
A modified prosthesis eversion technique for proximal anastomosis in ascending aorta replacement
Highlight box
Key findings
• The modified prosthesis eversion technique is an effective alternative for proximal anastomosis in ascending aorta replacement, with less blood loss, shorter operation time, and lower rate of postoperative complications compared with the conventional technique.
What is known and what is new?
• Many proximal anastomotic techniques have been developed. However, at present, there is still no one-of-a-kind method for proximal anastomosis, and finding the optimal technique is of great interest.
• This study described a modified prosthesis eversion technique for proximal anastomosis and compared its operative outcomes with the conventional prosthesis eversion technique.
What is the implication, and what should change now?
• The modified prosthesis eversion technique is an effective alternative for proximal anastomosis in ascending aorta replacement and can be widely used in clinical practice.
Introduction
For patients with ascending aorta aneurysm, it is necessary to replace the ascending aorta with a Dacron tube graft (1). A decisive factor in successful ascending aorta replacement is a reliable proximal anastomosis between the vascular prosthesis and the sinotubular junction (STJ) (2). The prosthesis eversion technique initially reported by Rene Pretre in 1998 is a traditional proximal anastomosis approach (3). The most obvious feature of this approach is that the vascular prosthesis needs to be inserted into and extracted from the left ventricle through the aortic valve, which may damage the valve leaflets. Moreover, the vascular graft is anastomosed to the aortic root with a 4-0 polypropylene suture reinforced by a strip of Teflon felt, which may lead to troublesome bleeding. To avoid these problems, many proximal anastomotic techniques, such as doubled felt anastomosis (4), proximal stepwise technique (5), “sleeve” repair (6), and turn-up anastomosis (7), have been developed. However, at present, there is still no one-of-a-kind method for proximal anastomosis, and finding the optimal technique is of great interest.
We designed a modified prosthesis eversion technique for proximal anastomosis in ascending aorta replacement. This study aimed to evaluate its feasibility and compare its operative outcomes with the conventional prosthesis eversion technique. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-550/rc).
Methods
Patients
We conducted a retrospective analysis of 108 consecutive patients who had ascending aortic aneurysm and underwent ascending aorta replacement with the modified (n=55) or conventional (n=53) prosthesis eversion technique between January 2019 and December 2022 in our center. Detailed preoperative patient characteristics are listed in Table 1, and there was no difference between the two groups. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Southwest Hospital of Army Medical University (Institutional Review Board File KY2021058). The informed consent form was signed by all patients to allow the intervention and data recording.
Table 1
| Variables | Modified group (n=55) | Conventional group (n=53) | P value |
|---|---|---|---|
| Male:female | 36:19 | 35:18 | 0.763 |
| Age (years) | 52.6±10.8 | 52.9±13.3 | 0.816 |
| Body mass index (kg/m2) | 24.9±3.5 | 25.3±4.2 | 0.545 |
| Comorbidities | |||
| Hypertension | 12 (21.8) | 10 (18.9) | 0.596 |
| Diabetes mellitus | 6 (10.9) | 5 (9.4) | 0.521 |
| COPD | 9 (16.4) | 9 (17.0) | 0.614 |
| Coronary artery disease | 5 (9.1) | 6 (11.3) | 0.305 |
| Chronic renal insufficiency | 6 (10.9) | 7 (13.2) | 0.358 |
| Previous cardiac surgery | 2 (3.6) | 1 (1.9) | 0.069 |
| EuroSCORE II | 5.5±4.0 | 5.9±4.3 | 0.618 |
Values are expressed as mean ± standard deviation or number (%). COPD, chronic obstructive pulmonary disease; EuroSCORE, European System for Cardiac Operative Risk Evaluation.
The surgical indication for aortic diseases was determined by the maximum aortic diameter. These recommendations could be found in the 2021 European Society of Cardiology/European Association for Cardio-Thoracic Surgery Guidelines for the management of valvular heart disease (8). In this study, the indication for intervention was the maximum diameter of ascending aorta >55 mm. The exclusion criteria were: Marfan syndrome, intimal tears extended to the aortic annulus, connective tissue disorders, or aortic root diameter >45 mm.
The decision to proceed with the modified or conventional prosthesis eversion technique was discretionary based on the underlying clinical condition. In general, one control subject was added to the conventional cohort for every patient from the modified cohort. Matching variables included age (±5 years), sex (exact), height (±20 cm), weight (±20 kg) and European System for Cardiac Operative Risk Evaluation (EuroSCORE) II (±2.5).
All the patients were followed up by means of general examination, transthoracic echocardiography and computed tomography angiography (CTA) before discharge, at 3 months after surgery, and annually thereafter. The primary endpoint was any-cause death. The secondary endpoints were surgery-related complications, including neurological deficits, anastomotic site bleeding and endoleaks that required additional surgical treatment.
Operative techniques
All operations were performed under general anesthesia and via a median sternotomy. The pericardium was opened and the aorta is separated from the surrounding tissue. After systemic heparinization, cardiopulmonary bypass (CPB) was established by intubation of the femoral and/or right subclavian artery and right atrium. After the nasopharyngeal temperature dropped to moderate hypothermia (24–28 ℃), circulatory arrest and selective cerebral perfusion was performed. After the distal arch repair was completed, the extracorporeal circulation was resumed and warming was started. Root treatment occurred during the rewarming phase.
The ascending aorta was clamped, and the proximal aorta was transected circumferentially at approximately 6 mm above the STJ. Then, initial assessment of the valve leaflets’ suitability for preservation was performed. The valve sparing is indicated by either a normal aortic valve or mild insufficiency due to geometric changes caused by an aortic root aneurysm. The non-valve sparing indications were: (I) the aortic valve was unhealthy, majorly fibrotic or calcified; (II) an aortic aneurysm exceeding 60 mm in maximum diameter; (III) a bicuspid aortic valve and severe aortic regurgitation.
The diameter of the STJ was assessed, and a vascular prosthesis slightly smaller than STJ was selected. In this study, the Terumo (Japan or Inter Gard, France) one-branch vascular prosthesis was used to replace the ascending aorta, which was 24–30 mm in diameter.
Modified prosthesis eversion technique
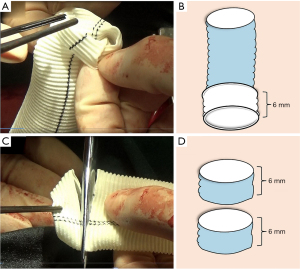
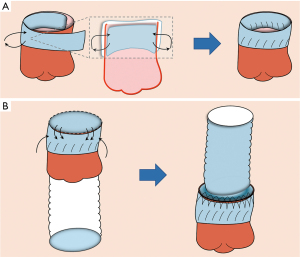
The vascular prosthesis was everted about 6 mm on one end, served as proximal end (Figure 1A,1B). Then, two small strips about 6 mm high were cut away from the other end of the same prosthesis and opened in a “C” shape (Figure 1C,1D).
The everted end of vascular prosthesis was positioned inside the aortic root overlapping about 5–6 mm with the aortic stump (Figure 2A,2B). In addition, the everted upper border of the prosthesis was aligned in parallel with the cut edge of the aortic stump. And, one graft strip was placed outside the aortic stump, and a small segment of the other graft strip was used to completely wrap the outer aortic wall. So, the new three-layers root was composed of the everted short segment of the vascular prosthesis, the native aortic wall, and the graft strip from the inside out.
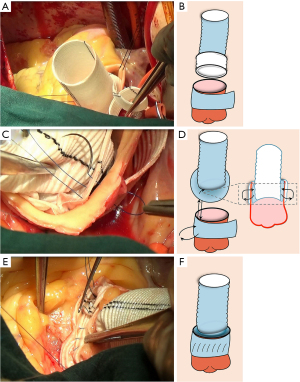
The sutures should be performed through the three-layers root by a 5-0 Prolene suture with a double needle. The first needle was passed from the outside to the inside about 2–3 mm above the STJ, and turned back, and then passed from the inside to the outside at the level of the STJ (Figure 2C,2D). The lower half of anastomosis was like a series of “I” shapes (Figure 2E,2F). Next, the upper half of anastomosis was made up of two perfect serial tandem coils, using the out-to-inside and returning-to-the-out technique about 4 mm above the STJ (Figure 3A,3B). To be specific, one coil was created with the forehand in a clockwise direction. The other coil was created using the backhand in the counterclockwise direction with a second needle (Figure 3C,3D). Notedly, each stitch should not exceed 3 mm in length to avoid an everting suture line and a folding of the graft at the aortic root.
After completion of the suture, the proximal vascular graft was conventionally anastomosed to the distal ascending aorta or to an arch graft if there was a more extended aneurysm involving the aortic arch (Figure 4A,4B). The method of aortic arch reconstruction was based on a study we published previously (9), which included photographs and sketches of each step of the procedure.
Conventional prosthesis eversion technique
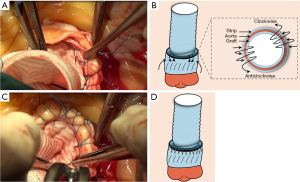
A suture line was fixed on one end of the vascular prosthesis served as distal end, and then completely introverted. Then, two small strips about 6 mm high were cut just like the modified prosthesis eversion technique, and were placed circumferentially inside the external and internal walls of the aortic stump to reinforce the aortic root, respectively. Next, 5-0 Prolene sutures were applied to fix the external and internal strips of graft at the level of the STJ using the same suture method as the lower half anastomosis of the modified prosthesis eversion technique (Figure 5A). The reconstructed aortic root was anastomosed to the vascular prosthesis as the proximal anastomosis. To be specific, the introverted prosthesis was inserted into the residual stump of the aorta and the left ventricle through the aortic valve, and the free margin of the prosthesis was positioned at the level of the rim of the repaired aortic root. Then the vascular graft was sewn to the reconstructed aorta with a 5-0 Prolene suture using the same suture method as the upper half anastomosis of the modified prosthesis eversion technique. After this, the vascular graft was extracted from the left ventricle by pulling on the suture line on the distal end (Figure 5B). The rest of the operation was carried out as routine. In the classical Sun’s procedure (10), the 4-branched arch graft could also be anastomosed proximally to the STJ in an end-to-end fashion, which didn’t involve the prosthesis eversion technique.
In the modified and conventional prosthesis eversion technique, we routinely sprayed a small amount of fibrin-based tissue glue (Tissucol Duo Quick; 2 mL/set) around the anastomotic sites with a 2-syringe application device and a pressure-controlled spray system to prevent blood leaking.
Statistical analysis
Categoric variables were presented as percentages and were analyzed using the chi-square test. Continuous variables were expressed as mean ± standard deviation and were analyzed using Student’s t-test or the Mann-Whitney U test. Statistical analyses were performed using SAS version 9.2 (SAS Institute, Inc., Cary, NC, USA).
Results
Intraoperative data
The intraoperative variables of the two groups are shown in Table 2. All patients were effectively treated with the modified or conventional prosthesis eversion technique. No significant differences of the concomitant surgeries were found in both groups. However, the durations of CPB, aortic cross-clamping and total operation in the conventional group were longer than those in the modified group.
Table 2
| Variables | Modified group (n=55) | Conventional group (n=53) | P value |
|---|---|---|---|
| Surgical method | |||
| Isolated ascending aorta replacement | 8 (14.5) | 8 (15.1) | 0.622 |
| Total arch replacement | 47 (85.5) | 45 (84.9) | 0.635 |
| Elephant trunk procedure | 47 (85.5) | 45 (84.9) | 0.635 |
| Aortic valve replacement | 10 (18.2) | 11 (20.8) | 0.389 |
| Coronary artery bypass | 5 (9.1) | 4 (7.5) | 0.496 |
| Procedural time | |||
| CPB time (min) | 186.8±35.5 | 235.9±38.8 | 0.042 |
| Cross-clamping time (min) | 115.6±25.0 | 148.5±32.7 | 0.047 |
| Total operation time (min) | 286.5±45.9 | 347.2±43.8 | 0.035 |
Values are mean ± standard deviation or number (%). CPB, cardiopulmonary bypass.
Perioperative data
The postoperative variables of the two groups are shown in Table 3. Perioperative blood loss was 1,694.5±525.8 mL in the conventional group and 952.9±360.7 mL in the modified group, which was determined from the estimated intraoperative blood loss and measured postoperative suction drainage within 48 hours. The indications of re-exploration for bleeding were: (I) high-drain output (>200 mL/h) over the first 3 hours postoperatively; (II) hemodynamic instability, such as persistent tachycardia and hypotension; (III) continuous decline in hemoglobin levels; (IV) post-operative echocardiography suggested pericardial tamponade. The incidence of re-exploration for bleeding was higher in the conventional group, which all resulted from anastomotic hemorrhage. Accordingly, the total volume of intraoperative and postoperative allogenic blood transfusion was significantly lower in the modified group. In the conventional group, one patient died of large anastomotic hemorrhage after refusing further treatment on postoperative day 2. The modified group had a shorter duration in intensive care unit (ICU) and hospital, and lower total hospitalization costs than those in the conventional group.
Table 3
| Variables | Modified group (n=55) | Conventional group (n=53) | P value |
|---|---|---|---|
| Ventilation time (hours) | 56.5±18.9 | 55.5±21.8 | 0.524 |
| Neurologic dysfunction | 1 (1.8) | 1 (1.9) | 0.806 |
| Perioperative blood loss (mL) | 952.9±360.7 | 1,694.5±525.8 | 0.029 |
| Re-exploration for bleeding | 0 | 2 (3.8) | 0.023 |
| Intraoperative and postoperative allogenic blood transfusion | |||
| Packed red blood cells (units) | 3.5±2.1 | 5.6±3.2 | 0.045 |
| Fresh-frozen plasma (units) | 3.4±2.0 | 5.1±2.5 | 0.044 |
| Apheresis platelets (doses) | 1.3±0.7 | 2.4±0.8 | 0.036 |
| Pooled cryoprecipitate (doses) | 1.5±0.7 | 2.9±1.4 | 0.033 |
| Operative mortality | 0 | 0 | 1.000 |
| In-hospital mortality | 0 | 1 (1.9) | 0.042 |
| Length of ICU stay (days) | 3.6±1.0 | 5.5±2.1 | 0.048 |
| Length of hospital stay (days) | 11.9±2.6 | 14.5±5.5 | 0.047 |
| Total hospitalization cost (×105, yuan) | 12.3±3.2 | 15.5±4.8 | 0.043 |
Values are mean ± standard deviation or number (%). ICU, intensive care unit.
Post-operative echocardiography showed good valve function with minimal residual regurgitation in both groups. Before discharge, all patients underwent CTA that confirmed correct positioning of the vascular graft without stenosis, endoleak or dissection around the anastomosis sites in both groups.
Follow-up data
A total of 107 patients were discharged, and no patient was lost during follow-up. The average durations of follow-up were 27.5±8.5 months for the modified group and 25.5±9.5 months for the conventional group. There was no aorta-related reintervention in either the conventional group or the modified group. In the modified group, one patient died of cerebral hemorrhage 24 months after surgery. In the conventional group, one patient died in a traffic accident 15 months after surgery.
Discussion
In our modified technique, the everted end of vascular prosthesis was inserted into the aortic root and the overlap with the aortic stump was about 5–6 mm. In this way, the location of the transition zone between the graft and the aorta was optimal and every part of the anastomotic edge was clearly exposed to the surgeons. Also, it was easy to apply extra hemostatic stitches in case of bleeding as the anastomosis itself was completely external. Furthermore, the vascular prosthesis was not inserted into or pulled out of the left ventricle through the aortic valve, and it did not have any damage to the aortic stump, valve cusps, or left ventricular outflow tract. Moreover, the root adventitia was reinforced by a layer of graft strip, which avoided the adventitial tearing during anastomosis and reduced the chance of stenosis and endoleak originating from the anastomosis site as reported in the literatures (11).
Bleeding from the suture line is commonly encountered in clinical practice, especially on the back wall of the anastomosis (12). In our modified technique, the sutures were performed through the 3-laminar structure by a 5-0 Prolene suture. The lower half of anastomosis was like a series of “I” shape. The upper half of anastomosis was made up of two perfect serial tandem coils. In this way, there was less chance of surgical field errhysis due to anastomotic hemorrhage, and the incidence of re-exploration for bleeding was lower compared with previously reported anastomosis techniques (11,13).
A cell saver device, the Autolog® autotransfusion system (Medtronic, Minneapolis, MN, USA) is routinely used in patients undergoing open surgical repair of aortic aneurysm, which increases the patient’s haemoglobin level and minimizes the risks related to allogenic blood transfusion (14). Massive blood loss followed by massive allogenic blood transfusion greatly is independently associated with serious postoperative complications such as sepsis, renal failure, and respiratory failure (15,16). Our study showed the modified technique dramatically reduced perioperative blood loss. Moreover, the modified technique could significantly shorten the duration in ICU and hospital, and this may be partly due to a decrease in blood loss and transfusion products.
Study limitations
This is a retrospective, single-institutional, and low-volume study which focused on surgical outcomes in patients with aortic aneurysms. Surgical methods and outcomes for dissection patients will be described in future studies. In addition, the surgeon’s preference could be a potential bias of this study. Prospective and randomized studies are needed to improve our results.
Conclusions
The modified prosthesis eversion technique is an effective alternative for proximal anastomosis in ascending aorta replacement, with less blood loss, shorter operation time, and lower rate of postoperative complications compared with the conventional technique.
Acknowledgments
Funding: This work was supported by the Foundation of Logistics Support Department (No. 20WQ004), and the Science and Technology Innovation Capacity Improvement Project of University (No. 2019XYY13).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-550/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-550/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-550/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-550/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Review Board of Southwest Hospital of Army Medical University (Institutional Review Board File KY2021058). The informed consent form was signed by all patients to allow the intervention and data recording.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Spanos K, Nana P, von Kodolitsch Y, et al. Management of Ascending Aorta and Aortic Arch: Similarities and Differences Among Cardiovascular Guidelines. J Endovasc Ther 2022;29:667-77. [Crossref] [PubMed]
- Olsthoorn JR, Lam KY, Akca F, et al. Sutureless aortic valve with supracoronary ascending aortic replacement as an alternative strategy for composite graft replacement in elderly patients. Neth Heart J 2022;30:125-30. [Crossref] [PubMed]
- Prêtre R. Performance of a safe proximal anastomosis in aortic dissection. Ann Thorac Surg 1998;65:1798-9. [Crossref] [PubMed]
- Tang Y, Liao Z, Han L, et al. Long-term results of modified sandwich repair of aortic root in 151 patients with acute type A aortic dissection. Interact Cardiovasc Thorac Surg 2017;25:109-13. [Crossref] [PubMed]
- Inoue Y, Minatoya K, Itonaga T, et al. Utility of Proximal Stepwise Technique for Acute Aortic Dissection Involving the Aortic Root. Ann Thorac Surg 2016;101:e183-5. [Crossref] [PubMed]
- Song L, Gao Y, Xu M, et al. "Sleeve" Sinus of Valsalva Repair in Patients with Acute Type A Aortic Dissection. Heart Surg Forum 2021;24:E418-21. [Crossref] [PubMed]
- Tsutsui M, Ishidou K, Narita M, et al. Modified turn-up technique for proximal anastomosis in acute aortic dissection type A has potential to facilitate stable outcomes for low-volume early-career surgeons. Front Surg 2022;9:917686. [Crossref] [PubMed]
- Vahanian A, Beyersdorf F, Praz F, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease: Developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Rev Esp Cardiol (Engl Ed) 2022;75:524. [Crossref] [PubMed]
- Zheng HJ, Yu SJ, Lin DQ, et al. Simplified total arch reconstruction with a stented graft for extended aortic arch dilation. J Thorac Dis 2023;15:1572-83. [Crossref] [PubMed]
- Zhong L, Xiong H, Li J, et al. Early outcomes of Sun’s procedure in elderly patients with acute aortic dissection: a single-center retrospective study. Early outcomes of Sun's procedure in elderly patients with acute aortic dissection: a single-center retrospective study. J Int Med Res 2022;50:3000605221109377. [Crossref] [PubMed]
- Mokashi SA, Rosinski BF, Desai MY, et al. Aortic root replacement with bicuspid valve reimplantation: Are outcomes and valve durability comparable to those of tricuspid valve reimplantation? J Thorac Cardiovasc Surg 2022;163:51-63.e5. [Crossref] [PubMed]
- Patlolla SH, Saran N, Dearani JA, et al. Outcomes and risk factors of late failure of valve-sparing aortic root replacement. J Thorac Cardiovasc Surg 2022;164:493-501.e1. [Crossref] [PubMed]
- Huang LC, Xu Z, Dai XF. A new patch technique for valve-sparing aortic root repair in acute type A aortic dissection. Ann Transl Med 2021;9:949. [Crossref] [PubMed]
- Task Force on Patient Blood Management for Adult Cardiac Surgery of the European Association for Cardio-Thoracic Surgery (EACTS) and the European Association of Cardiothoracic Anaesthesiology. 2017 EACTS/EACTA Guidelines on patient blood management for adult cardiac surgery. J Cardiothorac Vasc Anesth 2018;32:88-120. [Crossref] [PubMed]
- Shi J, Zhou C, Pan W, et al. Effect of High- vs Low-Dose Tranexamic Acid Infusion on Need for Red Blood Cell Transfusion and Adverse Events in Patients Undergoing Cardiac Surgery: The OPTIMAL Randomized Clinical Trial. JAMA 2022;328:336-47. [Crossref] [PubMed]
- Gupta R. Red Blood Cell Transfusion and Cardiac Surgery-Associated Acute Kidney Injury. JACC Basic Transl Sci 2022;7:639-41. [Crossref] [PubMed]


