
Sex differences in coronary artery bypass graft surgery outcomes: a narrative review
Introduction
Ischemic heart disease is the leading cause of death globally in women and men (1), and coronary artery bypass grafting (CABG) is the most frequently performed cardiac surgery worldwide (2). Approximately 370,000 CABGs are performed in the United States annually (2), and women comprise 20–30% of the CABG population (3). However, women have well-described worse outcomes after CABG, including higher mortality and higher rates of major adverse postoperative events, including stroke and myocardial infarction (MI), when compared with their male counterparts (4-6). Herein we present a comprehensive narrative review of the current evidence on sex disparities in CABG outcomes, along with proposed etiopathologies for, and factors contributing to, this outcomes difference. We also discuss future directions to improve CABG outcomes in women. We present this article in accordance with the Narrative Review reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-294/rc).
Methods
We searched PubMed for references with the terms “coronary artery bypass graft outcomes”, “sex differences in coronary artery bypass graft”, “coronary artery bypass graft AND women”, and “sex disparities in coronary artery bypass graft”, or their combination in the title or abstract. We also identified relevant articles from the references lists of selected articles. We prioritized randomized trials and publications from the last 15 years but cited other references where historically relevant and necessary. The search strategy is summarized in Table 1.
Table 1
| Items | Specification |
|---|---|
| Date of search | 11/1/2022 |
| Databases and other sources searched | PubMed |
| Search terms used | “coronary artery bypass graft outcomes”, “sex differences in coronary artery bypass graft”, “coronary artery bypass graft AND women”, “sex disparities in coronary artery bypass graft” |
| Timeframe | 2007–2022 |
| Inclusion criteria | Prioritized English-language and randomized trials, included observational studies |
| Selection process | Junior and senior author(s) agreement |
| Any additional considerations | Identification of historically relevant or related articles by agreement between junior and senior author |
Outcomes in women after CABG
Women undergoing CABG have a well-described higher operative mortality when compared with men (4-10). The higher operative mortality in women is sufficiently well-demonstrated such that both the European risk prediction models for operative mortality after cardiac surgery, the 1999 EuroSCORE I (11) and the 2012 EuroSCORE II (12), which are based on a multi-national, multi-center database that found that women had significantly higher mortality than men, and the 2018 Society of Thoracic Surgeons (STS) Adult Cardiac Surgery Risk Model (13), include female sex as a variable that is predictive of operative mortality. In a retrospective study from the STS national database including 1,042,506 patients (25.1% women) undergoing isolated CABG from 2011–2018 in the United States, Enumah et al. (7) found that women had an unadjusted higher operative (30-day) mortality than men [odds ratio (OR): 1.68, 95% confidence interval (CI): 1.63–1.73]. On multivariable analysis, women had a higher risk of death than men (OR: 1.26, 95% CI: 1.21–1.30) at 30 days. A retrospective cohort study of all fee-for-service Medicare patients undergoing CABG from 1999–2014 including 1,863,719 patients (33.6% women) found that over the course of the study period, women had persistently higher in-hospital mortality compared with men (1999: 6.6%, 95% CI: 5.4–6.6 versus 4.3%, 95% CI: 4.2–4.5, respectively, and 2014: 4.8%, 95% CI: 4.5–5.1 versus 2.7%, 95% CI: 2.6–2.9, respectively), despite a greater adjusted annual decline in in-hospital mortality among women (−2.70%, 95% CI: −2.97% to −2.44%) than men (−2.44%, 95% CI: −2.67% to −2.21%) (9). In a meta-analysis by Shi et al. (8) including 112 observational studies and 5,008,262 patients (28.8% women), women had a 30-day mortality of 4.9% compared with 3.3% in men (P<0.001, unadjusted OR: 1.81, 95% CI: 1.72–1.91). Even among the 25 studies that reported risk-adjusted outcomes to account for the baseline heterogeneity between men and women, there was an association between female sex and a higher risk of 30-day mortality (adjusted OR: 1.40, 95% CI: 1.35–1.45).
While worse operative mortality has been repeatedly demonstrated in women undergoing CABG, data has been mixed regarding the presence of a sex difference in long-term CABG outcomes. In a 2019 retrospective cohort study of 52,546 (20.5% women) undergoing isolated CABG from 2008–2016, Johnston et al. (14) found that at a median of 5 years of follow-up [interquartile range (IQR), 3 to 7 years], mortality was higher in women than men [35.2% versus 26.5%, adjusted hazard ratio (HR): 1.15, 95% CI: 1.08–1.21]. However, in a 2004 Ontario database study by Guru et al. (15) of 54,425 isolated CABG patients (22.2% women), mortality was higher in women at one-year follow-up (adjusted HR: 1.44, 95% CI: 1.29–1.61; P=0.02) but not beyond that time (adjusted HR: 0.89, 95% CI: 0.78–1.0; P=0.06). In a meta-analysis of 20 studies and 966,492 patients (28.7% women) undergoing isolated CABG, Alam et al. (16) found that 30-day mortality was significantly higher in women (OR: 1.77, 95% CI: 1.67–1.88), and that mortality at one year (OR: 1.31, 95% CI: 1.18–1.45) and five years (OR: 1.14, 95% CI: 1.08–1.20) was persistently higher in women. Most recently, in a large retrospective analysis of over one million CABG patients (24.5% women) from the STS database from 2010 to 2020 (17), women had a higher unadjusted operative mortality (2.8%, 95% CI: 2.8–2.9% versus 1.7%, 95% CI: 1.7–1.7%; P<0.001), and the attributable risk to female sex for operative mortality ranged from 1.28 in 2011 to 1.41 in 2020, with no change over the study period (P for trend =0.38). The aforementioned studies are summarized in Table 2.
Table 2
| Author | Year | Study period | Sample size (n) | Follow-up | Mortality outcomes |
|---|---|---|---|---|---|
| Gaudino et al. (17) | 2023 | 2011–2020 | 1,297,204, women: 24.5% | 30 days | Unadjusted 30-day mortality: 2.8% (95% CI: 2.8–2.9%) in women vs. 1.7% (95% CI: 1.7–1.7%) in men, P<0.001 |
| 30-day mortality attributable risk to female sex: 1.28 in 2011 to 1.41 in 2020, P for trend =0.38 | |||||
| Enumah et al. (7) | 2020 | 2011–2018 | 1,042,506, women: 25.1% | 30 days | Unadjusted 30-day mortality, women: OR: 1.68 (95% CI: 1.63–1.73) |
| MVA for 30-day mortality, women: OR: 1.26 (95% CI: 1.21–1.30) | |||||
| Shi et al. (8) | 2022 | N/A | 5,008,262, women: 28.8% | 30 days | Unadjusted 30-day mortality, women: OR: 1.81 (95% CI: 1.72–1.91) |
| Adjusted 30-day mortality, women: OR: 1.40 (95% CI: 1.35–1.45) | |||||
| Angraal et al. (9) | 2018 | 1999–2014 | 1,863,719, women: 33.6% | 30 days | 30-day mortality |
| 1999: 6.6% (95% CI: 5.4–6.6%) in women vs. 4.3% (95% CI: 4.2–4.5%) in men | |||||
| 2014: 4.8% (95% CI: 4.5–5.1%) in women vs. 2.7% (95% CI: 2.6–2.9%) in men | |||||
| Adjusted annual decline in 30-day mortality | |||||
| Women: −2.70% (95% CI: −2.97% to −2.44%) | |||||
| Men: −2.44% (95% CI: −2.67% to −2.21%) | |||||
| 1 year | Adjusted annual decline in 1-year mortality | ||||
| Women: −1.67% (95% CI: −1.88% to −1.46%) | |||||
| Men: −1.20% (95% CI: −1.37% to −1.03%) | |||||
| Guru et al. (15) | 2004 | 1991–1999 | 54,425, women: 22.2% | 30 days | Adjusted 30-day mortality, women: OR: 1.45 (95% CI: 1.23–1.63) |
| 1 year | Adjusted 1-year mortality, women: HR: 1.44 (95% CI: 1.29–1.61) | ||||
| >1 year | Adjusted late (>1-year) mortality, women: HR: 0.89 (95% CI: 0.78–1.02) | ||||
| Johnston et al. (14) | 2019 | 2008–2016 | 52,546, women: 20.5% | 5 years | Adjusted mortality, women: HR: 1.15 (95% CI: 1.08–1.21) |
Adapted with permission from Gaudino et al. (18). CABG, coronary artery bypass grafting; CI, confidence interval; OR, odds ratio; MVA, multivariable analysis; N/A, not applicable; HR, hazard ratio.
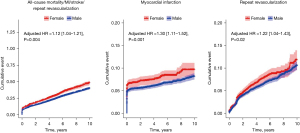
Women also have higher rates of morbidity after CABG, including stroke and MI (5,6,19). In a meta-analysis of 84 observational studies and 903,346 patients (24.8% women), Bryce et al. (6) found that women were at a higher risk of major adverse cardiac events, defined as a composite of stroke, repeat revascularization, and MI [incidence rate ratio (IRR): 1.30, 95% CI: 1.19–1.66; P<0.001], stroke alone (IRR: 1.31, 95% CI: 1.15–1.51; P>0.001), and MI alone (IRR: 1.28, 95% CI: 1.13–1.45; P<0.001), but not repeat revascularization (IRR: 0.99, 95% CI: 0.76–1.29; P=0.95). Yet in a 2021 individual patient data meta-analysis including four CABG randomized trials and 13,193 patients (20.6%women), Gaudino et al. (5) found that over 5 years of follow-up, women had a higher risk of MI (adjusted HR: 1.30, 95% CI: 1.11–1.52; P=0.001) and repeat revascularization (adjusted HR: 1.22, 95% CI: 1.04–1.43; P=0.02) individually (Figure 1).
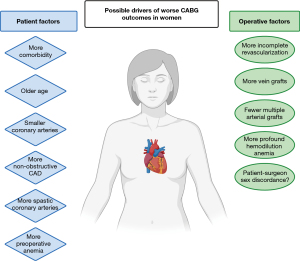
The driver of the described sex difference in outcomes after CABG remains unclear. Proposed etiopathologies that may contribute to worse outcomes in women include differences in baseline characteristics between men and women at time of presentation for CABG, differences in pathophysiology and the natural history of coronary artery disease (CAD), differences in surgical technique employed, and differences in body size including body surface area (BSA) and its implications on preoperative and intraoperative anemia and on coronary artery size and technical complexity of the surgery itself (Figure 2). In the following text we summarize the existing evidence on each of these proposed mechanisms.
Preoperative and patient-related factors
Differences in comorbidity
Women present for CABG later than men, with older age and more cardiovascular risk factors than men at time of presentation (4-8,16), and it has been suggested that these baseline preoperative differences account for the relatively poorer outcomes in women when compared with men. Said differences may be due, in part, to delayed diagnosis of CAD or lack of referral for surgical revascularization (18,20,21). For instance, in a retrospective, multi-center study of 1,064 patients (215 women) with multivessel CAD, Fink et al. demonstrated that male sex was associated with referral to CABG (OR: 2.27; P<0.001) (20). In their retrospective study of over one million patients from the STS database, Enumah et al. (7) found that women presented with advanced age and with a higher incidence of cardiac risk factors, including hypertension and diabetes, than their male counterparts (7). The aforementioned meta-analysis of over 20 studies and almost one million patients by Alam et al. (16) found women were older than men at time of presentation (67.7 versus 63.0 years; P=0.04), had a higher incidence of hypertension (69% versus 60%; P<0.01), diabetes (34% versus 25%; P<0.01), and urgent (rather than elective) CABG (51% versus 44%; P<0.001).
Yet in a propensity-matched study of 3,600 isolated CABG patients by the same group, female sex was an independent predictor of operative mortality both before (OR: 1.67, 95% CI: 1.35–1.67) and after (OR: 1.84, 95% CI: 1.22–2.78) propensity matching between sexes. Similarly, in the previously discussed individual patient data meta-analysis of over 13,000 isolated CABG patients by Gaudino et al. (5), women were older and had higher incidence of diabetes, hypertension, and peripheral vascular disease than men. However, even after risk-adjustment for these factors, the risk of the composite of mortality, MI, stroke, and repeat revascularization was significantly higher in women (adjusted HR: 1.12, 95% CI: 1.04–1.21; P=0.004). While risk-adjustment and propensity matching cannot fully account for patient heterogeneity, allocation bias, or low-quality data, the persistent association between women and relatively worse postoperative outcomes even after such adjustments are made suggests that the outcomes disparity may be driven by factors beyond differences in preoperative patient comorbidity.
Pathophysiology and natural history
The underlying pathophysiology of CAD in women may play a role in relatively poorer outcomes after surgery. While obstructive CAD predominates in men, women are over-represented among patients with ischemia secondary to non-obstructive CAD (22,23). MI secondary to non-obstructive CAD, including coronary spasm, dissection, or emboli (24), is similarly more prevalent among women (25-27). In a retrospective, multicenter registry study of 322,523 patients who underwent coronary angiography for MI, non-obstructive CAD was found in 18,918 patients, of whom women comprised 10.5%, a significantly higher proportion than men (3.4%, P<0.0001) (26). In addition, prior evidence in patients with CAD undergoing percutaneous revascularization has shown that compared to men, women are more likely to have microvascular disease that, like non-obstructive CAD, may not be effectively treated by revascularization (28).
Coronary arteries in women have been found to be more prone to spasm when compared with men (29-32). In a study of 1,379 consecutive patients with stable angina, Aziz et al. found on multivariable logistic regression that women were more likely to have both coronary microvascular dysfunction (OR: 4.2, 95% CI: 3.1–5.5; P<0.001) and epicardial vasospasm (OR: 2.3, 95% CI: 1.7–3.1; P<0.001), respectively, on provocative testing (32). The higher spasticity of coronary arteries in women may render the operation technically more challenging, contributing to poorer outcomes (33).
Coronary artery size
Coronary artery diameter has been inversely related to perioperative morbidity and has been proposed to explain both the observed lower graft patency and increased mortality among women (34,35). Women have been found to have smaller conduits and smaller target coronary arteries than men (36-39). In a 1993 multi-center study of prospectively-collected data on 3,055 CABG patients, O’Connor et al. found that lower BSA (<1.6) was associated with an increased risk of in-hospital mortality when compared with higher BSA (>2.0; OR: 2.84; P for trend =0.007) (40). In a subgroup of 945 patients in whom the target coronary artery diameter was measured, there was a strong positive correlation (Pearson’s correlation, r=0.83–0.98) between coronary luminal diameter and BSA (40). However, in this study, BSA, target artery diameter, and outcomes were not reported by sex. A follow-up prospective observational study by the same group of 1,325 CABG patients (27.3% women) found that small target coronary vessel size was associated with an increased in-hospital mortality (15.8% for 1.0 mm vessels, 4.6% for 1.5 to 2.0 mm vessels, and 1.5% for 2.5 to 3.5 mm vessels, P for trend <0.001), and that the average mid-left anterior descending (LAD) artery diameter in women was significantly smaller than in men (2.04 versus 2.81 mm, respectively; P<0.001) (41). On multiple linear regression, target vessel size was positively correlated with both body mass index (BMI) and BSA. When patients were stratified by BMI and by BSA, there was still a small but significant difference in mid-LAD diameter between men and women (BSA: mean size difference range: 0.15–0.18 mm; P for trend <0.001; BMI: mean size difference range: 0.22–0.23 mm; P for trend <0.004) (41). More recent studies have demonstrated an association between sex and smaller coronary artery size utilizing techniques such as intravascular ultrasound, computed tomography, and measurement with vascular sheaths (36-39). Proposed mechanisms to explain the relationship between small vessel diameter and poorer outcomes may include an increased risk of thrombosis and occlusion in smaller vessels, technical difficulty in operating and sewing anastomoses on smaller vessels, or decreased short-term patency in conduits grafted to smaller target vessels (42).
However, in a 1995 prospective observational study of 1,132 men and 355 women referred for CABG, Mickleborough et al. found that women had smaller body size than men (men’s mean BSA 1.9±0.2, women’s mean BSA 1.7±0.2; P<0.001) but were not more likely to have small (<1.5 mm diameter) target vessels (30.9% of men versus 31.3% of women; no P value provided) (43). This raises the question of whether smaller vessel size truly drives the outcomes disparity in women, or if smaller body size in women is a surrogate for another risk factor for operative mortality.
Operative factors
Completeness of revascularization
Incomplete revascularization has repeatedly been associated with worse survival after CABG (44-46). A retrospective study of 1,034 CABG patients found that incomplete revascularization, defined as the absence of grafts to territories supplied by vessels with 50% stenosis or more, predicted an increased risk of cardiac death (HR: 1.85, 95% CI: 1.03–3.34; P=0.04) (45). On multivariable logistic regression, female sex was a predictor of incomplete revascularization (OR: 2.09, 95% CI: 1.37–3.66; P=0.002). Failure of complete revascularization in all patients in this study was most often attributed to coronary arteries being too small, too severely diseased, or both (45); women have previously been found to have smaller coronary arteries (discussed above) (40), which is suggestive of a possible association. In a retrospective study of 1,212,487 patients (24.9% women) from the STS Adult Cardiac Surgery Database (47) undergoing first-time isolated CABG, Jawitz et al. found that women had lower odds of undergoing complete revascularization, defined as cases where the number of distal anastomoses was greater than or equal to the number of diseased myocardial territories, than their male counterparts (adjusted OR: 0.86, 95% CI: 0.83–0.90; P<0.001). A retrospective study of 7,157 patients (30% women) undergoing primary CABG who received left internal thoracic artery (LITA)-to-LAD grafting found that incomplete revascularization, defined as a completeness of revascularization index (the difference between the number of coronary grafts and the number of diseased coronary systems) ≤ one, was associated with increased long-term (15-year) mortality among all patients (adjusted HR: 1.53, 95% CI: 1.29–1.80) (48). The proportion of women decreased as completeness of revascularization increased in this cohort (P<0.001); female sex was also a predictor of incomplete revascularization (OR: 1.39, 95% CI: 1.06–1.81) (48). Unfortunately, there is no universal definition of incomplete revascularization, which is a limitation of the existing evidence on this topic. The reason for the greater rate of incomplete revascularization in women rather than men is, at this time, unclear. While proposed mechanisms have included greater technical difficulty in women and operating on smaller coronary artery vessels, and poor tolerance of cardiopulmonary bypass, there is no definitive explanation at this time (48).
Conduit selection
Grafts utilized for CABG are either arterial, harvested from the chest wall [LITA and right internal thoracic artery (RITA)], arms [radial artery (RA)], and less commonly, the abdomen [right gastroepiploic artery (RGEA)], or venous, harvested from the legs [saphenous vein grafts (SVGs)]. SVGs are more susceptible to atherosclerotic burden and have demonstrated higher rates of graft failure, or occlusion preventing blood flow to the portion of the heart targeted for revascularization, than arterial grafts. The LITA has a reported short-term (1-year) graft failure rate of <5%, and a long-term (10-year) graft failure rate of 10–15% (49,50), the RITA has a similar short-term failure rate and a long-term failure rate of 10–25% (50), and the RA has a reported short-term failure rate of 4–8%, with long-term failure estimated at 11–15% (50,51). SVGs have a reported short-term failure rate ranging from 10–25% and a long-term failure rate of 40–50% (49-51).
In patients undergoing CABG, professional society guidelines recommend utilizing the LITA to bypass the LAD, due to decades of observational data that suggest improved patency and outcomes compared with other conduits [American College of Cardiology (ACC)/American Heart Association (AHA)/Society for Cardiovascular Angiography and Interventions (SCAI) Class 1, Level of Evidence B-NR; European Society of Cardiology (ESC)/European Association for Cardio-Thoracic Surgery (EACTS): Class 1, Level of Evidence B] (52,53). The RA is recommended as the second arterial conduit in lieu of the SVG to bypass the second most important coronary target after the LAD (ACC/AHA/SCAI Class 1, Level of Evidence B; ESC/EACTS: Class 1, Level of Evidence B) (52,53). Despite these professional guideline recommendations and the evidence of poorer patency rates with SVG grafting when compared with arterial grafting, SVGs remain the most commonly used graft in CABG (54). Women are even more likely than men to receive SVGs in lieu of any arterial grafting (55) or multiple-arterial grafting (56). In a retrospective study of 57,943 patients undergoing primary elective CABG (19% women), Attia et al. (55) found that women received less all-arterial conduits, and less arterial grafting at all, than men. In the previously mentioned retrospective study of over one million patients, Jawitz et al. found that women were less likely to receive LITA grafting to the LAD (adjusted OR: 0.79, 95% CI: 0.75–0.83; P<0.001) and were less likely to undergo multiple arterial grafting (adjusted OR: 0.78, 95% CI: 0.75–0.81; P<0.001) than men (47), findings that persisted over the duration of the study.
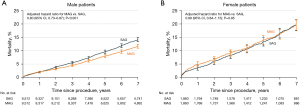
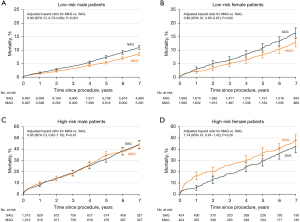
Another consideration beyond whether women receive multiple arterial grafting is whether they derive similar benefits from multiple arterial grafting as men. In a retrospective analysis of the New York State Cardiac Surgery Reporting System of all CABG patients from 2005 to 2014, just 13.8% of women, compared with 21.9% of men, received multiple arterial grafting (57). After propensity-matching based on preoperative risk profiles as well as extent of CAD and surgeon experience, there were 9,512 male pairs and 1,860 female pairs who receiving single versus multiple arterial grafting. Men who received multiple arterial grafting were found to derive a mortality benefit (adjusted HR: 0.80, 95% CI: 0.73–0.87; P<0.001), whereas women did not (adjusted HR: 0.99, 95% CI: 0.84–1.15; P=0.85) (Figure 3) (57). However, when patients were stratified into high-risk and low-risk groups, low-risk patients of both sexes were found to derive a mortality and morbidity benefit from arterial grafting (men: adjusted HR: 0.80, 95% CI: 0.73–0.89; P<0.001 and women: adjusted HR: 0.80, 95% CI: 0.65–0.97; P=0.02), but the high-risk patients of both sexes did not (men: adjusted HR: 0.95, 95% CI: 0.82–1.10; P=0.47 and women: adjusted HR: 1.14, 95% CI: 0.91–1.42; P=0.26) (Figure 4). The apparent lack of benefit of multiple arterial grafting in women overall may have been a reflection of the greater proportion of women who were high-risk compared to men (13.0% versus 6.0%). Ultimately, a randomized control trial on multiple arterial grafting in women is needed to provide the prospective data on this topic that will better guide surgeons and patients in determining the best operative plan. The results of the ongoing ROMA:Women trial (ClinicalTrials.gov: NCT04124120) will hopefully bring clarity to this issue.
Lastly, the choice of arterial conduit may have an impact on outcomes that is modified by sex. In an individual patient data analysis of six CABG studies and 1,036 patients (534 with RA grafts, 502 with SVGs), Gaudino et al. (58) found that the use of RA grafts was associated with a significant reduction in the incidence of the composite of death, MI, or repeat revascularization (HR: 0.73, 95% CI: 0.61–0.88; P<0.001). They found that the benefit of RA grafts was even more pronounced in women (HR: 0.51, 95% CI: 0.36–0.72) compared with men (HR: 0.84, 95% CI: 0.61–1.05) (58). However, bilateral ITA grafting has been shown to be associated with sternal wound infection in women (55,59). In a retrospective study including 2,979 patients receiving BITA (10% women), women had a higher incidence of postoperative mediastinitis compared with men (3.3% versus 1.5%; P=0.02), making this a less attractive choice of conduit in women undergoing CABG.
Anemia and hemodilution
Preoperative anemia and intraoperative hemodilution have previously been demonstrated to be associated with increased mortality and morbidity after CABG (60). A 2003 retrospective, single-center study of 5,000 CABG patients (34.1% women) found that operative mortality, stroke, MI, low cardiac output, cardiac arrest, renal failure, prolonged ventilation, pulmonary edema, reoperation due to bleeding, and multiorgan failure were all significantly increased as nadir intraoperative hematocrit dropped below 22% (P for all <0.001). In addition, the incidence of operative mortality was significantly greater with increasing severity of hemodilution anemia. On stepwise multivariate linear regression, both BSA and female sex were found to be independent predictors of the nadir hematocrit.
In a 2001 retrospective analysis of 3,560 consecutive CABG patients (30.9% women) by Schwann et al., both mortality and adverse cardiovascular events were increased among patients with lower BMI (defined as BMI 24 kg/m2 and below) (61). On univariate analysis, the authors also found lower preoperative and postoperative hematocrit in those patients with lower BMI (BMI 24 kg/m2 and below). Women were disproportionately represented among patients at the extremes of BMI, including very low BMI (61). The previously discussed 2004 retrospective analysis by Guru et al. (15) of over 50,000 isolated CABG patients and more than 12,000 women found that female sex conferred an increased risk of death within the first year of surgery. However, after adjustment for BSA (reported in 13,921 patients and 3,172 women), female sex was no longer a risk factor for early mortality (HR: 1.04, 95% CI: 0.84–1.30; P=0.72). Unfortunately, this study did not report on perioperative hematocrit, making it difficult to establish anemia as a link between small body size and mortality. The largest recent study on body size and outcomes in cardiac surgery demonstrated the ‘obesity paradox’, given that obesity is a risk factor for cardiovascular disease and death (62). A 2017 observational study of 401,227 patients (23.7% women) undergoing cardiac surgery, obesity was associated with decreased mortality in patients with BMI 25 to 30 kg/m2 (OR: 0.79, 95% CI: 0.76–0.83), BMI 30 to 35 kg/m2 (OR: 0.81, 95% CI: 0.76–0.86), and BMI 35 to 40 kg/m2 (OR: 0.83, 95% CI: 0.75–0.94) (62). However, there was no stratification within BMI groups by sex. In addition, women were again unevenly distributed across BMI groups, with the majority of women either being in the BMI <18 kg/m2 category (58%) and the BMI >40 kg/m2 category (45%) (62). This study also did not report on perioperative hematocrit levels, intraoperative transfusion requirements, or hemodilution secondary to cardiopulmonary bypass. Therefore, it did not clarify if body habitus is a surrogate for coronary artery diameter or profundity of perioperative anemia and hemodilution, nor did it clarify if female sex is a surrogate for body size. Further research is needed to clarify the relationship between sex, body size, and hemodilution or anemia.
Future directions
The relationship between hemodilution, anemia, sex, and body size merits further investigation. While the previously discussed studies have examined each of these factors independently, a large-scale study is needed to elucidate the effect of profound intraoperative anemia and the ensuing ischemia and malperfusion suffered by patients undergoing cardiopulmonary bypass, and whether there is a subsequent sex disparity in outcomes.
It has been a traditionally accepted belief that CABG patients’ clinical status is tied to the patency of their grafts (understanding, of course, that numerous patient and graft-related factors may modulate this relationship) (63). Unfortunately, data on this is drawn from older studies; few contemporary prospective studies have performed systematic graft imaging, and those that have, have yielded conflicting results (63-68). In a 2012 post-hoc analysis of the PREVENT IV trial including 1,829 CABG patients with imaging at four-year follow-up, vein graft failure was associated with the composite of death, MI, or repeat revascularization (adjusted HR: 1.58, 95% CI: 1.21–2.06; P=0.008), but not with death alone (adjusted HR: 1.04, 95% CI: 0.71–1.52; P=0.85), or death and MI (adjusted HR: 1.08, 95% CI: 0.77–1.53; P=0.65).(67) However, in the SYNTAX-LE MANS sub-study including 114 CABG patients with left mainstem CAD, the composite of death, MI, stroke, or repeat revascularization was not significantly associated with graft occlusion (P=0.85) at 15-month angiographic follow-up (69). There is even less data regarding the relationship between sex, outcomes, and graft patency. A recent meta-analysis of 17 studies (eight randomized controlled trials and nine observational studies), 8,235 patients, and 14,871 SVGs found no significant difference in SVG patency between men and women (IRR: 0.96, 95% CI: 0.90–1.03; P=0.24) at mean follow-up of 33.5 months, a finding that challenges the accepted patency-outcomes relationship (70). However, of 61.5% of the published studies in graft patency were excluded from this meta-analysis: 100 were excluded for failing to report patency by sex, and an additional 44 were excluded by virtue of having less than ten women enrolled. More data overall, and more prospective data in particular, are needed to better understand the relationships between sex, graft patency, and clinical outcomes, especially given the relatively higher proportion of women with non-obstructive CAD when compared with men.
In a recent cross-sectional study of over 1.5 million Medicare hospitalizations, patients treated by female physicians had lower rates of readmission and mortality when compared with male physicians (71). The general surgery literature has further examined the issue of patient-sex concordance. The largest of these studies, by Wallis et al. (72), showed that sex discordance between surgeon and patient was associated with a significantly increased likelihood of the composite of adverse postoperative outcomes (adjusted OR: 1.07, 95% CI: 1.04–1.09). Patient sex significantly modified this association, with worse outcomes for women treated by men in comparison with women treated by women (adjusted OR: 1.11, 95% CI: 1.06–1.16). Overall, women surgeons also had lower rates of morbidity and mortality than men. Although the aforementioned paper did provide subgroup analyses by subspecialty and found that patient-surgeon sex discordance was associated with the composite outcome of mortality, readmission, or complications (adjusted OR: 1.10, 95% CI: 1.06–1.13; P=0.02). However, cardiothoracic cases comprised a small proportion of the total included cases. Such a study has not been attempted specifically in cardiothoracic surgery, a field in which this may be particularly salient given the described poorer CABG outcomes in women.
Limitations
The limitations of this work must be acknowledged. We have conducted a narrative review, which is intended to provide an overview and discussion of the evidence regarding sex differences in CABG outcomes. Due to both space limitations and the nature of a narrative review, factors contributing to sex differences in CABG outcomes that have limited supporting evidence or for which the evidence is mixed may have been excluded from the discussion. In addition, the vast majority of randomized cardiac surgery trials have been conducted in overwhelmingly male populations, limiting the availability of high-quality prospective data on outcomes in women after cardiac surgery.
Conclusions
Women have worse operative mortality and morbidity than men after undergoing CABG, and this disparity has persisted over time despite changes and advancements in the field of cardiac surgery. The root cause of this problem has yet to be elucidated despite prior research into baseline patient characteristics and comorbidity, the natural history of CAD in women, and sex differences in operative technique and the possible adverse effects of profound hemodilution and anemia. The relationship between graft status and clinical outcomes, between sex and intraoperative anemia, and between patient-surgeon sex concordance and clinical outcomes have yet to be clearly delineated and present promising opportunities for research that may help close the outcomes gap between men and women.
Acknowledgments
Funding: LH is partially supported by
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-294/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-294/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-294/coif). MG serves as an unpaid editorial board member of Journal of Thoracic Disease from October 2022 to September 2024. LH is partially supported by a T-32 Multidisciplinary Research Training Grant in Cardiovascular Disease from the National Heart, Lung, and Blood Institute (No. 1 T32 HL160520-01A1). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Roth GA, Mensah GA, Johnson CO, et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990-2019: Update From the GBD 2019 Study. J Am Coll Cardiol 2020;76:2982-3021. Correction appears in J Am Coll Cardiol 2021;77:1958-9.
- Benjamin EJ, Muntner P, Alonso A, et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation 2019;139:e56-e528. Correction appears in Circulation 2020;141:e33.
- Gaudino M, Di Mauro M, Fremes SE, Di Franco A. Representation of Women in Randomized Trials in Cardiac Surgery: A Meta-Analysis. J Am Heart Assoc 2021;10:e020513. [Crossref] [PubMed]
- Alam M, Lee VV, Elayda MA, et al. Association of gender with morbidity and mortality after isolated coronary artery bypass grafting. A propensity score matched analysis. Int J Cardiol 2013;167:180-4. [Crossref] [PubMed]
- Gaudino M, Di Franco A, Alexander JH, et al. Sex differences in outcomes after coronary artery bypass grafting: a pooled analysis of individual patient data. Eur Heart J 2021;43:18-28. [Crossref] [PubMed]
- Bryce Robinson N, Naik A, Rahouma M, et al. Sex differences in outcomes following coronary artery bypass grafting: a meta-analysis. Interact Cardiovasc Thorac Surg 2021;33:841-7. [Crossref] [PubMed]
- Enumah ZO, Canner JK, Alejo D, et al. Persistent Racial and Sex Disparities in Outcomes After Coronary Artery Bypass Surgery: A Retrospective Clinical Registry Review in the Drug-eluting Stent Era. Ann Surg 2020;272:660-7. [Crossref] [PubMed]
- Shi D, Zhang B, Motamed M, et al. Higher Mortality in Women After Coronary Artery Bypass: Meta-analysis and Bias Analysis of Confounding. Ann Thorac Surg 2022;113:674-80. [Crossref] [PubMed]
- Angraal S, Khera R, Wang Y, et al. Sex and Race Differences in the Utilization and Outcomes of Coronary Artery Bypass Grafting Among Medicare Beneficiaries, 1999-2014. J Am Heart Assoc 2018;7:e009014. [Crossref] [PubMed]
- Dixon LK, Di Tommaso E, Dimagli A, et al. Impact of sex on outcomes after cardiac surgery: A systematic review and meta-analysis. Int J Cardiol 2021;343:27-34. [Crossref] [PubMed]
- Nashef SA, Roques F, Michel P, et al. European system for cardiac operative risk evaluation (EuroSCORE). Eur J Cardiothorac Surg 1999;16:9-13. [Crossref] [PubMed]
- Nashef SA, Roques F, Sharples LD, et al. EuroSCORE II. Eur J Cardiothorac Surg 2012;41:734-44; discussion 744-5. [Crossref] [PubMed]
- Shahian DM, Jacobs JP, Badhwar V, et al. The Society of Thoracic Surgeons 2018 Adult Cardiac Surgery Risk Models: Part 1-Background, Design Considerations, and Model Development. Ann Thorac Surg 2018;105:1411-8. [Crossref] [PubMed]
- Johnston A, Mesana TG, Lee DS, et al. Sex Differences in Long-Term Survival After Major Cardiac Surgery: A Population-Based Cohort Study. J Am Heart Assoc 2019;8:e013260. [Crossref] [PubMed]
- Guru V, Fremes SE, Tu JV. Time-related mortality for women after coronary artery bypass graft surgery: a population-based study. J Thorac Cardiovasc Surg 2004;127:1158-65. [Crossref] [PubMed]
- Alam M, Bandeali SJ, Kayani WT, et al. Comparison by meta-analysis of mortality after isolated coronary artery bypass grafting in women versus men. Am J Cardiol 2013;112:309-17. [Crossref] [PubMed]
- Gaudino M, Chadow D, Rahouma M, et al. Operative Outcomes of Women Undergoing Coronary Artery Bypass Surgery in the US, 2011 to 2020. JAMA Surg 2023;158:494-502. [Crossref] [PubMed]
- Gaudino M, Di Franco A, Cao D, et al. Sex-Related Outcomes of Medical, Percutaneous, and Surgical Interventions for Coronary Artery Disease: JACC Focus Seminar 3/7. J Am Coll Cardiol 2022;79:1407-25. [Crossref] [PubMed]
- Ahmed WA, Tully PJ, Knight JL, et al. Female sex as an independent predictor of morbidity and survival after isolated coronary artery bypass grafting. Ann Thorac Surg 2011;92:59-67. [Crossref] [PubMed]
- Fink N, Nikolsky E, Assali A, et al. Revascularization Strategies and Survival in Patients With Multivessel Coronary Artery Disease. Ann Thorac Surg 2019;107:106-11. [Crossref] [PubMed]
- Hansen KW, Soerensen R, Madsen M, et al. Developments in the invasive diagnostic-therapeutic cascade of women and men with acute coronary syndromes from 2005 to 2011: a nationwide cohort study. BMJ Open 2015;5:e007785. [Crossref] [PubMed]
- Reynolds HR, Picard MH, Spertus JA, et al. Natural History of Patients With Ischemia and No Obstructive Coronary Artery Disease: The CIAO-ISCHEMIA Study. Circulation 2021;144:1008-23. [Crossref] [PubMed]
- Shaw LJ, Merz CN, Pepine CJ, et al. The economic burden of angina in women with suspected ischemic heart disease: results from the National Institutes of Health--National Heart, Lung, and Blood Institute--sponsored Women's Ischemia Syndrome Evaluation. Circulation 2006;114:894-904. [Crossref] [PubMed]
- Ibánez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Rev Esp Cardiol (Engl Ed) 2017;70:1082. [Crossref] [PubMed]
- Agewall S, Beltrame JF, Reynolds HR, et al. ESC working group position paper on myocardial infarction with non-obstructive coronary arteries. Eur Heart J 2017;38:143-53. [Crossref] [PubMed]
- Smilowitz NR, Mahajan AM, Roe MT, et al. Mortality of Myocardial Infarction by Sex, Age, and Obstructive Coronary Artery Disease Status in the ACTION Registry-GWTG (Acute Coronary Treatment and Intervention Outcomes Network Registry-Get With the Guidelines). Circ Cardiovasc Qual Outcomes 2017;10:e003443. [Crossref] [PubMed]
- Reynolds HR, Shaw LJ, Min JK, et al. Association of Sex With Severity of Coronary Artery Disease, Ischemia, and Symptom Burden in Patients With Moderate or Severe Ischemia: Secondary Analysis of the ISCHEMIA Randomized Clinical Trial. JAMA Cardiol 2020;5:773-86. [Crossref] [PubMed]
- Kosmidou I, Leon MB, Zhang Y, et al. Long-Term Outcomes in Women and Men Following Percutaneous Coronary Intervention. J Am Coll Cardiol 2020;75:1631-40. [Crossref] [PubMed]
- Pepine CJ, Anderson RD, Sharaf BL, et al. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women's Ischemia Syndrome Evaluation) study. J Am Coll Cardiol 2010;55:2825-32. [Crossref] [PubMed]
- Wong PS, Roberts RE, Randall MD. Sex differences in the role of transient receptor potential (TRP) channels in endothelium-dependent vasorelaxation in porcine isolated coronary arteries. Eur J Pharmacol 2015;750:108-17. [Crossref] [PubMed]
- Wong PS, Roberts RE, Randall MD. Sex differences in endothelial function in porcine coronary arteries: a role for H2O2 and gap junctions? Br J Pharmacol 2014;171:2751-66. [Crossref] [PubMed]
- Aziz A, Hansen HS, Sechtem U, et al. Sex-Related Differences in Vasomotor Function in Patients With Angina and Unobstructed Coronary Arteries. J Am Coll Cardiol 2017;70:2349-58. [Crossref] [PubMed]
- Hessian R, Jabagi H, Ngu JMC, et al. Coronary Surgery in Women and the Challenges We Face. Can J Cardiol 2018;34:413-21. [Crossref] [PubMed]
- Fisher LD, Kennedy JW, Davis KB, et al. Association of sex, physical size, and operative mortality after coronary artery bypass in the Coronary Artery Surgery Study (CASS). J Thorac Cardiovasc Surg 1982;84:334-41.
- Kennedy JW, Kaiser GC, Fisher LD, et al. Clinical and angiographic predictors of operative mortality from the collaborative study in coronary artery surgery (CASS). Circulation 1981;63:793-802. [Crossref] [PubMed]
- Taqueti VR. Sex Differences in the Coronary System. In: Kerkhof PLM, Miller VM, editors. Sex-Specific Analysis of Cardiovascular Function. Advances in Experimental Medicine and Biology. Cham: Springer International Publishing; 2018. p. 257–78.
- Hiteshi AK, Li D, Gao Y, et al. Gender differences in coronary artery diameter are not related to body habitus or left ventricular mass. Clin Cardiol 2014;37:605-9. [Crossref] [PubMed]
- Kim SG, Apple S, Mintz GS, et al. The importance of gender on coronary artery size: in-vivo assessment by intravascular ultrasound. Clin Cardiol 2004;27:291-4. [Crossref] [PubMed]
- Urbanowicz T, Michalak M, Olasińska-Wiśniewska A, et al. Gender differences in coronary artery diameters and survival results after off-pump coronary artery bypass (OPCAB) procedures. J Thorac Dis 2021;13:2867-73. [Crossref] [PubMed]
- O'Connor GT, Morton JR, Diehl MJ, et al. Differences between men and women in hospital mortality associated with coronary artery bypass graft surgery. The Northern New England Cardiovascular Disease Study Group. Circulation 1993;88:2104-10. [Crossref] [PubMed]
- O'Connor NJ, Morton JR, Birkmeyer JD, et al. Effect of coronary artery diameter in patients undergoing coronary bypass surgery. Northern New England Cardiovascular Disease Study Group. Circulation 1996;93:652-5. [Crossref] [PubMed]
- Caliskan E, de Souza DR, Böning A, et al. Saphenous vein grafts in contemporary coronary artery bypass graft surgery. Nat Rev Cardiol 2020;17:155-69. [Crossref] [PubMed]
- Mickleborough LL, Takagi Y, Maruyama H, et al. Is sex a factor in determining operative risk for aortocoronary bypass graft surgery? Circulation 1995;92:II80-4. [Crossref] [PubMed]
- Mohammadi S, Kalavrouziotis D, Dagenais F, et al. Completeness of revascularization and survival among octogenarians with triple-vessel disease. Ann Thorac Surg 2012;93:1432-7. [Crossref] [PubMed]
- Kleisli T, Cheng W, Jacobs MJ, et al. In the current era, complete revascularization improves survival after coronary artery bypass surgery. J Thorac Cardiovasc Surg 2005;129:1283-91. [Crossref] [PubMed]
- Caputo M, Reeves BC, Rajkaruna C, et al. Incomplete revascularization during OPCAB surgery is associated with reduced mid-term event-free survival. Ann Thorac Surg 2005;80:2141-7. [Crossref] [PubMed]
- Jawitz OK, Lawton JS, Thibault D, et al. Sex Differences in Coronary Artery Bypass Grafting Techniques: A Society of Thoracic Surgeons Database Analysis. Ann Thorac Surg 2022;113:1979-88. [Crossref] [PubMed]
- Schwann TA, Yammine MB, El-Hage-Sleiman AM, et al. The effect of completeness of revascularization during CABG with single versus multiple arterial grafts. J Card Surg 2018;33:620-8. [Crossref] [PubMed]
- Goldman S, Zadina K, Moritz T, et al. Long-term patency of saphenous vein and left internal mammary artery grafts after coronary artery bypass surgery: results from a Department of Veterans Affairs Cooperative Study. J Am Coll Cardiol 2004;44:2149-56. [Crossref] [PubMed]
- Gaudino M, Antoniades C, Benedetto U, et al. Mechanisms, Consequences, and Prevention of Coronary Graft Failure. Circulation 2017;136:1749-64. [Crossref] [PubMed]
- Buxton BF, Hayward PA, Raman J, et al. Long-Term Results of the RAPCO Trials. Circulation 2020;142:1330-8. [Crossref] [PubMed]
- Lawton JS, Tamis-Holland JE, Bangalore S, et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022;145:e4-e17. Erratum in Circulation 2022;145:e771. [Crossref] [PubMed]
- Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40:87-165. Erratum in Eur Heart J 2019;40:3096.
- Schwann TA, Habib RH, Wallace A, et al. Operative Outcomes of Multiple-Arterial Versus Single-Arterial Coronary Bypass Grafting. Ann Thorac Surg 2018;105:1109-19. [Crossref] [PubMed]
- Attia T, Koch CG, Houghtaling PL, et al. Does a similar procedure result in similar survival for women and men undergoing isolated coronary artery bypass grafting? J Thorac Cardiovasc Surg 2017;153:571-9.e9. [Crossref] [PubMed]
- Jabagi H, Tran DT, Hessian R, et al. Impact of Gender on Arterial Revascularization Strategies for Coronary Artery Bypass Grafting. Ann Thorac Surg 2018;105:62-8. [Crossref] [PubMed]
- Gaudino M, Samadashvili Z, Hameed I, et al. Differences in Long-term Outcomes After Coronary Artery Bypass Grafting Using Single vs Multiple Arterial Grafts and the Association With Sex. JAMA Cardiol 2020;6:401-9. [Crossref] [PubMed]
- Gaudino M, Benedetto U, Fremes S, et al. Association of Radial Artery Graft vs Saphenous Vein Graft With Long-term Cardiovascular Outcomes Among Patients Undergoing Coronary Artery Bypass Grafting: A Systematic Review and Meta-analysis. JAMA 2020;324:179-87. [Crossref] [PubMed]
- Vrancic JM, Navia DO, Espinoza JC, et al. Is sex a risk factor for death in patients with bilateral internal thoracic artery grafts? J Thorac Cardiovasc Surg 2019;158:1345-53.e1. [Crossref] [PubMed]
- Habib RH, Zacharias A, Schwann TA, et al. Adverse effects of low hematocrit during cardiopulmonary bypass in the adult: should current practice be changed? J Thorac Cardiovasc Surg 2003;125:1438-50. [Crossref] [PubMed]
- Schwann TA, Habib RH, Zacharias A, et al. Effects of body size on operative, intermediate, and long-term outcomes after coronary artery bypass operation. Ann Thorac Surg 2001;71:521-30; discussion 530-1. [Crossref] [PubMed]
- Mariscalco G, Wozniak MJ, Dawson AG, et al. Body Mass Index and Mortality Among Adults Undergoing Cardiac Surgery: A Nationwide Study With a Systematic Review and Meta-Analysis. Circulation 2017;135:850-63. [Crossref] [PubMed]
- Gaudino M, Di Franco A, Bhatt DL, et al. The association between coronary graft patency and clinical status in patients with coronary artery disease. Eur Heart J 2021;42:1433-41. [Crossref] [PubMed]
- Brindis RG, Brundage BH, Ullyot DJ, et al. Graft patency in patients with coronary artery bypass operation complicated by perioperative myocardial infarction. J Am Coll Cardiol 1984;3:55-62. [Crossref] [PubMed]
- Morice MC, Serruys PW, Sousa JE, et al. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med 2002;346:1773-80. [Crossref] [PubMed]
- Halabi AR, Alexander JH, Shaw LK, et al. Relation of early saphenous vein graft failure to outcomes following coronary artery bypass surgery. Am J Cardiol 2005;96:1254-9. [Crossref] [PubMed]
- Lopes RD, Mehta RH, Hafley GE, et al. Relationship between vein graft failure and subsequent clinical outcomes after coronary artery bypass surgery. Circulation 2012;125:749-56. [Crossref] [PubMed]
- Fitzgibbon GM, Kafka HP, Leach AJ, et al. Coronary bypass graft fate and patient outcome: angiographic follow-up of 5,065 grafts related to survival and reoperation in 1,388 patients during 25 years. J Am Coll Cardiol 1996;28:616-26. [Crossref] [PubMed]
- Morice MC, Feldman TE, Mack MJ, et al. Angiographic outcomes following stenting or coronary artery bypass surgery of the left main coronary artery: fifteen-month outcomes from the synergy between PCI with TAXUS express and cardiac surgery left main angiographic substudy (SYNTAX-LE MANS). EuroIntervention 2011;7:670-9. [Crossref] [PubMed]
- Lehtinen ML, Harik L, Soletti G, et al. Sex differences in saphenous vein graft patency: A systematic review and meta-analysis. J Card Surg 2022;37:4573-8. [Crossref] [PubMed]
- Tsugawa Y, Jena AB, Figueroa JF, et al. Comparison of Hospital Mortality and Readmission Rates for Medicare Patients Treated by Male vs Female Physicians. JAMA Intern Med 2017;177:206-13. [Crossref] [PubMed]
- Wallis CJD, Jerath A, Coburn N, et al. Association of Surgeon-Patient Sex Concordance With Postoperative Outcomes. JAMA Surg 2022;157:146-56. [Crossref] [PubMed]


