- Bovolato P, Casadio C, Billè A, et al. Does surgery improve survival of patients with malignant pleural mesothelioma?: a multicenter retrospective analysis of 1365 consecutive patients. J Thorac Oncol 2014;9:390-6. [Crossref] [PubMed]
- Baas P, Scherpereel A, Nowak AK, et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet 2021;397:375-86. [Crossref] [PubMed]
- Rudd RM. Malignant mesothelioma. Br Med Bull 2010;93:105-23. [Crossref] [PubMed]
- Treasure T, Lang-Lazdunski L, Waller D, et al. Extra-pleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: clinical outcomes of the Mesothelioma and Radical Surgery (MARS) randomised feasibility study. Lancet Oncol 2011;12:763-72. [Crossref] [PubMed]
- Batirel HF. Extrapleural pneumonectomy (EPP) vs. pleurectomy decortication (P/D). Ann Transl Med 2017;5:232. [Crossref] [PubMed]
- Lim E, Darlison L, Edwards J, et al. Mesothelioma and Radical Surgery 2 (MARS 2): protocol for a multicentre randomised trial comparing (extended) pleurectomy decortication versus no (extended) pleurectomy decortication for patients with malignant pleural mesothelioma. BMJ Open 2020;10:e038892. [Crossref] [PubMed]
- Cho BCJ, Donahoe L, Bradbury PA, et al. Surgery for malignant pleural mesothelioma after radiotherapy (SMART): final results from a single-centre, phase 2 trial. Lancet Oncol 2021;22:190-7. [Crossref] [PubMed]
- Cho BCJ. The rationale for neoadjuvant radiation therapy in malignant pleural mesothelioma: how smart is SMART? Ann Transl Med 2017;5:247. [Crossref] [PubMed]
- Voigt SL, Raman V, Jawitz OK, et al. The Role of Neoadjuvant Chemotherapy in Patients With Resectable Malignant Pleural Mesothelioma-An Institutional and National Analysis. J Natl Cancer Inst 2020;112:1118-27. [Crossref] [PubMed]
- Tsao AS, Pass HI, Rimner A, et al. New Era for Malignant Pleural Mesothelioma: Updates on Therapeutic Options. J Clin Oncol 2022;40:681-92. [Crossref] [PubMed]
- Nakamura A, Takuwa T, Hashimoto M, et al. Clinical Outcomes With Recurrence After Pleurectomy/Decortication for Malignant Pleural Mesothelioma. Ann Thorac Surg 2020;109:1537-43. [Crossref] [PubMed]
- Kostron A, Friess M, Crameri O, et al. Relapse pattern and second-line treatment following multimodality treatment for malignant pleural mesothelioma. Eur J Cardiothorac Surg 2016;49:1516-23. [Crossref] [PubMed]
- Politi L, Borzellino G. Second surgery for recurrence of malignant pleural mesothelioma after extrapleural pneumonectomy. Ann Thorac Surg 2010;89:207-10. [Crossref] [PubMed]
- Zhou H, Wu W, Tang X, et al. Effect of hyperthermic intrathoracic chemotherapy (HITHOC) on the malignant pleural effusion: A systematic review and meta-analysis. Medicine (Baltimore) 2017;96:e5532. [Crossref] [PubMed]
- Picchi E, Di Giuliano F, Ferrari D, et al. Pleural thymoma: Radiological and histological findings. Eur J Radiol Open 2018;5:147-52. [Crossref] [PubMed]
- D'Andrea MA, Reddy GK. Management of metastatic malignant thymoma with advanced radiation and chemotherapy techniques: report of a rare case. World J Surg Oncol 2015;13:77. [Crossref] [PubMed]
- Song K, Flores RM. A narrative review of hyperthermic intrathoracic chemotherapy for advanced lung cancer. Ann Transl Med 2021;9:958. [Crossref] [PubMed]
- Kim HJ, Lee HJ, Kim E, et al. Abrupt hemodynamic changes accompanying intrapleural hyperthermic chemotherapy: Case series. Medicine (Baltimore) 2018;97:e10982. [Crossref] [PubMed]
- Klotz LV, Gruenewald C, Bulut EL, et al. Cytoreductive Thoracic Surgery Combined with Hyperthermic Chemoperfusion for Pleural Malignancies: A Single-Center Experience. Respiration 2021;100:1165-73. [Crossref] [PubMed]
- Miller DL, Parks CS, Ange B, et al. Hyperthermic intrathoracic extracorporeal chemotherapy for secondary malignant pleural disease. J Surg Oncol 2023;128:604-11. [Crossref] [PubMed]
- Migliore M, Nardini M. Does cytoreduction surgery and hyperthermic intrathoracic chemotherapy prolong survival in patients with N0-N1 nonsmall cell lung cancer and malignant pleural effusion? Eur Respir Rev 2019;28:190018. [Crossref] [PubMed]
- Singh S, Armstrong A, Robke J, et al. Hyperthermic Intra-Thoracic Chemotherapy (HITeC) for the management of recurrent ovarian cancer involving the pleural cavity. Gynecol Oncol Case Rep 2014;9:24-5. [Crossref] [PubMed]
- Stojiljkovic D, Nikolic S, Cvetkovic A, et al. Hyperthermic Intrathoracic Chemotherapy (Hithoc) in Ovarian Carcinoma – Case Report. Eur J Surg Oncol 2020;46:e164.
- Ried M, Kovács J, Markowiak T, et al. Hyperthermic Intrathoracic Chemotherapy (HITOC) after Cytoreductive Surgery for Pleural Malignancies-A Retrospective, Multicentre Study. Cancers (Basel) 2021;13:4580. [Crossref] [PubMed]
- Clemente A, Carli F. The physiological effects of thoracic epidural anesthesia and analgesia on the cardiovascular, respiratory and gastrointestinal systems. Minerva Anestesiol 2008;74:549-63.
- Ripley RT, Palivela N. Extended pleurectomy and decortication: Video atlas of operative steps. JTCVS Tech 2021;7:324-30. [Crossref] [PubMed]
- Goodman MD, McPartland S, Detelich D, et al. Chemotherapy for intraperitoneal use: a review of hyperthermic intraperitoneal chemotherapy and early post-operative intraperitoneal chemotherapy. J Gastrointest Oncol 2016;7:45-57. [Crossref] [PubMed]
- van Ruth S, van Tellingen O, Korse CM, et al. Pharmacokinetics of doxorubicin and cisplatin used in intraoperative hyperthermic intrathoracic chemotherapy after cytoreductive surgery for malignant pleural mesothelioma and pleural thymoma. Anticancer Drugs 2003;14:57-65. [Crossref] [PubMed]
- Rusch VW, Niedzwiecki D, Tao Y, et al. Intrapleural cisplatin and mitomycin for malignant mesothelioma following pleurectomy: pharmacokinetic studies. J Clin Oncol 1992;10:1001-6. [Crossref] [PubMed]
- Ambrogi MC, Bertoglio P, Aprile V, et al. Diaphragm and lung-preserving surgery with hyperthermic chemotherapy for malignant pleural mesothelioma: A 10-year experience. J Thorac Cardiovasc Surg 2018;155:1857-1866.e2. [Crossref] [PubMed]
- Kerscher C, Ried M, Hofmann HS, et al. Anaesthetic management of cytoreductive surgery followed by hyperthermic intrathoracic chemotherapy perfusion. J Cardiothorac Surg 2014;9:125. [Crossref] [PubMed]
- Sugarbaker PH, Stuart OA, Eger C. Pharmacokinetics of Hyperthermic Intrathoracic Chemotherapy following Pleurectomy and Decortication. Gastroenterol Res Pract 2012;2012:471205. [Crossref] [PubMed]
- de Bree E, van Ruth S, Schotborgh CE, et al. Limited cardiotoxicity after extensive thoracic surgery and intraoperative hyperthermic intrathoracic chemotherapy with doxorubicin and cisplatin. Ann Surg Oncol 2007;14:3019-26. [Crossref] [PubMed]
- Ratto GB, Civalleri D, Esposito M, et al. Pleural space perfusion with cisplatin in the multimodality treatment of malignant mesothelioma: a feasibility and pharmacokinetic study. J Thorac Cardiovasc Surg 1999;117:759-65. [Crossref] [PubMed]
- Richards WG, Zellos L, Bueno R, et al. Phase I to II study of pleurectomy/decortication and intraoperative intracavitary hyperthermic cisplatin lavage for mesothelioma. J Clin Oncol 2006;24:1561-7. [Crossref] [PubMed]
- Tilleman TR, Richards WG, Zellos L, et al. Extrapleural pneumonectomy followed by intracavitary intraoperative hyperthermic cisplatin with pharmacologic cytoprotection for treatment of malignant pleural mesothelioma: a phase II prospective study. J Thorac Cardiovasc Surg 2009;138:405-11. [Crossref] [PubMed]
- Mehta SS, Bhatt A, Glehen O. Cytoreductive Surgery and Peritonectomy Procedures. Indian J Surg Oncol 2016;7:139-51. [Crossref] [PubMed]
- Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res 1996;82:359-74. [Crossref] [PubMed]
- Harmon RL, Sugarbaker PH. Prognostic indicators in peritoneal carcinomatosis from gastrointestinal cancer. Int Semin Surg Oncol 2005;2:3. [Crossref] [PubMed]


