
Systematic review and meta-analysis of time-to-event long-term outcomes following the Ross procedure
Highlight box
Key findings
• The Ross procedure was confirmed to provide survival rate of approximately 87% at 20 years despite the need for reintervention in roughly 20% of patients.
What is known and what is new?
• The characteristic feature of the Ross procedure with a living substitute in the aortic valve position allows favorable hemodynamics and viability, is resistant to valve degeneration and does not require anticoagulation.
• Meta-analysis of time-to-event data was performed to assess long-term outcomes following the Ross procedure in a large population.
What is the clinical implication, and what should change now?
• Our current study revealed that the long-term outcomes following the Ross procedure up to 20 years were excellent, but the indications and surgical techniques differ among studies. Generalization of the procedure is essential for the education of surgeons who will start this procedure and potentially will bring even better outcomes.
Introduction
Management of aortic valve disease in young and middle-aged patients remains controversial. Historically, mechanical prostheses have long been the mainstay of choice for this population. Recently, bioprostheses have been increasingly used with the promise of valve-in-valve transcatheter aortic valve replacement (TAVR) within surgical bioprostheses to treat the expected structural valve deterioration, while avoiding lifelong anticoagulation (1,2). Mechanical and biological prostheses, however, have failed to restore life expectancy after aortic valve replacement (AVR) similar to the general population and are associated with excess mortality, particularly in young patients (3,4). The Ross procedure offers an alternative option to these conventional prosthetic valves. In this procedure, a non-functioning aortic valve is replaced with a patient’s pulmonary valve (pulmonary autograft), and a healthy donor valve (such as homograft) or prosthetic valve is implanted in the pulmonary valve position. The characteristic feature of the Ross procedure with a living substitute in the aortic valve position allows favorable hemodynamics and viability, is resistant to valve degeneration and does not require anticoagulation (5-10). However, despite these benefits and excellent outcomes reported by selected institutions or registry-based studies, its indication in the non-elderly adult patient population remains debated. The complexity of the Ross procedure, the perceived concern for reintervention on both the autograft and right ventricular outflow tract (RVOT), and the lack of prospective randomized controlled trials have contributed to these controversies. Therefore, the recent American College of Cardiology/American Heart Association (ACC/AHA) valve guideline only recommended the Ross procedure for the young population by experienced surgeons (class IIb) (11). In addition, long-term outcomes following the Ross procedure, including survival and freedom from valve reinterventions, remain mostly unknown (12,13). A large amount of contemporary data on the Ross procedure has recently accumulated (5-10). Hence, this study aimed to evaluate the long-term results of the Ross procedure using the meta-analysis of time-to-event outcomes. We present this article in accordance with the PRISMA reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-326/rc).
Methods
PRISMA flow diagram was shown in Figure 1. Studies were included if the following criteria were met: (I) the study design was retrospective or prospective, randomized or non-randomized, matched or unmatched populations; (II) the study included patients ≥16 years old with aortic valve disease who underwent the Ross procedure; (III) the study reported survival and/or reintervention at least one of autograft and/or homograft; (IV) the Kaplan-Meier curves were available beyond 20 years.

The eligible studies were identified using a 2-level strategy. First, databases including MEDLINE, EMBASE, Cochrane Library, Web of Science, and Google Scholar were searched through June 2022 using Web-based search engines. Search terms were “pulmonary autograft” OR ‘Ross procedure” AND “outcome”. Second, relevant studies were identified through a manual search of secondary sources, including references to initially identified articles and a search of reviews and commentaries. The searched language was not limited to English. The extracted studies were carefully reviewed by two independent authors (Shimamura J and Kuno T). Abstracts or full manuscripts were screened to assess whether a study met the eligibility criteria. The risk of bias in individual studies was evaluated by two independent authors (Shimamura J and Kuno T) using the Newcastle-Ottawa Scale (Table S1).
Outcomes of interest
The primary outcome of this study was death from any cause. Secondary outcomes of interest included valve reinterventions on either autograft or RVOT. Reintervention was defined as surgical or percutaneous reintervention on any operated valves.
Statistical analysis
The meta-analysis of the time-to-event outcomes was performed as follows. Raw data (time, survival probability) were extracted from each Kaplan-Meier curve. Then, the data were processed in conjunction with the numbers at risk at given time point. Finally, the reconstructed time-to-event outcomes were merged to create the study dataset. The methodological details were described in the previous literature (14,15) (Appendix 1).
Results
Six studies were identified and analyzed, including 4,910 patients (3,601 males) (5-10). The summary of the included studies is shown in Table 1. Included studies are from single or multi-center studies from North or South America, Europe, and Australia. The mean/median age ranges from 39.0 to 47.7 years old. Mean/median follow-up period of these studies ranged from 9.2 to 17 years.
Table 1
| Author | Patient (N) | Patient population | Age, years | Male (%) | Follow-up, years | Exclusion | Outcomes | AI (%) | IE (%) | Annuloplasty (%) | Autograft implantation technique (%) | 30-day mortality (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aboud 2021 (5) | 2,444 | European Registry | 44.1±11.7 | 1,853 (75.8) | 9.2 (0–27.4) | NA | Survival, freedom from AG/HG reintervention | 540 (22.1); mixed 1,246 (51.0) | NA | NA | SC 892; RR 480; RR + R 1,072 | 25 (1.0) |
| Buratto 2018 (6) | 392 | Melbourne group | 39.0±13.0 | 271 (69.0) | 10.0±7.0 (0–25.0) | Urgent surgery, concomitant cardiovascular procedure, dissection, endocarditis | Survival | 118 [30]; mixed 83 [21] | NA | NA | Root within root 361 [92], inserted inside Valsalva graft 20 [5] | 1 (0.3) |
| Martin 2017 (7) | 310 | Single institution | 40.8±10.6 | 187 (60.3) | NA | NA | Survival, freedom from AG/HG reintervention | 60 (19.4); mixed 23 (7.4) | 12 (3.9) | 4 (1.3) | Root implant 259 [84], root inclusion 33 (10.7), subcoronary 18 (5.8) | 4 (1.3) |
| Mazine 2022 (8) | 108 | Single institution | 40 [33–47] | 14.5±7.2 | NA | Survival | 29 [26]; mixed 22 [20] | NA | NA | 0 (0) | ||
| Romeo 2021 (9) | 1,431 | Australia, Belgium, Brazil, Canada, Germany | 47.7±9.5 | 1,064 (74.3) | 9.2 (IQR, 4.2–14.0) | Emergency operation, concomitant mitral valve surgery, aortic dissection | Survival, freedom from AG/HG reintervention | 376 (33.7) | NA | NA | NA | 10 (0.7) |
| Ryan 2021 (10) | 225 | Single institution | 42.0±11.0 | 157 [70] | 17.0 (IQR, 12.0–20.0) | NA | Survival, freedom from AG/HG reintervention | 81 [36]; mixed 46 [20] | 24 (10.7) | 60 [27] | External graft support | 5 (2.2) |
Values are n (%), mean ± SD, n, or median (range). AI, aortic insufficiency; IE, infective endocarditis; NA, not available; AG, autograft; HG, homograft; SC, supra-coronary; RR, root replacement; R, reinforcement; IQR, interquartile range; SD, standard deviation.
Regarding exclusion criteria, urgent/emergency surgery was excluded in most studies, but endocarditis was excluded in only one study. Regarding the valve etiology, the proportion of aortic insufficiency or mixed lesion (aortic stenosis and aortic insufficiency) varied among studies (aortic insufficiency: 19–36%, mixed lesion: 7–50%). In addition, the Ross procedure technique (sub-coronary, full root replacement with/without graft reinforcement, and the rate of annuloplasty) differed among studies.
Survival rate and freedom from any valve reintervention from each study are summarized in Table S1. Aggregated survival rate at 5, 10, 15, and 20 years was 99.9%±0.1%, 97.6%±0.5%, 94.3%±0.9%, and 87.4%±1.9% (Figure 2). Aggregated freedom from autograft reintervention at 5, 10, 15, and 20 years was 97.7%±0.5%, 95.3%±0.7%, 91.4%±1.2%, 84.8%±2.5% (Figure 3). Aggregated freedom from RVOT reintervention was 99.0%±0.3%, 99.0%±0.3%, 97.5%±0.7%, 93.3%±1.8% (Figure 4). Aggregated freedom from any valve reintervention at 5, 10, 15, and 20 years was 95.8%±0.6%, 92.6%±0.9%, 88.5%±1.2%, 80.8%±2.5% (Figure 5). The details of reinterventions, including indications and performed procedures, are shown in Table 2. Structural valve degeneration, non-structural valve degeneration, autograft dilatation, RVOT stenosis, and endocarditis were the main causes of reinterventions. The survival rate and freedom from reintervention of each study were summarized in Tables S2-S5. The causes of death in a late phase were described in Table S6.

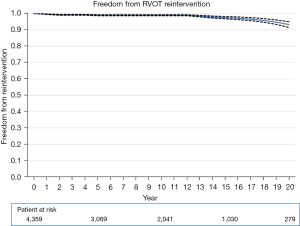

Table 2
| Author | Autograft reintervention | Homograft reintervention | Reintervention on both AG and HG |
|---|---|---|---|
| Aboud 2021 (5) | 161 reinterventions: SVD 44 NSVD 76 endocarditis 37 technical 4 | 143 reinterventions [35 transcatheter (17 valve intervention, 18 balloon valvuloplasty)]: SVD 85, NSVD 10, endocarditis 26, other 22 |
35 simultaneous AG and HG reintervention |
| Buratto 2018 (6) | NA | ||
| Martin 2017 (7) | Pulmonary autograft failure: 55 (17.7%) (SVD 36, non-SVD 19). SVD: isolated moderate or severe autograft insufficiency: 30 (15 patients required reintervention), remaining 6 patients with SVD developed autograft insufficiency with mild dilatation of ascending aorta (5 patients required reintervention). 20 reintervention (Bentall in 13, VSRR in 2, AVR in 5). Non-SVD: isolated dilatation of ascending aorta: 10 (5 required reintervention), both dilated ascending aorta and secondary autograft insufficiency: 7 (all required reintervention) VSRR in 7 | 67 (21.6%) patients develop HG SVD and 21 patients underwent reintervention. HG stenosis was diagnosed in 18 patients and 11 patients required valvular replacement | |
| Mazine 2022 (8) | 15 (12 surgical 3 percutaneous) | ||
| Romero 2021 (9) | NA | ||
| Ryan 2021 (10) | 40 (18%) isolated AG reintervention. Autograft dilation with AI (n=33, 59%), isolated AI (n=18.32%), endocarditis (n=4, 7.1%), ruptured leaflet after traumatic fall (n=1, 1.8%), VSRR (n=16, 29%), root replacement (n=26, 46%), AVR (n=12, 21%), TAVI (n=1, 1.8%), repair of pseudoaneurysm (n=1, 1.8%) | 8 (3.6%) isolated HG reintervention. Homograft stenosis (n=17, 71%), homograft stenosis and insufficiency (n=3, 13%), endocarditis (n=3, 13%). Unintentional homograft injury during redo sternotomy for CABG (n=1, 4.2%). PVR (n=17, 71%) [transcatheter PVR (n=4, 17%), balloon valvuloplasty (n=3, 13%)] | 16 (7.2%) reintervention on both valves (10 combined operation and 6 separate operations occurred a median of 10 years apart) |
RVOT, right ventricular outflow tract; AG, autograft; HG, homograft; SVD, structural valve degeneration; NSVD, non-structural valve degeneration; NA, not available; VSRR, valve sparing root replacement; AVR, aortic valve replacement; AI, aortic insufficiency; TAVI, transcatheter aortic valve implantation; CABG, coronary artery bypass grafting; PVR, pulmonary valve replacement.
Discussion
In this study, we have reported 20-year survival and valve reintervention rates of 4,910 patients from six studies undergoing the Ross procedure using a meta-analysis of time-to-event data. The survival rate, freedom from autograft, RVOT, and any valve reintervention at 20 years were 87.4%, 84.8%, 93.3%, and 80.8%, respectively. Notably, freedom from any valve reintervention at 20 years in this relatively young population is excellent. Furthermore, Ross recipients experienced high survival outcomes despite the subset of patients requiring valve reinterventions.
Survival
The ultimate goal of aortic valve intervention for patients with aortic valve disease is to provide survival and quality of life that mirrors the healthy general population. The excellent short and long-term survival and quality of life following the Ross procedure have been repeatedly reported (5-10). For example, Aboud et al. reported that long-term survival was non-inferior to an age- and sex-matched general population with a median follow-up of 9.2 years (5). In contrast, both bioprosthetic and mechanical AVR for non-elderly patients have failed to provide comparable long-term survival (3,4). However, the Ross procedure tends to be performed in healthy individuals with fewer comorbidities, and selection bias cannot be easily eliminated compared to other valve substitute options.
From the hemodynamic and physiological point of view, an autograft is a living tissue that possesses a remodeling effect. However, mechanical or bioprosthetic valves have inevitable rigidities introduced by leaflet discs, stent frames, and fixed sewing rings that restrict the aortic root’s dynamic functional structure. The transvalvular gradient of the autograft is significantly lower than that of a conventional prosthetic valve (16). These benefits may result in the favorable long-term survival.
Interestingly, the causes of late death following the Ross procedure were mainly non-valve-related. Ryan et al. reported the clinical outcomes among 225 patients following the Ross procedure. There were 24 late deaths, but only three mortalities were valve-related (one death after the reoperation and two endocarditis-related deaths) (10). On the other hand, conventional AVR is often associated with valve-related complications. For example, structural valve degeneration requiring reintervention is inevitable in relatively young patients who choose to have a bioprosthetic AVR (8). In addition, mechanical AVR is associated with valve-related complication such as valve thrombosis or a bleeding event, and the annual risk of bleeding and thromboembolism were reported to be 0.7–3.5% (17,18). However, we need to consider that the Ross procedure is associated with higher perioperative risk compared with isolated AVR despite its long-term survival advantages (19).
Autograft reintervention
In the current analysis, autograft reintervention tended to be more common than reintervention on the RVOT, and autograft insufficiency was the most common form of autograft failure. It is well known that predominant preoperative aortic insufficiency and aortic annulus dilatation are associated with an increased risk of autograft failure and reintervention (20,21). However, in our series, the degree of preoperative aortic insufficiency and annulus size varied among studies. Because autograft failure can be more common in patients with primary aortic regurgitation, valve-sparing root replacement/remodeling with valve repair should be considered if a durable repair can be anticipated (10).
Autograft implantation has been modified to include specific technical elements: trimming off any excess muscle off the autograft; trimming off any excess autograft above the neo-sinotubular junction; placement of the autograft deep into the left ventricular outflow tract by meticulous attention to each suture (intra-annular implantation for external annulus support), and providing external supports of the autograft annulus and the neo-sinotubular junction using various materials, particularly in patients with aortic insufficiency and/or large aortic annulus (22,23). In the present study, the autograft implantation technique was different among studies, therefore comparing techniques was not possible. Most common autograft interventions were isolated valve replacement, redo root replacement, and valve-sparing operations. TAVR was performed in only four patients because the only failure mode of the autograft is regurgitation and TAVR is essentially an operation for stenosis (24).
RVOT reintervention
In the current study, RVOT reintervention was relatively rare and freedom from reintervention at 20 years was over 90%. Although detailed information for the RVOT was not available in these studies, stenosis was the primary cause of reintervention in the RVOT. Previous studies have reported that generous oversizing of the implanted homograft by removing RVOT muscle is an effective option to prevent homograft stenosis (25). Homograft dysfunction caused by immune response is another possible cause requiring homograft reintervention. The use of decellularized homograft has been thought to be effective in preventing robust immune responses (26). Homografts from older donors showed a lower peak transvalvular gradient during follow-up and might be considered in the future for homograft selection (27). Reintervention in the RVOT is one of the downsides of the Ross procedure, but the incidence of the reintervention is relatively rare despite 20 years follow-up period. Furthermore, the mortality associated with RVOT reintervention was relatively low in the present systematic review.
There has been a perception in our community that the Ross procedure introduces two-valve disease by harvesting a healthy pulmonary valve. In addition, autograft or RVOT reintervention following the Ross procedure is more complicated than redo AVR after prosthetic AVR (28-30). In our analysis, only approximately 20% of the patients experienced any valve reintervention over 20 years of follow-up. In previous reports, the Ross procedure was associated with a higher cumulative risk of autograft and/or RVOT reintervention than mechanical AVR (31). However, the incidence of the reintervention of the Ross procedure is still significantly lower than that of bioprosthetic AVR (8). El-Hamamsy et al. reported that the incidence of reintervention was 17.2%, 29.8%, and 7.4% after the Ross, biological, and mechanical AVR, respectively, at 15 years in a propensity-matched comparison (31).
Future directions
Our current study revealed that the long-term outcomes up to 20 years were excellent, but the indications and surgical techniques differ among studies. Generalization of the Ross procedure is essential for the education of surgeons who will start this procedure and potentially will bring even better outcomes. Discussion should include not only the advantages of the Ross but also the improvement in mechanical valves that may require less intense anticoagulation, and bioprosthetic valves with better durability (18). Advancement in the transcatheter approach may decrease the invasiveness and surgical risks of reintervention for autograft and RVOT following the Ross procedure, but more experience and newer devices are needed (23).
Limitations
This study has several limitations. First, the Ross procedure is still mainly performed at selected centers with experience, and generalization of our findings may be difficult. Second, our study shares the limitations of meta-analysis related to the inclusion of retrospective observational studies. Third, the described studies were different in terms of indication and technique of the Ross procedure. Fourth, there is a possible overlap of the patients among studies. Fifth, studies that did not report Kaplan-Meier curve were not included in the current study. Lastly, because the algorithm for reconstruction of individual patient data from the Kaplan-Meier curve is not able to reproduce informative censoring of competing risk, we could only calculate cause-specific Kaplan-Meier estimates in the outcome of reintervention. Despite these limitations, the present study represents the longest available meta-analysis analyzing long-term outcomes following the Ross procedure. Since life expectancy in the developed countries is around 80 years old, patients who undergo the Ross procedure at a younger age (approximately 40 years old on average in this study) will require extremely long-term follow-up and guidance regarding the reintervention since they may experience multiple valve reinterventions after very late follow-up.
Conclusions
In summary, the present meta-analysis of time-to-event outcomes revealed that the Ross procedure provides excellent freedom from valve reintervention and survival for up to 20 years. However, even longer follow-up and data from more centers are warranted to assess the clinical outcomes of the Ross procedure recipients.
Acknowledgments
This study was presented at the 72nd Annual Scientific Session of the American College of Cardiology Together with WCC (ACC.23/WCC), March 4–6, 2023, New Orleans, LA, USA.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-326/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-326/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-326/coif). SF serves as a consultant for Terumo Aortic, Artivion and Medtronic Inc. and has received speakers’ honoraria from Edwards Lifesciences and Medtronic Inc. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Brown JM, O'Brien SM, Wu C, et al. Isolated aortic valve replacement in North America comprising 108,687 patients in 10 years: changes in risks, valve types, and outcomes in the Society of Thoracic Surgeons National Database. J Thorac Cardiovasc Surg 2009;137:82-90. [Crossref] [PubMed]
- Isaacs AJ, Shuhaiber J, Salemi A, et al. National trends in utilization and in-hospital outcomes of mechanical versus bioprosthetic aortic valve replacements. J Thorac Cardiovasc Surg 2015;149:1262-9.e3. [Crossref] [PubMed]
- Glaser N, Persson M, Jackson V, et al. Loss in Life Expectancy After Surgical Aortic Valve Replacement: SWEDEHEART Study. J Am Coll Cardiol 2019;74:26-33. [Crossref] [PubMed]
- Goldstone AB, Chiu P, Baiocchi M, et al. Mechanical or Biologic Prostheses for Aortic-Valve and Mitral-Valve Replacement. N Engl J Med 2017;377:1847-57. [Crossref] [PubMed]
- Aboud A, Charitos EI, Fujita B, et al. Long-Term Outcomes of Patients Undergoing the Ross Procedure. J Am Coll Cardiol 2021;77:1412-22. [Crossref] [PubMed]
- Buratto E, Shi WY, Wynne R, et al. Improved Survival After the Ross Procedure Compared With Mechanical Aortic Valve Replacement. J Am Coll Cardiol 2018;71:1337-44. [Crossref] [PubMed]
- Martin E, Mohammadi S, Jacques F, et al. Clinical Outcomes Following the Ross Procedure in Adults: A 25-Year Longitudinal Study. J Am Coll Cardiol 2017;70:1890-9. [Crossref] [PubMed]
- Mazine A, David TE, Stoklosa K, et al. Improved Outcomes Following the Ross Procedure Compared With Bioprosthetic Aortic Valve Replacement. J Am Coll Cardiol 2022;79:993-1005. [Crossref] [PubMed]
- Romeo JLR, Papageorgiou G, da Costa FFD, et al. Long-term Clinical and Echocardiographic Outcomes in Young and Middle-aged Adults Undergoing the Ross Procedure. JAMA Cardiol 2021;6:539-48. [Crossref] [PubMed]
- Ryan WH, Squiers JJ, Harrington KB, et al. Long-term outcomes of the Ross procedure in adults. Ann Cardiothorac Surg 2021;10:499-508. [Crossref] [PubMed]
- Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021;143:e35-71. [Crossref] [PubMed]
- Etnel JRG, Grashuis P, Huygens SA, et al. The Ross Procedure: A Systematic Review, Meta-Analysis, and Microsimulation. Circ Cardiovasc Qual Outcomes 2018;11:e004748. [Crossref] [PubMed]
- Flynn CD, De Bono JH, Muston B, et al. Systematic review and meta-analysis of long-term outcomes in adults undergoing the Ross procedure. Ann Cardiothorac Surg 2021;10:411-9. [Crossref] [PubMed]
- Barendregt JJ, Doi SA, Lee YY, et al. Meta-analysis of prevalence. J Epidemiol Community Health 2013;67:974-8. [Crossref] [PubMed]
- Williamson PR, Smith CT, Hutton JL, et al. Aggregate data meta-analysis with time-to-event outcomes. Stat Med 2002;21:3337-51. [Crossref] [PubMed]
- Um KJ, Mcclure GR, Belley-Cote EP, et al. Hemodynamic outcomes of the Ross procedure versus other aortic valve replacement: a systematic review and meta-analysis. J Cardiovasc Surg (Torino) 2018;59:462-70. [Crossref] [PubMed]
- Misawa Y. Valve-related complications after mechanical heart valve implantation. Surg Today 2015;45:1205-9. [Crossref] [PubMed]
- Puskas J, Gerdisch M, Nichols D, et al. Reduced anticoagulation after mechanical aortic valve replacement: interim results from the prospective randomized on-X valve anticoagulation clinical trial randomized Food and Drug Administration investigational device exemption trial. J Thorac Cardiovasc Surg 2014;147:1202-1210; discussion 1210-1. [Crossref] [PubMed]
- Reece TB, Welke KF, O'Brien S, et al. Rethinking the ross procedure in adults. Ann Thorac Surg 2014;97:175-81. [Crossref] [PubMed]
- Charitos EI, Takkenberg JJ, Hanke T, et al. Reoperations on the pulmonary autograft and pulmonary homograft after the Ross procedure: An update on the German Dutch Ross Registry. J Thorac Cardiovasc Surg 2012;144:813-21; discussion 821-3. [Crossref] [PubMed]
- Stelzer P, Mejia J, Varghese R. Operative risks of the Ross procedure. J Thorac Cardiovasc Surg 2021;161:905-915.e3. [Crossref] [PubMed]
- Liebrich M, Charitos EI, Dingemann C, et al. The reinforced full-root technique for the Ross operation: surgical considerations and operative insights. Ann Cardiothorac Surg 2021;10:485-90. [Crossref] [PubMed]
- Varrica A, Satriano A, Frigiola A, et al. Autograft Wrapping Reinforcement in Adolescents Undergoing Ross Operation: A Tailored Coat. Ann Thorac Surg 2022;114:866-71. [Crossref] [PubMed]
- Saia F, Galiè N, Laborde JC, et al. Transcatheter aortic valve implantation for severe autograft regurgitation after Ross operation. EuroIntervention 2014;10:141-5. [Crossref] [PubMed]
- Pergola V, Di Salvo G, Fadel B, et al. The long term results of the Ross procedure: The importance of candidate selection. Int J Cardiol 2020;320:35-41. [Crossref] [PubMed]
- da Costa FDA, Etnel JRG, Charitos EI, et al. Decellularized Versus Standard Pulmonary Allografts in the Ross Procedure: Propensity-Matched Analysis. Ann Thorac Surg 2018;105:1205-13. [Crossref] [PubMed]
- Fernández-Carbonell A, Rodríguez-Guerrero E, Merino-Cejas C, et al. Predictive Factors for Pulmonary Homograft Dysfunction After Ross Surgery: A 20-Year Follow-up. Ann Thorac Surg 2021;111:1338-44. [Crossref] [PubMed]
- Shih E, Brinkman WT, Harrington KB, et al. Outcomes of redo operations after the Ross procedure. J Thorac Cardiovasc Surg 2023;165:1803-1812.e2. [Crossref] [PubMed]
- Stelzer P, Mejia J, Williams EE. Outcomes of reoperations after Ross procedure. Ann Cardiothorac Surg 2021;10:491-8. [Crossref] [PubMed]
- Varrica A, Caldaroni F, Saitto G, et al. Outcomes and Quality of Life After Ross Reintervention: Would You Make the Same Choice Again? Ann Thorac Surg 2020;110:214-20. [Crossref] [PubMed]
- El-Hamamsy I, Toyoda N, Itagaki S, et al. Propensity-Matched Comparison of the Ross Procedure and Prosthetic Aortic Valve Replacement in Adults. J Am Coll Cardiol 2022;79:805-15. [Crossref] [PubMed]



