
Integrated cardiothoracic team approach for complex lung transplantation procedures in Japan—current status and future direction
Introduction
Lung transplantation is an effective treatment option for end-stage lung disease when other medical therapies have failed (1). In Japan, the start of clinical lung transplantation was delayed for approximately 15 years as compared to North America and Europe due to time required for establishment of social consensus regarding the concept of brain death. A transplant law was finally enacted in October 1997 and cadaveric lung transplantation became an officially approved procedure (2). The first living-donor lobar lung transplantation (LDLLT) was conducted at Okayama University in 1998 (3), followed by successful single lung transplants from cadaveric donors conducted at two institutions, Tohoku University and Osaka University, in 2000 (4). Although the number of patients with lung transplantations has increased since 2011 in Japan, the year following enforcement of the revised Organ Transplant law, which permits organ donation from brain-dead donors based on family consent even if the patient has not declared their intention to donate organs or in child cases (2), organ donations from a donor having a status of brain death remain significantly fewer as compared to North America, Europe, and some other Asian countries. By the end of 2021, a total of 658 cadaveric-donor lung transplant, 270 LDLLT, and three combined heart and lung transplant procedures had been performed in Japan, and the outcomes of such cases has thus far been good, with a five-year survival rate greater than 70% for both cadaveric-donor and LDLLT patients (2). Although significantly lagging behind North America and Europe in regard to start and progress of clinical lung transplantation, the unique situation has led to outstanding development of LDLLT in Japan and establishing it as the leading country in regard to living-related clinical transplantation.
A lung transplant is a complex procedure that carries a high risk of perioperative complications, some of which can be fatal, and success is dependent on the involvement of a team comprised of general thoracic surgeons, cardiac surgeons, cardiologists, anesthesiologists, perfusionists, operating room staff, pulmonologists, intensive care physicians, rehabilitation specialists, transplant nurse coordinators, and social workers, with their expertise and different perspectives used in a collaborative effort to ultimately benefit the patient. The technically challenging surgical procedures, appropriate preoperative preparations, optimization of the patient, and selection of a suitable donor, as well as management of postoperative complications are important for the success of lung transplantation, thus utilization of a multidisciplinary team is indispensable. In addition, surgical knowledge, techniques, and patient management related to cardiovascular surgery are necessary for successful lung transplantation, especially in regard to management of mechanical circulatory support (MCS), vascular anastomosis in difficult cases, and concomitant cardiac surgery.
The clinical and surgical skill sets required to complete a lung transplant procedure are quite advanced, and completion of a cardiothoracic transplant fellowship program is recommended before becoming an experienced lung transplant specialist in most other countries. It is not uncommon for general thoracic surgeons in Japan to receive lung transplantation training and gain experience as a surgical trainee at high volume centers in other countries, as it is difficult to receive formal cardiovascular surgery or lung transplantation training in their own country (5). Under the limited number of lung transplants in Japan, whereas quite a few of thoracic surgeons have been trained through the fellowship programs not only in North America but also in Europe, it remains challenging for them to continue to develop their technical proficiency as well as appropriate decision making on MCS options under urgent circumstances. All of these backgrounds contributed to facilitating our unique approach with integrated cardiothoracic expertise and efforts in order to prioritize the optimal outcomes and best benefits for the patients who undergo lung transplantation in Japan.
All lung transplantations conducted at Osaka University, including 70 cadaveric-donor lung transplants (41 single, 29 bilateral), 11 bilateral LDLLTs, and three combined heart and lung transplants, have been performed by an integrated cardiothoracic team. At our institution, those cases have generally been supported by cardiovascular surgeons for establishment of extracorporeal circulation and concomitant cardiac surgery, as well as vascular anastomosis in difficult cases, with the 5- and 10-year survival rates for cadaveric-donor lung transplant recipients 72.1% and 65.4%, respectively. The present review was conducted with focus on the role of cardiac surgeons for advancing lung transplantation outcomes based on our experience.
Integrated cardiothoracic approach to complex lung transplant
Precise MCS management throughout lung transplant process
Precise perioperative MCS management has a pivotal role in lung transplantation. Intraoperative MCS is generally used when the patient is not expected to tolerate single lung ventilation, and allows for management of graft reperfusion flow and low oxygen concentration ventilation (6). Notably, MCS is a key strategy for patients with pulmonary hypertension (PH) to prevent a sudden or further increase in pulmonary artery pressure, which can lead to acute right ventricular failure, and also necessary when performing a lung transplant procedure concomitant with cardiac surgery.
The preference for intraoperative MCS type is dependent on the strategy of the institution performing the procedures. Schwarz et al. reported that cardiopulmonary bypass (CPB) in lung transplantation cases had a greater association with impaired posttransplant results as compared to venoarterial (VA)-extracorporeal membrane oxygenation (ECMO) (7). Ius et al. noted that peripheral ECMO is routinely utilized for intraoperative MCS, in which blood is usually drained from the femoral vein and returned to the femoral artery (8), with advantages including low invasiveness and easy removal, as well as no need for use of a sternotomy or clamshell approach. While thoracic surgeons understand conditions related to an implanted lung and are familiar with management of lung failure, cardiac surgeons are called on to manage intraoperative and postoperative MCS for treating hemodynamic instability and heart failure. Therefore, the MCS strategy should be individualized for each patient to achieve the best possible outcome, and determined based on careful discussion among thoracic and cardiac surgeons.
At our institution, MCS strategy and pump-on timing are generally discussed preoperatively by the lung transplantation team. Based on recipient condition, a CPB has been used for 34 patients and VA-ECMO for 36. A CPB was generally used for those with idiopathic or secondary PH including conditions treated by concomitant cardiac surgery, while an unplanned CPB was performed for an atrial procedure due to bleeding in one case that required intraoperative conversion from ECMO to CPB and in another for removal of atrial thrombus. Theoretically, use of a closed circuit and centrifugal pump for ECMO is less invasive, and also reduces inflammatory responses and the necessity for full heparinization as compared to a CPB. A study that compared ECMO and CPB cases concluded that the former is associated with lower occurrence of primary graft dysfunction (PGD), fewer blood transfusions after a lung transplantation procedure, and a shorter stay in the intensive care unit (8), thus recently we have attempted to use ECMO as much as possible.
Complex vascular anastomosis during lung transplant procedure
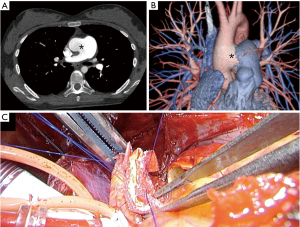
Vascular complications after lung transplantation occur in 1.8% of cases with vascular anastomosis, and are associated with high morbidity and mortality. Donor-recipient size mismatch and surgical technique, as well as twisting, stricture, or thrombosis at an arterial or venous anastomosis site have been cited as causes. In cases with primary anastomosis (PA), the donor PA should be short to prevent kinking, thus the surgeon must identify the location of the first PA branch on the left and truncus branch on the right to properly align the donor PA to that of the recipient, and prevent narrowing of the diameter of the anastomosis. Pulmonary vasculopathy occurs as a result of shear stress due to chronically elevated PA pressure, which includes hypertrophy of the media, intimal fibrosis, and adventitial layer thickening, resulting in PA enlargement. For a patient with a giant PA aneurysm, the PA is inflamed, edematous, and fragile, thus anastomosis is often difficult for thoracic surgeons and generally performed by a cardiac surgeon. In one such case with an atrial septal defect (ASD), the chest was accessed via a clamshell incision and a cardiopulmonary bypass established, while closure of the ASD was performed by a cardiac surgeon. Both hili were carefully developed by a thoracic surgeon, which was challenging due to the presence of a giant pulmonary artery aneurysm (Figure 1A-1C). Furthermore, the PA caliber mismatch between the donor and recipient was excessive, thus vascular anastomosis was performed by the cardiac surgeon using a direct plication method, in which the main PA of the recipient was sutured obliquely and the remaining part was anastomosed. In patients with atrial anastomosis, donor-recipient size mismatch results in different locations between the donor and recipient because of a long left atrial cuff, thus autologous tissue from either the donor or recipient pericardium is used for pulmonary vein anastomosis. Techniques for reconstruction of an inadequate donor left atrial cuff in lung transplantation cases has been reported (9). When the donor left atrial cuff is inadequate for anastomosis after lung procurement, for example, because of a short left atrial cuff length, it is necessary to perform reconstruction with a donor pericardial patch at the back table before placing the donor lung into the chest cavity of the recipient (Figure 1D). Furthermore, early identification of and intervention for a vascular complication can improve the outcome in patients who have received a transplanted lung. Intraoperative assessment using pressure gradient measurement and transoesophageal echocardiography are recommended (10). Current treatments for vascular complications include conservative or medical management, catheter-based intervention, and surgical revision, the latter of which is generally performed by a cardiac surgeon. Furthermore, complex lung transplants from cadaveric donors have been performed by experienced centers, such as an inverted transplantation due to donor shortage and recipient condition (11,12). For those procedures, cardiovascular techniques including complicated vascular anastomosis to complement any position gap between the recipient and donor hilum are necessary.

Concurrent cardiac surgery
Cardiac disease that requires surgical intervention during transplantation may be encountered. Patients with a coexisting cardiac pathology should not be automatically excluded from lung transplantation (13). Recently, there has been remarkable progress in catheter-directed intervention methods, such as percutaneous coronary intervention and aortic valve repair, resulting in an ongoing transformation of transplant candidacy for patients with concurrent coronary artery disease or valvular disease (14). Although it is important to treat any lesions as much as possible before the lung transplantation, concomitant cardiac disease can be corrected by corrective surgery at the time of the transplant, another factor that requires careful preoperative decision-making by a multidisciplinary team including experienced cardiac surgeons. Meng and coworkers presented a meta-analysis of lung transplantation cases that underwent concomitant cardiac surgical procedures (1). No significant difference was noted in regard to mortality rates of patients who underwent lung transplantation with or without concomitant cardiac surgery, though postoperative complication rates were higher in the concomitant group. Long-time use of CPB during lung transplantation for an additional cardiac procedure may be associated with adverse effects on post-transplant outcome (15), and the decision to perform concomitant procedures should be tailored to the clinical condition of each patient. Coronary artery disease is a pathological factor often found in patients with end-stage lung disease. Although several studies have shown that concomitant coronary artery bypass grafting (CABG) does not affect outcome after lung transplantation, patients with irreversible coronary artery disease are excluded from those considered to be recipient candidates in Japan and surgical intervention for coronary artery disease is rarely performed during lung transplantation. Ueyama et al. reported the first beating CABG case performed concomitant with bilateral LDLLT in Japan (16). Therefore, the primary concomitant cardiac procedure performed in lung transplantation cases is generally for congenital heart disease or valvular dysfunction. In congenital heart disease patients with a complex anatomy who are not suitable candidates for surgical repair, an unrepaired intracardiac or extracardiac shunt could lead to a progressive increase in pulmonary vascular resistance and life-threatening PH (17). Lung transplantation with cardiac surgery is an option in select cases that are not responsive to medical treatment for congenital heart disease.
We reported the first case in Japan of Eisenmenger syndrome secondary to an isolated peri-membranous ventricular septal defect (VSD) in a patient who underwent bilateral lung transplantation and closure of the VSD (18). A sequential bilateral lung transplantation procedure was performed, as left ventricular function was preserved and improvement after surgery was expected. At the time of writing, the patient has been alive for more than 15 years. We have also performed bilateral lung transplantation for two patients, one with Eisenmenger syndrome and the other pulmonary artery hypertension concomitant with VSD closure, four with ASD closure, and one with patent foramen ovale closure. In another patient with Eisenmenger syndrome and an aortopulmonary (AP) window defect, that defect was closed by using a flap of the anterior wall of the main pulmonary artery, which was repaired by a cardiac surgeon using a pericardial patch, then followed by bilateral lung transplantation (Figure 2). Our thoracic surgery team generally performs hilar dissection of the recipient lungs as much as possible with or without VA-ECMO before systemic heparinization for CPB, then cardiac surgery is done under CPB by cardiac surgeons before explantation of the native lungs followed by bilateral lung transplantation. All nine of those patients are presently alive (average 7.2 years, range 1.1–20.8 years).
Biventricular management in severe PH cases
PH is a complex heterogeneous set of disease processes that have significant effects on the life of affected patients. Medical therapy has transitioned from upfront use of continuous intravenous prostaglandin administration to combinations of oral medications targeting multiple pathways that underly the disease process (19). Despite many advances, lung transplantation remains the definitive treatment for patients with disease refractory to best medical therapy or with progression. Bilateral lung transplantation is the standard procedure for PH patients, as it is associated with comparable or better results as compared with combined heart and lung transplantation, and the waiting time for lungs alone is generally shorter than that for both heart and lungs (20). Tricuspid valve repair (TVR) concomitant with double-lung transplantation is a surgical option for PH patients with severe tricuspid regurgitation and right ventricle (RV) dysfunction. A previous study noted that functional tricuspid regurgitation (TR) is found in most patients with severe PH, and annular dilatation and altered RV geometry are important factors in the pathogenesis of tricuspid regurgitation development resulting in RV dysfunction (21). Moderate to severe RV dysfunction is an independent risk factor for primary graft failure after double lung transplantation for severe PH, thus TVR can potentially accelerate cardiac adaptation and recovery from severe RV dysfunction, leading to prevention of PGD.
At our institution, seventeen patients with idiopathic pulmonary arterial hypertension (iPAH) have undergone bilateral lung transplantation, with TVR performed in two for severe TR. Postoperative care of PH transplant patients poses a new set of challenges because the lung physiology normalizes with immediate reductions of pulmonary vascular resistance and left ventricular filling pressure, and increase in cardiac output, which can then unmask left-sided dysfunction. In two prior reports, nearly all PH transplant patients received post-operative continuation of extracorporeal circulation such as with VA-ECMO to allow for controlled normalization of cardiac hemodynamics and accommodation, and showed large reductions in the incidence of both PGD and early mortality (22,23). In our series of iPAH cases, ten of the seventeen patients required post-operative VA-ECMO. Several groups have recently demonstrated that pulmonary edema occurring in PGD cases is primarily related to diastolic dysfunction of the left ventricle rather than right ventricle stress (8). Adaptation of the left ventricle to the new hemodynamics may require a longer duration of VA-ECMO, which can lead to excellent results by allowing for controlled filling and recovery of the left ventricle. We consider that not only preoperative careful surgical planning, but also operative and postoperative discussion with the cardiac surgery team is very important when performing lung transplantation, especially in patients with PH.
Development of LDLLT
A shortage of lung donors is a major obstacle, especially in Japan, where the average waiting time is greater than 900 days, resulting in a considerable number of deaths of individuals on the waiting list. Under such circumstances, an LDLLT has been performed as a life-saving procedure for critically ill patients who are unlikely to survive the long wait for a cadaveric lung (2). In addition, complex procedures such as living-donor segmental lung transplantation have been developed by experienced Japanese centers (24). Although LDLLT patients are generally in a worse preoperative condition than those scheduled for a cadaveric-donor lung transplant, the survival rates of these are similar. In LDLLT cases, the graft lungs are usually small, thus vascular complications can be lethal (25). Various techniques for anastomosis of the PA in cases with a size mismatch, such as plication for the PA of the recipient and autopericardial patching for that of the donor, have been reported by experienced centers in Japan (26). Although vascular anastomosis is performed by thoracic surgeons in most lung transplant centers in Japan for a standard LDLLT, at our institution when the PA caliber mismatch is too great for performing anastomosis of the PA, such vascular anastomosis is performed by cardiac surgeons to prevent a vascular complication. In addition, donor safety is one of the most important issues facing living-donor lobar lung transplantation. No perioperative death of a donor for LDLLT has been reported, though a relatively high rate of morbidity after a lobectomy has been noted. Pulmonary arteries are the most complex when determining the dividing lines, because of their variations in branching (25). When a complex pulmonary arterioplasty for the remaining arterial stump of the donor is necessary, that is performed at our institution with an autopericardial graft with support provided by a cardiac surgeon to avoid vascular complications.
Combined heart and lung transplantation
We previously reported the first case of successful combined heart and lung transplantation performed in Japan (27). Congenital heart disease with irreversible PH including Eisenmenger Syndrome is the most frequent indication for heart-lung transplantation. Combined heart and lung transplantation for a patient with a double outlet right ventricle and PH (Eisenmenger syndrome), and two patients with restrictive cardiomyopathy with PH have been performed at our institution. During the recipient operation, the diseased heart and lungs were removed under CPB in collaboration with thoracic and cardiac surgeons, followed by dissection around the main trunk of the trachea up to above the bifurcation, along with meticulous hemostasis of the many collateral vessels at the posterior mediastinum by thoracic surgeons. Both donor lungs were placed through the dorsal side of both phrenic nerves, then anastomosis of the main trunk of the trachea was performed using a running suture for the membranous portion and figure-of-eight sutures for the cartilaginous portion by thoracic surgeons, followed by anastomosis of aorta and inferior vena cava by cardiac surgeons. Next, the aortic cross-clamp was released by de-airing via the root vent, followed by anastomosis of the superior vena cava. A cold ischemia time greater than four hours is associated with poor prognosis for heart transplantation and the allograft ischemic time should be less (28), thus teamwork between thoracic and cardiac surgeons is a key factor for successful combined heart and lung transplantation. Furthermore, a favorable operative outcome is completely dependent on achieving ideal hemostasis, as the two major causes of mortality in heart-lung transplantation cases are massive bleeding and graft failure (29). Therefore, after confirmation of sufficient heart contraction as well as ventilation for both lungs, the CPB is weaned off, and additional hemostasis performed by thoracic and cardiac surgeons following a protamine infusion. In all three of our patients, heart-lung transplantation was successfully performed, the postoperative courses were uneventful, and each patient is alive at the time of writing for 6.4, 8.7, and 13.8 years.
Future directions
Complex lung transplant surgery and management in Japan are performed by use of an integrated cardiothoracic team approach, which has led to a synergistic impact on successful lung transplantation cases by capitalizing greatly on the experiences, techniques, and expertise of cardiac and thoracic experts (Figure 3). Nevertheless, there is a need to provide opportunity to young Japanese thoracic surgeons to obtain expertise in cardiovascular surgery so that they can fully understand the key skills of cardiac surgeons required for complex lung transplantation cases. The unique situation in Japan has led to development of LDLLT and it is considered that thoracic surgeons can benefit from learning about LDLLT procedures used at experienced lung transplant centers. Additionally, it is obviously important for them to communicate and share experiences with high volume lung transplant centers in other countries.
Acknowledgments
The authors express their sincere appreciation to Professor Norihisa Shigemura from Division of Cardiovascular Surgery, Temple University and Lewis Katz School of Medicine for the useful discussions. We would like to thank Intermed (www.intermed-jp.com/) for English language editing.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Masaaki Sato) for the series “Why is the Outcome Good? Secrets of Lung Transplantation in Japan” published in the Journal of Thoracic Disease. The article has undergone external peer review.
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1720/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1720/coif). The special series “Why is the Outcome Good? Secrets of Lung Transplantation in Japan” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work, ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Meng E, Jiang SM, Servito T, et al. Lung transplantation and concomitant cardiac surgical procedures: A systematic review and meta-analysis. J Card Surg 2022;37:3342-52. [Crossref] [PubMed]
- Date H. Current status and problems of lung transplantation in Japan. J Thorac Dis 2016;8:S631-6. [Crossref] [PubMed]
- Date H, Sato M, Aoyama A, et al. Living-donor lobar lung transplantation provides similar survival to cadaveric lung transplantation even for very ill patients†. Eur J Cardiothorac Surg 2015;47:967-73. [Crossref] [PubMed]
- Miyoshi S, Minami M, Ohta M, et al. Single lung transplantation from a brain-dead donor for a patient with idiopathic pulmonary fibrosis. A breakthrough after new legislation in Japan. Jpn J Thorac Cardiovasc Surg 2001;49:398-403. [Crossref] [PubMed]
- Ikeda N, Asamura H, Chida M. Training program of general thoracic surgery in Japan: Present status and future tasks. J Thorac Cardiovasc Surg 2022;163:353-8. [Crossref] [PubMed]
- Taka H, Miyoshi K, Kurosaki T, et al. Lung transplantation via cardiopulmonary bypass: excellent survival outcomes from extended criteria donors. Gen Thorac Cardiovasc Surg 2019;67:624-32. [Crossref] [PubMed]
- Schwarz S, Hoetzenecker K, Klepetko W. Procedural mechanical support for lung transplantation. Curr Opin Organ Transplant 2021;26:309-13. [Crossref] [PubMed]
- Ius F, Aburahma K, Boethig D, et al. Long-term outcomes after intraoperative extracorporeal membrane oxygenation during lung transplantation. J Heart Lung Transplant 2020;39:915-25. [Crossref] [PubMed]
- Oto T, Rabinov M, Negri J, et al. Techniques of reconstruction for inadequate donor left atrial cuff in lung transplantation. Ann Thorac Surg 2006;81:1199-204. [Crossref] [PubMed]
- Felten ML, Michel-Cherqui M, Sage E, et al. Transesophageal and contact ultrasound echographic assessments of pulmonary vessels in bilateral lung transplantation. Ann Thorac Surg 2012;93:1094-100. [Crossref] [PubMed]
- Chida M, Araki O, Karube Y, et al. Right-to-left inverted single lung transplantation. JTCVS Tech 2020;4:395-7. [Crossref] [PubMed]
- Yamamoto H, Miyoshi K, Otani S, et al. Right single lung transplantation using an inverted left donor lung: interposition of pericardial conduit for pulmonary venous anastomosis - a case report. BMC Pulm Med 2020;20:46. [Crossref] [PubMed]
- Scheinin SA, Singh G, Loebe M. Adding complexity to complexity: The role of concomitant cardiac surgery in lung transplantation. J Card Surg 2022;37:3353-4. [Crossref] [PubMed]
- Wallen TJ, Arnaoutakis GJ, Beaver T, et al. Successful bridge to lung transplantation with transcatheter aortic valve replacement. Am J Transplant 2020;20:3658-61. [Crossref] [PubMed]
- Biniwale R, Ross D, Iyengar A, et al. Lung transplantation and concomitant cardiac surgery: Is it justified? J Thorac Cardiovasc Surg 2016;151:560-6. [Crossref] [PubMed]
- Ueyama K, Miyahara S, Ide Y, et al. On-pump beating CABG concomitant with bilateral living-donor lobar lung transplantation. Heart Lung 2019;48:166-8. [Crossref] [PubMed]
- Diller GP, Körten MA, Bauer UM, et al. Current therapy and outcome of Eisenmenger syndrome: data of the German National Register for congenital heart defects. Eur Heart J 2016;37:1449-55. [Crossref] [PubMed]
- Inoue M, Minami M, Fukushima N, et al. Bilateral lung transplantation with closure of ventricular septal defect in a patient with Eisenmenger syndrome. Gen Thorac Cardiovasc Surg 2010;58:25-8; discussion 29. [Crossref] [PubMed]
- Hwalek A, Rosenheck JP, Whitson BA. Lung transplantation for pulmonary hypertension. J Thorac Dis 2021;13:6708-16. [Crossref] [PubMed]
- de Perrot M, Granton JT, McRae K, et al. Outcome of patients with pulmonary arterial hypertension referred for lung transplantation: a 14-year single-center experience. J Thorac Cardiovasc Surg 2012;143:910-8. [Crossref] [PubMed]
- Shigemura N, Sareyyupoglu B, Bhama J, et al. Combining tricuspid valve repair with double lung transplantation in patients with severe pulmonary hypertension, tricuspid regurgitation, and right ventricular dysfunction. Chest 2011;140:1033-9. [Crossref] [PubMed]
- Tudorache I, Sommer W, Kühn C, et al. Lung transplantation for severe pulmonary hypertension--awake extracorporeal membrane oxygenation for postoperative left ventricular remodelling. Transplantation 2015;99:451-8. [Crossref] [PubMed]
- Hoetzenecker K, Schwarz S, Muckenhuber M, et al. Intraoperative extracorporeal membrane oxygenation and the possibility of postoperative prolongation improve survival in bilateral lung transplantation. J Thorac Cardiovasc Surg 2018;155:2193-2206.e3. [Crossref] [PubMed]
- Nakajima D, Tanaka S, Ikeda T, et al. Living-donor segmental lung transplantation for pediatric patients. J Thorac Cardiovasc Surg 2023;165:2193-201. [Crossref] [PubMed]
- Chen F, Miwa S, Bando T, et al. Pulmonary arterioplasty for the remaining arterial stump of the donor and the arterial cuff of the donor graft in living-donor lobar lung transplantation. Eur J Cardiothorac Surg 2012;42:e138-9. [Crossref] [PubMed]
- Yokoyama Y, Chen-Yoshikawa TF, Nakajima D, et al. Various techniques for anastomosis of pulmonary arteries with size mismatch during lung transplantation. JTCVS Tech 2021;9:192-4. [Crossref] [PubMed]
- Sawa Y, Matsumiya G, Shigemura S, et al. First successful heart-lung transplantation in Japan: report of a case. Surg Today 2013;43:1461-6. [Crossref] [PubMed]
- Shudo Y, Leipzig M, He H, et al. Combined Heart-Lung Transplantation Outcomes in Asian Populations: National Database Analysis. JACC Asia 2022;2:504-12. [Crossref] [PubMed]
- Stoica SC, McNeil KD, Perreas K, et al. Heart-lung transplantation for Eisenmenger syndrome: early and long-term results. Ann Thorac Surg 2001;72:1887-91. [Crossref] [PubMed]



