
PD-1 inhibitors-associated myocarditis in non-small cell lung cancer patients
Highlight box
Key findings
• Factors associated with immune checkpoint inhibitor (ICI)-mediated myocarditis include a history of smoking, underlying liver or kidney disease, and previous use of beta-blockers, angiotensin-converting enzyme inhibitor or angiotensin receptor blocker.
• Routine monitoring of cardiac troponin I level and electrocardiogram could contribute to early diagnosis of ICI-associated myocarditis.
• Steroid pulse therapy supplemented by immunoglobulin is essential for severe ICI-associated myocarditis.
What is known and what is new?
• The administration of ICI would cause a rare but fatal myocarditis, with an incidence of 1.14% and a mortality of 46%.
• We reported risk factors, clinical features, laboratory examinations, imaging findings, and management of ICI-associated myocarditis.
What is the implication, and what should change now?
• Earlier identification and treatment are essential for managing myocarditis caused by programmed cell death protein 1 inhibitors.
Introduction
Immunotherapy has currently been the first-line treatment for non-small cell lung cancer (NSCLC). Compared with radiotherapy, chemotherapy, and molecular targeted therapy, immunotherapy has the advantages of potent antitumor activity and long-lasting effects. As of September 2019, a total of 2,975 immune checkpoint inhibitors (ICIs) clinical trials were in progress, with an increase of 97.5% compared with 2017 (1). Although ICI represents a major advance in the treatment of NSCLC, it might lead to a wide range of immune-related adverse events (irAEs), including the toxicities of multiple organs such as the liver, intestinal tract, skin, thyroid, and pituitary (2). It is difficult to predict the certain location and moment before the onset of irAEs. Therefore, the diagnosis and management of irAEs are still challenging (3,4). Recently, emerging case reports have raised awareness of ICI-associated severe myocarditis, which is reported to be a rare but life-threatening complication (5,6). Although the incidence of ICI-related myocarditis (1.14%) was lower than other irAEs, the mortality rate of ICI-associated myocarditis is up to 46% (5,6). Therefore, it is necessary to better characterize ICI-associated myocarditis, including its early diagnosis indicators and management.
In this retrospective study, 14 cases were observed to have their symptoms relieved by traditional glucocorticoid immunomodulation and supportive therapy after ICI-mediated myocarditis. In this article, we detail the clinical manifestations, biochemical indicators, imaging findings, and treatment of myocarditis, aiming to provide references for early diagnosis and treatment of ICI-associated myocarditis by discussing the successful practical experience. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-596/rc).
Methods
Patients
A retrospective case-control study was conducted to clarify the clinical features of myocarditis. From June 2019 to January 2021, among 673 NSCLC patients treated with programmed cell death protein 1 (PD-1) inhibitors therapy, a total of 14 patients who developed myocarditis were included, including patients transferred to our hospital because of severe cardiotoxicity. Diagnosis criteria of ICI-associated myocarditis were represented in Supplementary file (Appendix 1). In comparison with 14 ICI-associated myocarditis cases, 45 NSCLC controls who used PD-1 inhibitors but did not develop cardiotoxicity in our hospital were randomly selected to be included in the study. Control subjects were evenly matched to myocarditis cases on age, sex ratio, and tumor staging. All cases and controls were from the First Affiliated Hospital of Guangzhou Medical University. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was reviewed and approved by the Medical Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University, Guangzhou, Guangdong, China (approval No. 202280). Individual consent for this retrospective analysis was waived.
Clinical data information
Patients with advanced NSCLC were recommended for first-line PD-1 therapy. During ICI treatment, baseline data were regularly monitored every 2 to 4 weeks. In order to detect cardiotoxicity as soon as possible, physical examination, electrocardiogram (ECG), and biochemical indicators monitoring were routine examinations. Close attention was paid to clinical manifestations of suspected myocarditis, such as elevated troponin, chest pain, dyspnea, muscle weakness, palpitations, or malignant arrhythmias. Patients with abnormalities were admitted to our hospital for examination. The first-line evaluation was arranged as soon as possible, including immediate cardiology/cardio-oncology consultation, ECG/24 h ambulatory ECG, transthoracic echocardiogram (TTE), chest X-ray, and biomarkers such as myocardial injury indicators, lymphocytes, and cytokines. Moreover, advanced diagnostic tests were performed on patients whose myocarditis-related abnormalities were identified in the above assessment. The possibility of coronary artery disease was excluded by coronary arteriography. Cardiac magnetic resonance (CMR) was recommended to be implemented as fast as possible, and endomyocardial biopsy (EMB) was further performed if the diagnosis was in doubt. The individualized treatment plan was adopted according to the grade of cardiotoxicity, and attention was paid to giving life support and preventing side effects during medication. To observe the clinical outcome and decide whether to restart ICI treatment, follow-up was performed once myocarditis occurred.
Statistical analysis
Continuous variables are summarized as mean ± standard deviation or median (interquartile range), and categorical variables are expressed as percentages. The continuous variables of cases and controls were compared using the unpaired Student’s t-tests or Wilcoxon rank-sum tests, and the categorical variables were tested by chi-square test or Fisher’s exact test. Paired-sample t-test was used to compare biomarkers levels at different times. Variables with significance were included in the binary multivariate logistic regression. All statistical tests were 2-sided, and 5% was set as the level of significance. Statistical analyses were performed using IBM SPSS Statistics version 26 (RRID: SCR_019096). The vertical bar charts and heat maps were drawn by GraphPad Prism software version 8.0 (RRID: SCR_002798), and the horizontal bar charts and pie charts were drawn by Microsoft Excel (RRID: SCR_016137).
Results
Patient characteristics
Baseline characteristics of patients are described in Table 1. The mean age of ICI-associated myocarditis cases (n=14) was 60.43±14.10 years, with 71.4% being male. In comparison with controls, myocarditis patients had a higher prevalence of liver and kidney disease, and previous use of beta-blockers and angiotensin-converting enzyme inhibitors or angiotensin receptor blockers (ACEI or ARB) was more common (Table 1). Logistic regression including the above factors shows that liver disease [odd ratio (OR): 20.40, 95% confidence interval (95% CI): 3.20–130.13, P=0.001] and previous use of beta-blockers (OR: 16.46, 95% CI: 1.05–258.96, P=0.046) are independently associated with ICI-associated myocarditis (Table 2).
Table 1
| Variables | Myocarditis (n=14) | Control (n=45) | P value |
|---|---|---|---|
| Age at start of ICI (years) | 60.43±14.10 | 61.4±11.20 | 0.791 |
| Body mass index (kg/m2) | 22.27±4.42 | 22.65±3.22 | 0.742 |
| Male | 10 (71.4) | 38 (84.4) | 0.484 |
| CV risk factors | |||
| Current or prior smoking | 3 (21.4) | 25 (55.6) | 0.026* |
| Hypertension | 3 (21.4) | 5 (11.1) | 0.591 |
| Diabetes mellitus | 2 (14.3) | 2 (4.4) | 0.236 |
| Hyperlipidemia | 1 (7.1) | 2 (4.4) | 0.564 |
| No CV risk factors | 9 (64.3) | 18 (40.0) | 0.111 |
| Underling disease | |||
| Heart disease | 1 (7.1) | 3 (6.7) | 1.000 |
| Pulmonary disease | 7 (50.0) | 10 (26.7) | 0.096 |
| Liver disease | 7 (50.0) | 3 (6.7) | 0.001* |
| Kidney disease | 4 (28.6) | 1 (2.2) | 0.011* |
| Pre-ICI CV medications | |||
| Statin | 1 (7.1) | 2 (4.4) | 0.564 |
| Aspirin | 1 (7.1) | 1 (2.2) | 0.421 |
| Beta-blockers | 5 (35.7) | 1 (2.2) | 0.002* |
| ACEI or ARB | 4 (28.6) | 2 (4.4) | 0.036* |
| Calcium-channel blockers | 3 (21.4) | 4 (8.8) | 0.427 |
| Primary cancer type | |||
| Non-small cell lung cancer | 14 (100.0) | 42 (93.3) | 1.000 |
| Small cell lung cancer | 0 (0.0) | 2 (4.4) | 1.000 |
| Other | 0 (0.0) | 1 (2.2) | 1.000 |
| Tumor staging | |||
| III | 6 (42.9) | 16 (35.6) | 0.622 |
| IV | 8 (57.1) | 29 (64.4) | 0.622 |
| Prior cancer treatment | |||
| Chemotherapy | 10 (71.4) | 26 (57.8) | 0.360 |
| Radiation | 2 (14.3) | 3 (6.7) | 0.730 |
| VEGF inhibitors | 7 (50.0) | 10 (22.2) | 0.096 |
| EGFR inhibitors | 0 (0.0) | 1 (2.2) | 1.000 |
| Surgery | 4 (28.6) | 8 (17.8) | 0.620 |
| Overall types of PD-1 inhibitors | |||
| Sintilimab | 5 (35.7) | 19 (42.2) | 0.665 |
| Pembrolizumab | 6 (42.9) | 9 (20.0) | 0.173 |
| Tislelizumab | 2 (14.3) | 6 (13.3) | 1.000 |
| Camrelizumab | 1 (7.1) | 5 (11.1) | 1.000 |
| Toripalimab | 1 (7.1) | 4 (8.9) | 1.000 |
| Nivolumab | 0 (0.0) | 2 (4.4) | 1.000 |
| Single agent vs. combined | |||
| Single PD-1 inhibitors | 3 (21.4) | 3 (6.7) | 0.276 |
| PD-1 inhibitors plus chemotherapy | 8 (57.1) | 31 (68.9) | 0.626 |
| PD-1 inhibitors plus VEGF inhibitors | 1 (7.1) | 3 (6.7) | 1.000 |
Values are mean ± SD or n (%). *, P<0.05. ICI, immune checkpoint inhibitor; CV, cardiovascular; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; VEGF, vascular endothelial growth factor; EGFR, epidermal growth factor receptor; PD-1, programmed cell death protein 1; SD, standard deviation.
Table 2
| Variables | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| History of smoking | 0.27 (0.066–1.134) | 0.074 | – | – | |
| Liver disease | 13.00 (2.70–62.72) | 0.001* | 20.40 (3.20–130.13) | 0.001* | |
| Kidney disease | 8.00 (1.28–50.04) | 0.026* | 7.60 (0.64–90.76) | 0.109 | |
| Previous use of beta-blockers | 22.79 (2.37–219.38) | 0.007* | 16.46 (1.05–258.96) | 0.046* | |
| Previous use of ACEI or ARB | 8.00 (1.28–50.04) | 0.026* | 2.45 (0.14–42.40) | 0.538 | |
*, P<0.05. OR, odd ratio; CI, confidence interval; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Cancer and treatment characteristics
A total of 14 NSCLC patients developed immune-associated myocarditis during PD-1 medication monitoring, including adenocarcinoma (n=7), large cell carcinoma (n=4), and squamous cell carcinoma (n=3). Compared with controls, 64% of myocarditis cases had experienced other concurrent irAEs, with a higher incidence of myositis (50%) and peripheral neuritis (35.7%, Table 1). There were no statistically significant differences in characteristics between cases and controls, including prior cancer treatment, type of PD-1 inhibitors, and prevalence of other ICI-related side effects (Table 1).
Monitoring of myocarditis
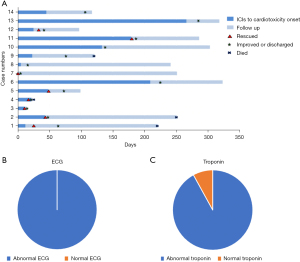
The median time from the initiation of ICI to the onset of myocarditis was 34 days (interquartile range, 12 to 146 days). Approximately 80% of cases developed myocarditis within 3 months after ICI therapy, most of which develop after the first dose of PD-1 inhibitors. Shortness of breath and fatigue were the most common symptoms in patients with myocarditis (Table 3). Besides, we have observed that immune-associated myocarditis was usually fulminant, with rapid deterioration in 57% of patients after admission, leading to disordered vital signs. The course of disease and characteristics of troponin and electrocardiogram of patients with ICI-associated myocarditis are shown in Figure 1.
Table 3
| Variables | Myocarditis (n=14) |
|---|---|
| Clinical presentation | |
| No symptoms | 1 (7.1) |
| Shortness of breath | 9 (64.3) |
| Fatigue | 7 (50.0) |
| Myalgia | 6 (42.9) |
| Chest pain | 5 (35.7) |
| Edema | 5 (35.7) |
| ECG | |
| Performed | 14 |
| T wave or ST segment abnormality | 11 (78.6) |
| Arrhythmia | 8 (57.1) |
| Conduction block | 5 (35.7) |
| QRS interval (ms) | 179±128 |
| QTc interval (ms) | 406±131 |
| TTE | |
| Performed | 14 |
| Pericardial effusion | 5 (35.7) |
| Increased systolic pulmonary artery pressure | 5 (35.7) |
| Left ventricular systolic dysfunction (LVEF ≤50%)† | 1 (7.7) |
| LVEF by TTE (%) | 69.2±7.8 |
| LVDd (mm) | 46.2±4.4 |
| CMR | |
| Performed | 4 |
| Pleural effusion | 1 (25.0) |
| Myocardial signal increase | 2 (50.0) |
| Patchy abnormal enhancement shadow | 1 (25.0) |
| LVEF by CMR (%) | 72.7±1.2 |
| LVEDV (mL) | 54.2±9.9 |
| LVESV (mL) | 14.7±2.0 |
| SV (mL) | 39.5±7.9 |
| EMB | |
| Performed | 1 |
| T lymphocyte infiltration | 1 (100.0) |
Values are mean ± SD or n (%). †, 13 of the 14 myocarditis patients had this information. ECG, electrocardiogram; ST, ST segment; QRS, QRS wave; QTc, QT interval corrected for heart rate; TTE, transthoracic echocardiography; LVEF, left ventricular ejection fraction; LVDd, left ventricular end diastolic dimension; CMR, cardiac magnetic resonance; LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; SV, stroke volume; EMB, endomyocardial biopsy; SD, standard deviation.
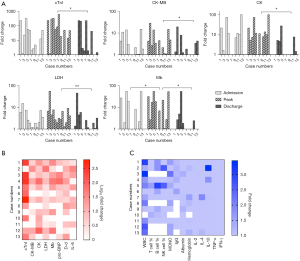
Biochemical indicators characteristics of myocarditis
Histograms show fluctuations in levels of five biomarkers of myocardial infarction from admission to discharge for each patient (Figure 2A). A measure of cardiac troponin I (cTnI) was available in all myocarditis patients and cTnI levels were elevated in 92%, with a peak median value of 10 µg/L (interquartile range, 1.4 to 23.3 µg/L). The abnormal increase of cTnI was considerably obvious in patients with severe myocarditis, which could reach 653 times the normal value (normal range of cTnI, 0–0.4 µg/L). Due to early diagnosis and prompt treatment, discharge cTnI levels decreased significantly compared to peak cTnI (median value: 1.8 vs. 10.0 µg/L, P=0.002). Moderate increases were also observed in other myocardial infarction biomarkers, including creatine kinase (CK), CK-MB, lactic dehydrogenase (LDH), and myoglobin (Mb) (Figure 2A). Besides, inflammatory cells and cytokines showed mildly abnormal or even normal (Figure 2B,2C). Interleukin-6 (IL-6) in 9 of 12 (64%) patients were elevated, with a median value of 45.8 pg/mL (interquartile range, 5.9 to 95.8 pg/mL).
Imaging characteristics of myocarditis
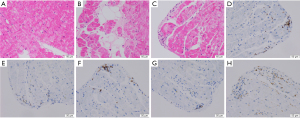
In accordance with the previous diagnostic recommendations (7,8), a series of clinical imaging or other auxiliary examinations have been performed (Table 3). All patients with myocarditis showed abnormal ECG with various manifestations. The most common ECG abnormalities noted in myocarditis patients consisted of T wave or ST segment abnormality (78.6%) and arrhythmia (57.1%). Most notably, patient #13 developed an abnormal ECG 219 days before the diagnosis of myocarditis, which was characterized by malignant arrhythmias. Ninety-three percent of cases showed abnormal echocardiogram, presenting as pericardial effusion and increased systolic pulmonary artery pressure (Table 3). Only 1 patient (7.7%) experienced a reduced left ventricular ejection fraction (LVEF, ≤50%, Table 3). Overall, 4 patients had a CMR study with 25% showing pleural effusion or abnormal enhancement shadow. One case underwent EMB after stable condition. Histology revealed a small number of lymphocytes infiltrated around individual small vessels within the myocardium (Figure 3).
Diagnosis and treatment of myocarditis
With reference to the previously proposed definition (7,9) and comprehensive experience, the diagnosis of myocarditis in 14 patients was clarified, including 3 definite, 6 probable, and 5 possible ICI-associated myocarditis (Table 4).
Table 4
| Variables | Myocarditis (n=14) |
|---|---|
| Myocarditis diagnosis | |
| Definite | 3 (21.4) |
| Probable | 6 (42.9) |
| Possible | 5 (35.7) |
| Corticosteroids | 14 (100.0) |
| Time from first irAE to corticosteroids (days)† | 1 [0–2] |
| Intravenous immunoglobulin | 9 (64.3) |
| Supportive therapy | |
| Mechanical ventilation | 4 (28.6) |
| Temporary or permanent pacemakers | 3 (21.4) |
| Inotropic agents or vasopressors | 10 (71.4) |
| Diuretics | 8 (57.1) |
| Beta-blockers | 4 (28.6) |
| Angiotensin-converting enzyme inhibitors | 3 (21.4) |
| Treatment outcome | |
| Death from cardiovascular cause | 1 (7.1) |
| Lost to follow-up | 1 (7.1) |
Values are median [IQR] or n (%). †, 13 of the 14 myocarditis patients had this information. irAE, immune-related adverse event; IQR, interquartile range.
All patients with myocarditis had ICI permanently discontinued and were treated with steroids as initial treatment. The median time from the onset of myocarditis to steroid initiation was 1 day (interquartile range, 0 to 2 days). The response rate to steroids was 76.9%. Patients #1, 2, and 3 received insufficient glucocorticoids (80–330 mg/d), leading to a poor prognosis. Therefore, the dose of methylprednisolone was subsequently adjusted to 0.5 mg/kg/d to treat mild ICI-related myocarditis (grade 1–2); severe myocarditis (grade 3) was treated with 1–2 mg/kg/d of steroids; fulminant myocarditis (grade 4) was treated with 1 g/d for 3–5 days. Meanwhile, 64% of cases were supplemented with intravenous gamma globulin on the basis of early glucocorticoid therapy, with a dose of 5–20 g/d (Table 4). Approximately all myocarditis cases had received appropriate cardiac support including mechanical circulatory support, inotropic and other cardiovascular drugs, and management of malignant arrhythmia including temporary or permanent pacemakers (Table 4). Finally, 12 patients successfully relieved the symptoms and maintained stable vital signs, 1 patient died of cardiovascular cause, and 1 patient lost follow-up after self-discharge. The mortality of ICI-associated myocarditis was 7.1%. Individual characteristics and case summaries of patients with myocarditis are presented in Table S1.
Discussion
ICI therapy is a breakthrough in cancer treatment. This retrospective case-control study provided a comprehensive clinical description of ICI-associated myocarditis. The results from this practice suggest two important findings. The first is to clarify how to identify patients with ICI-related myocarditis in the early stage. Early detection of abnormal ECG and elevated cTnI may have prognostic significance. The second important finding was that early use of high-dose steroids supplemented by immunoglobulin has been proven effective.
The risk factors for ICI-associated myocarditis are not well characterized. In our study, renal disease, liver disease, and previous use of beta-blockers, ACEI, or ARB were correlated with ICI-related myocarditis, among which liver disease and previous use of beta-blockers were independent risk factors for ICI-associated myocarditis. ICI combination, diabetes, and obesity are identified as the independent risk factors for ICI cardiotoxicity in an international registry (10). With regard to other potential risk factors, it has been reported that abnormal creatinine and aspartate transaminase (AST) (suggesting renal and hepatic dysfunction) are risk factors for fulminant myocarditis (11). It has been reported that patients with liver cirrhosis had higher risks of ICI-associated major adverse cardiovascular events (12). Likewise, renal dysfunction is considered to have a major impact on the progression of fulminant myocarditis (13). Therefore, monitoring renal and liver function may be critical in the early identification of ICI-related myocarditis and the effective management of fulminant myocarditis. Besides, our myocarditis cases tended to be more likely to have prior use of ACEI or ARB, which was consistent with a multicentre international registry in which the interpretation is based upon proportional analysis rather than multivariate regression analysis (14). Since beta-blockers, ACEI, and ARB are all cardiovascular drugs, it is suggested that cardiovascular disease may be related to ICI-associated myocarditis. In a retrospective study discussing the relationship between cardiovascular factors and ICI-associated myocarditis, history of heart failure and history of acute coronary syndrome help identify patients at higher risk of ICI-induced myocarditis (15). In a meta-analysis, all cases that experienced cardiovascular irAEs presented cardiovascular risk factors (16). However, our study did not show a difference in previous heart history between the myocarditis and control groups.
The onset time of cardiotoxic symptoms depends on the anamnesis, type of ICI, duration of medication, and single or combination medication (17). Several studies have shown that ICI-associated myocarditis occurs in the early stage, with a median time of 1–2 months, most of which occur within 3 months after the start of ICI treatment (17,18). It is worth noting that the absence of myocarditis or other irAEs in short term cannot ensure stable safety for immunotherapy, because myocarditis has also been reported to occur after nearly 20 months (6). The time to ICI-associated myocarditis onset varied widely in our cases, ranging from 1 to 266 days, with up to 29% of cases exceeding 3 months. Therefore, careful monitoring should begin immediately after PD-1 inhibitors administration and patients who have not yet experienced any side effects should also be evaluated every 4–6 weeks.
ICI-related myocarditis was usually accompanied by myositis and peripheral neuritis in our cases. Neuromuscular side effects usually begin as mild manifestations, with 77% of myositis patients having elevated CK and 43% having muscle weakness or myalgia. A case series report also indicated that myositis has a certain overlap with myocarditis and neuropathy, and neuromuscular side effects resulted in sequelae in at least one-third of the patients and were fatal in 5% of cases (19). Therefore, the possibility of myocarditis and other serious sequelae should be considered in patients with asymptomatic CK elevation and myositis symptoms. In addition, 29% of advanced NSCLC patients with myocarditis were complicated with pneumonia, which was more prone to respiratory failure and required close attention and adequate support to the respiratory and circulatory systems. Thirty-six percent of patients had pericardial effusion. It has been described that ICI can cause recurrent pericardial and pleural effusion. Therefore, the presence of new or enlarged pericardial effusion should raise suspicion of ICI-related myocarditis.
In the examination of biomarkers in patients with ICI-related myocarditis, cTnI levels were elevated in 92% of patients. In addition, an increase in CK, CK-MB, LDH, and Mb was also observed. However, it is worth noting that mild to large increases in serum CK and Mb are usually found in patients with ICI-related myositis. LDH is widely present in various tissues, and its elevation can be seen in various clinical situations. Considering the above, cTnI is the most reliable predictive biomarker of myocarditis in the early stage supplemented by CK-MB. It has been reported that higher cTnI and CK-MB levels are related to severe myocarditis and increased mortality among patients with myocarditis (20). Therefore, when patients have abnormally elevated troponin level, the risk of ICI-associated myocarditis should be noted and cardiac evaluation should be performed as soon as possible. If available, we recommend troponin monitoring both at baseline and after initiation of PD-1 inhibitors. However, we have observed that some patients had very high levels of troponin but ultimately achieved excellent results. The reason may be that the release of troponin in myocarditis is caused by increased permeability of the myocardial cell membrane and troponin level in acute myocarditis may be more affected by the measurement time compared with the severity of myocardial injury and dysfunction (21), or that despite high troponin levels in patients with myocarditis, adequate steroids can relieve symptoms of myocarditis and reduce myocardial enzyme levels. Moreover, ICI-associated myocarditis can also cause an increase in cytokines, which may suggest a strong positive correlation between the severity of the myocardial injury and the levels of cytokines. IL-6 levels increased in 64% of patients, and the levels of IL-4 and IL-10 slightly increased in 21% of patients, coupled with abnormal immune cells, suggesting a state of inflammatory activation. Since the cytokine IL-6 is the earliest and most highly sensitive indicator of inflammation, the significantly increased level of IL-6 can be used for early warning of severe irAEs. Although the research on the mechanism of PD-1-associated inflammatory storms needs to be further improved, this prompts us to be more alert to inflammatory factor storms once ICI-associated myocarditis occurs, otherwise it will eventually lead to various organ failures.
In our cases, all ICI-related myocarditis showed ECG abnormalities after the development of myocarditis. It is worth noting that patient #13 developed an abnormal ECG with arrhythmia 47 days and was diagnosed with myocarditis after 266 days after ICI initiation. Hence, we speculate that ECG may be suitable for early prediction of occult myocarditis. Almost all myocarditis patients in our study had a normal LVEF. This result contrasts with previous studies which showed that approximately 50% of patients with ICI-related myocarditis have reduced LVEF and left ventricular dysfunction (22). This finding warns us that we cannot ignore the risk of myocarditis despite the LVEF of patients remaining normal. In our case, CMR of four myocarditis patients did not show typical features of myocarditis. The reason may be that our cases were identified in the early stage of myocarditis by ECG and cardiac troponin monitoring, so there were no cardiac organic changes that can be detected by CMR. Besides, in cases of strong clinical suspicion for myocarditis but negative CMR presentations, EMB may be useful for definitive diagnosis (23). EMB emerges as a useful tool to reveal the mechanism of ICI-mediated myocarditis (24). In a study reporting two patients with fatal ICI-associated myocarditis, immunofluorescence studies and next-generation sequencing were performed on afflicted tissues to identify cell types in the infiltrates found in the myocardium, skeletal muscle, and tumor. Immunohistochemistry showed that infiltrating cells in the myocardium and skeletal muscle were positive for T-cell marker CD3 or macrophage marker CD68. T-cell infiltrates contained an abundance of CD4+ and CD8+ T cells. Notably, immune infiltrate was limited to the myocardium and skeletal muscle. No other tissues were affected, including adjacent smooth muscle. Clonal T-cell populations infiltrating the myocardium were identical to those present in skeletal muscle and tumor (25), which suggested that patients with ICI-associated myocarditis were having an overactivated and potentially fatal T-cell-driven immune response. Another case report showed that there were multiple subsets of immune cells involved in inflammation, including CD4+, CD8+, and CD68+ cells (26). Immunohistochemical analysis of EMB specimens allows identification of the subtype of pathogenic T-cells and revelation of underlying etiology in ICI-associated myocarditis, potentially guiding appropriate therapy (immunosuppression and antiviral treatment) (27,28). By using monoclonal and polyclonal antibodies to characterize inflammatory infiltrate on EMB tissue sections, markers of infection-negative immune-related myocarditis could be identified, in which case immunosuppression should be considered. We observed an increase in cTnI and pericardial effusion in patient #9 after the EMB, which was suspected to be due to part of the myocardium being damaged during the operation. As a result, we do not recommend EMB for fulminant myocarditis because of the risk of complications in invasive examination (21). In summary, we recommend cTnI combined with ECG as an early general screening method, which has the characteristics of low cost, less trauma, high specificity, and sensitivity.
Once the patient is diagnosed with myocarditis, glucocorticoids should be administered promptly. Delayed or insufficient use of glucocorticoids will affect outcomes. Two cases of ICI-related fulminant myocarditis were reported to be treated with 1 mg/kg/d of methylprednisolone, and both ended in cardiac death (25). In our cases, patient (#1, 2, 3) were administrated with an insufficient dose of 1–4 mg/kg, which led to a poor prognosis, presenting as malignant arrhythmia, conduction block, and myositis. The unfavorable prognosis of neuromuscular toxicity may also be long-term, with patient #13 having ventricular premature beats after one month of follow-up, and patient #10 having myositis, sinus arrhythmia, and T wave changes after 9 months. Through our follow-up, we found that patients with myocarditis generally had a favorable prognosis, but even though the ECG had turned to normal, neuromuscular toxicity in the periphery and heart may still be observed, suggesting irreversible permanent damage. Therefore, the dosage regimen was subsequently selected according to the condition, and the initial glucocorticoid dose was up to 1 g/d, which finally proved to be effective. Meanwhile, high-dose glucocorticoid pulse therapy should be used for 3–5 days if severe myocarditis is confirmed. Additionally, the occurrence of severe left ventricular dysfunction, heart failure, or malignant arrhythmia often indicates a critical condition, requiring immediate and sufficient glucocorticoid pulse therapy which is the key to recovering vital signs and controlling malignant course. It has been also reported that immunosuppression should be considered based on general myocarditis treatment strategies for patients who do not respond immediately to high-dose steroids (28). Treatment of ICI-associated myocarditis with other immunosuppressive agents results in a reduction in case mortality compared with high-dose steroids alone, suggesting that the addition of other immunosuppressive agents improves outcomes of patients with ICI-associated myocarditis (29). Glucocorticoid treatment was commonly carried out together with gamma globulin in our cases to help improve immunity. However, gamma globulin was only administered for severe myocarditis in our cases with insufficient dosage due to its expensive costs. Additionally, alemtuzumab or abatacept was considered as an effective supplementary treatment for ICI-related myocarditis, which can reduce the dose of steroids and avoid toxicity due to long-term high-dose steroids (29). A case report showed that alemtuzumab was administrated in a patient with ICI-myocarditis, which resulted in resolution of cardiac immunotoxicity unresponsive to glucocorticoid and mycophenolate mofetil (30). Similarly, cardiac damage has progressed in one ICI-associated myocarditis case treated with steroids and plasma exchange, and subsequent injection of the cytotoxic T lymphocyte associate protein-4 (CTLA-4) agonist abatacept induced rapid T-cell anergy and reduced cardiotoxicity (31). However, the data on the use of abatacept and alemtuzumab in the treatment of immune-related side effects are still limited, and further research is needed in the future to confirm their clinical value and provide more references for clinical practice.
There are limitations merit mentioned in our study. This study needs to be interpreted within the context of the study design. This was a single-centre, retrospective study, so our results may be biased by the potential confounding factors inherent in the nature of such studies. Due to the low incidence of ICI-associated myocarditis, the analysis was affected by a small sample size. Since this was a retrospective study, we did not prospectively perform ECG and CMR in ICI-associated myocarditis before patients started ICI and in patients without ICI-associated myocarditis. Therefore, we lacked data to compare the imaging differences between myocarditis group and the control group. In addition, although we observed that early and sufficient steroid administration may improve outcomes in patients with ICI-associated myocarditis, we did not establish this recommendation with a randomized study, hence detailed larger multicenter investigations should be performed to better determine the role of steroid timing and dose on prognosis of ICI-associated myocarditis.
Conclusions
In summary, our practice has proven that early identification and treatment are essential for managing myocarditis caused by PD-1 inhibitors. Based on the cases we have observed, attention should be paid to the characteristics of ICI-related myocarditis and the characteristics of concomitant myositis and peripheral neuritis. The combined diagnosis of ECG and cTnI is highly effective in prognostic judgment. In order to control the malignant course of severe myocarditis, the initial dose of glucocorticoid up to 1 g/d is usually effective.
Acknowledgments
We are grateful to patients from the first affiliated Hospital of Guangzhou Medical University who participated in this study, as well as all healthcare workers involved in the diagnosis and treatment.
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-596/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-596/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-596/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-596/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was reviewed and approved by the Medical Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University, Guangzhou, Guangdong, China (approval No. 202280). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Xin Yu J, Hodge JP, Oliva C, et al. Trends in clinical development for PD-1/PD-L1 inhibitors. Nat Rev Drug Discov 2020;19:163-4. [Crossref] [PubMed]
- Waliany S, Lee D, Witteles RM, et al. Immune Checkpoint Inhibitor Cardiotoxicity: Understanding Basic Mechanisms and Clinical Characteristics and Finding a Cure. Annu Rev Pharmacol Toxicol 2021;61:113-34. [Crossref] [PubMed]
- Hommes JW, Verheijden RJ, Suijkerbuijk KPM, et al. Biomarkers of Checkpoint Inhibitor Induced Immune-Related Adverse Events-A Comprehensive Review. Front Oncol 2020;10:585311. [Crossref] [PubMed]
- Ahern E, Allen MJ, Schmidt A, et al. Retrospective analysis of hospital admissions due to immune checkpoint inhibitor-induced immune-related adverse events (irAE). Asia Pac J Clin Oncol 2021;17:e109-16. [Crossref] [PubMed]
- Moslehi JJ, Salem JE, Sosman JA, et al. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet 2018;391:933. [Crossref] [PubMed]
- Mahmood SS, Fradley MG, Cohen JV, et al. Myocarditis in Patients Treated With Immune Checkpoint Inhibitors. J Am Coll Cardiol 2018;71:1755-64. [Crossref] [PubMed]
- Bonaca MP, Olenchock BA, Salem JE, et al. Myocarditis in the Setting of Cancer Therapeutics: Proposed Case Definitions for Emerging Clinical Syndromes in Cardio-Oncology. Circulation 2019;140:80-91. [Crossref] [PubMed]
- Palaskas N, Lopez-Mattei J, Durand JB, et al. Immune Checkpoint Inhibitor Myocarditis: Pathophysiological Characteristics, Diagnosis, and Treatment. J Am Heart Assoc 2020;9:e013757. [Crossref] [PubMed]
- Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2018;36:1714-68. [Crossref] [PubMed]
- Ganatra S, Parikh R, Neilan TG. Cardiotoxicity of immune therapy. Cardiol Clin 2019;37:385-97. [Crossref] [PubMed]
- Xu G, Chen F, Zhao W, et al. Establishment and assessment of a nomogram model for predicting the risk of fulminant myocarditis: A STROBE compliant cross-sectional study. Medicine (Baltimore) 2021;100:e25317. [Crossref] [PubMed]
- Wu NC, Feng YH, Kuo YH, et al. Clinical Features and Outcomes of Immune Checkpoint Inhibitor-Associated Cardiovascular Toxicities. Acta Cardiol Sin 2022;38:39-46. [PubMed]
- Xu M, Jiang T, Zhou Y, et al. Influence of echocardiographic measurements and renal impairments on the prognosis of fulminant myocarditis. Medicine (Baltimore) 2018;97:e9812. [Crossref] [PubMed]
- Dal'bo N, Patel R, Parikh R, et al. Cardiotoxicity of Contemporary Anticancer Immunotherapy. Curr Treat Options Cardiovasc Med 2020;22:62. [Crossref] [PubMed]
- Oren O, Yang EH, Molina JR, et al. Cardiovascular Health and Outcomes in Cancer Patients Receiving Immune Checkpoint Inhibitors. Am J Cardiol 2020;125:1920-6. [Crossref] [PubMed]
- Rubio-Infante N, Ramírez-Flores YA, Castillo EC, et al. Cardiotoxicity associated with immune checkpoint inhibitor therapy: a meta-analysis. Eur J Heart Fail 2021;23:1739-47. [Crossref] [PubMed]
- Zhou YW, Zhu YJ, Wang MN, et al. Immune Checkpoint Inhibitor-Associated Cardiotoxicity: Current Understanding on Its Mechanism, Diagnosis and Management. Front Pharmacol 2019;10:1350. [Crossref] [PubMed]
- Ganatra S, Neilan TG. Immune Checkpoint Inhibitor-Associated Myocarditis. Oncologist 2018;23:879-86. [Crossref] [PubMed]
- Moreira A, Loquai C, Pföhler C, et al. Myositis and neuromuscular side-effects induced by immune checkpoint inhibitors. Eur J Cancer 2019;106:12-23. [Crossref] [PubMed]
- Puzanov I, Subramanian P, Yatsynovich YV, et al. Clinical characteristics, time course, treatment and outcomes of patients with immune checkpoint inhibitor-associated myocarditis. J Immunother Cancer 2021;9:e002553. [Crossref] [PubMed]
- Janardhanan R. Myocarditis with very high troponins: risk stratification by cardiac magnetic resonance. J Thorac Dis 2016;8:E1333-6. [Crossref] [PubMed]
- Zhang L, Awadalla M, Mahmood SS, et al. Cardiovascular magnetic resonance in immune checkpoint inhibitor-associated myocarditis. Eur Heart J 2020;41:1733-43. [Crossref] [PubMed]
- Hu JR, Florido R, Lipson EJ, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors. Cardiovasc Res 2019;115:854-68. [Crossref] [PubMed]
- Palaskas NL, Segura A, Lelenwa L, et al. Immune checkpoint inhibitor myocarditis: elucidating the spectrum of disease through endomyocardial biopsy. Eur J Heart Fail 2021;23:1725-35. [Crossref] [PubMed]
- Johnson DB, Balko JM, Compton ML, et al. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. N Engl J Med 2016;375:1749-55. [Crossref] [PubMed]
- Balanescu DV, Donisan T, Palaskas N, et al. Immunomodulatory treatment of immune checkpoint inhibitor-induced myocarditis: Pathway toward precision-based therapy. Cardiovasc Pathol 2020;47:107211. [Crossref] [PubMed]
- Rose NR. Myocarditis: infection versus autoimmunity. J Clin Immunol 2009;29:730-7. [Crossref] [PubMed]
- Caforio AL, Pankuweit S, Arbustini E, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2013;34:2636-48, 2648a-2648d.
- Matzen E, Bartels LE, Løgstrup B, et al. Immune checkpoint inhibitor-induced myocarditis in cancer patients: a case report and review of reported cases. Cardiooncology 2021;7:27. [Crossref] [PubMed]
- Esfahani K, Buhlaiga N, Thébault P, et al. Alemtuzumab for Immune-Related Myocarditis Due to PD-1 Therapy. N Engl J Med 2019;380:2375-6. [Crossref] [PubMed]
- Salem JE, Allenbach Y, Vozy A, et al. Abatacept for Severe Immune Checkpoint Inhibitor-Associated Myocarditis. N Engl J Med 2019;380:2377-9. [Crossref] [PubMed]


