
Patient selection for left ventricular unloading: is lactate the vital piece of the puzzle?
The application of veno-arterial extracorporeal membrane oxygenation (VA-ECMO) is the only way to potentially save a patient in refractory cardiac arrest. Although ECMO could provide a bridge to transplant, long-time device support or even recovery, the application of ECMO is associated with specific device-related complications, including left ventricular (LV) overload (1,2). This process could be treated by implementing a mechanical circulatory device in addition to ECMO, resulting in LV unloading (3). However, strategies to counteract increased LV afterload are divers, including the use of percutaneous devices as intra-aortic balloon pump (IABP) or small micro-axial LV assist devices, like the Impella 2.5 and CP (Abiomed, Danvers, MA, USA), and the use of peripheral surgical or direct surgical devices (Impella 5.0 and 5.5) (4-7). Moreover, the value of LV unloading could still be questioned by itself, because of the high mortality rate in patients with INTERMACS-1 classification (8,9). Therefore, it is important to address the current questions in unloading of: Who, When and How.
The use of ECMO in patients without any or only minimal forward flow can result in LV overloading due to the retrograde flow which is necessary to provide perfusion proximal to the arterial peripheral cannula (10). To mitigate the increased LV afterload in low to akinetic ventricles and its harmful effects, devices unloading the LV or left atrium (LA) could be implemented. Although numbers frequently differ between studies, Russo et al. (5) describes that up to 42% of the patients with cardiogenic shock (CS) receives VA-ECMO with LV unloading. Differences between studies and centres, according to Rali et al. (11), are present due the availability of venting modalities and physician experiences and preferences. There is a lack in randomized-controlled trials with hard clinical endpoints on the benefit of any unloading strategy. The use of unloading is currently supported by a retrospective multicentre study with propensity matching which showed lower mortality in patients receiving VA-ECMO and Impella for LV unloading (12). In addition, two meta-analyses of non-randomised studies showed that mechanical LV-unloading using different devices resulted in a lower mortality rate (5,6).
LV unloading can be performed with several LV unloading strategies with different characteristics, of which IABP and micro-axial LV assist devices are most intensively studied (Figure 1). This combination of ECMO with micro-axial LV assist device, including Impella, is also known as ECMELLA. Several factors should be considered when selecting LV unloading strategy. IABP is the most common strategy to be used in LV unloading which is available in most of the centres and easy to deploy without additional surgical interventions (5,13). The implantation of IABP can especially be performed safely compared with micro-axial LV assist devices, including the Impella 2.5 and Impella CP (14,15). Although the Impella 5.0 and 5.5 require surgical implantation, these devices are however capable to provide more hemodynamic support compared to IABP (16). In addition, the Impella 5.0 and 5.5 allows an earlier weaning from ECMO as long as if the right ventricular function is sufficient, and even mobilize the patient during further weaning.
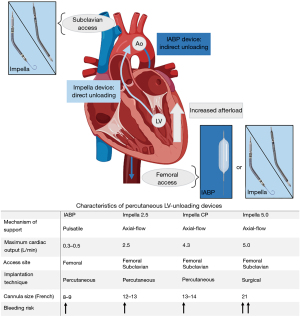
No published studies currently demonstrate any significant difference regarding mortality in relation to the type of device used for LV unloading (5,14). The use of IABP for LV unloading resulted in lower bleeding complications though (14). No randomized-controlled trials are however performed comparing the outcomes of these devices. Conclusively, although the meta-analysis by Russo et al. (5) demonstrates a lower mortality rate in 30 days with LV unloading, evidence is currently limited. The use of LV unloading in cases of increased LV afterload could therefore be defined as a class IIa recommendation. However, guidelines do not give a clear advice about which device should be used (17).
Although the ESC guideline recommends LV venting in patients deteriorating due to the effects of increased LV afterload, patient selection and timing is a key factor. The timing of LV venting is still under debate, but some larger meta-analyses do show benefits for early LV venting (12,13). As mentioned by Rali et al. (11), evaluating the optimal timing of LV unloading is often difficult due to the use of retrospective registries. In these registries data regarding timing and indication are not frequently mentioned. Nevertheless, it is important to find parameters to optimize patient selection and timing.
In the recent issue of the Journal of Thoracic Disease Aludaat et al. (18) retrospectively analysed patients with unloading by an Impella 5.0 in a high-volume centre experienced in the use of MCS devices for CS, aiming to identify parameters, including lactate levels measured after primary implantation of ECMO, in order to identify patients who are eligible for ECMELLA. The major result of this study was that serum lactate levels >7.9 mmol/L prior to implantation of the Impella 5.0 was associated with significantly poor outcomes (30-day survival 10% vs. 48%, P=0.001) and therefore LV unloading should be deferred.
Earlier studies demonstrated the prognostic benefits of lactate levels in patients with ECMO for the treatment of cardiac arrest and CS (19-21). Studies describing the role of lactate in the situation of LV ventricular unloading are however scarce. Ott et al. (22) described patients with a lactate value above 8 mmol/l prior to implantation of Impella had decreased 30-day survival rate. Although the study from Aludaat et al. (18) found a similar cut-off point (i.e., lactate value of 7.9 mmol/L) demonstrating a significant difference in survival rate, the area under the curve was only 0.66, suggesting that lactate is only a poor discriminator. The results from this study are unfortunately not validated to other parameters already used for predicting survival, including the SAVE-score. The SAVE-score has already been developed to predict survival for patients receiving ECMO. Validation of this score demonstrated an area under the receiver operating characteristic (AUROC) of 0.90, suggesting that the SAVE-score is a valuable tool to predict survival of patients receiving ECMO in the situation of CS (23).
It remains also unclear when to measure lactate levels in daily practice. Although the study mentions that lactate levels are measured prior to Impella implantation, time till implantation varied between 0 and 30 hours, in which lactate clearance particularly occurs during ECMO support (24). In addition, due to the retrospective design decision to perform LV unloading with Impella could be biased by the lactate levels measured during ECMO support.
Last, it would be interesting to further assess the use of the smaller Impella 2.5 and IABP for LV unloading. As earlier mentioned, the larger Impella 5.0 and 5.5 have some potential benefits due to the amount of hemodynamic support available and therefore the possibility to be used for weaning from ECMO, if the right ventricular function is sufficient. The need for surgical implantation is however laborious and has higher risks of bleeding complications (25).
The study from Aludaat et al. (18) provides valuable insights regarding the treatment of patients with INTERMACS 1. It demonstrates the potential value of measuring serum lactate levels to decide whether to perform LV unloading with Impella during ECMO support. Because the exact timing of lactate level measurements remains unclear, future research should focus on the implementation of serum lactate levels measurements in daily practice, as well as compare the prognostic benefit to other existing parameters, including the SAVE score.
Acknowledgments
The authors have used BioRender for designing their figure.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Thoracic Disease. The article did not undergo external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-987/coif). RJMVG reports consulting grants and personal fees from Boston Scientific, Abbott Vascular, Astra Zeneca and Amgen, and grants from InfraRedx. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Keebler ME, Haddad EV, Choi CW, et al. Venoarterial Extracorporeal Membrane Oxygenation in Cardiogenic Shock. JACC Heart Fail 2018;6:503-16. [Crossref] [PubMed]
- Thiele H, Ohman EM, de Waha-Thiele S, et al. Management of cardiogenic shock complicating myocardial infarction: an update 2019. Eur Heart J 2019;40:2671-83. [Crossref] [PubMed]
- Bertoldi LF, Pappalardo F, Lubos E, et al. Bridging INTERMACS 1 patients from VA-ECMO to LVAD via Impella 5.0: De-escalate and ambulate. J Crit Care 2020;57:259-63. [Crossref] [PubMed]
- Nishi T, Ishii M, Tsujita K, et al. Outcomes of Venoarterial Extracorporeal Membrane Oxygenation Plus Intra-Aortic Balloon Pumping for Treatment of Acute Myocardial Infarction Complicated by Cardiogenic Shock. J Am Heart Assoc 2022;11:e023713. [Crossref] [PubMed]
- Russo JJ, Aleksova N, Pitcher I, et al. Left Ventricular Unloading During Extracorporeal Membrane Oxygenation in Patients With Cardiogenic Shock. J Am Coll Cardiol 2019;73:654-62. [Crossref] [PubMed]
- Baldetti L, Gramegna M, Beneduce A, et al. Strategies of left ventricular unloading during VA-ECMO support: a network meta-analysis. Int J Cardiol 2020;312:16-21. [Crossref] [PubMed]
- Attinger-Toller A, Bossard M, Cioffi GM, et al. Ventricular Unloading Using the Impella(TM) Device in Cardiogenic Shock. Front Cardiovasc Med 2022;9:856870. [Crossref] [PubMed]
- Brescia AA, Watt TMF, Pagani FD, et al. Assessment of Mortality Among Durable Left Ventricular Assist Device Recipients Ineligible for Clinical Trials. JAMA Netw Open 2021;4:e2032865. [Crossref] [PubMed]
- Moussa MD, Beyls C, Lamer A, et al. Early hyperoxia and 28-day mortality in patients on venoarterial ECMO support for refractory cardiogenic shock: a bicenter retrospective propensity score-weighted analysis. Crit Care 2022;26:257. [Crossref] [PubMed]
- Lüsebrink E, Orban M, Kupka D, et al. Prevention and treatment of pulmonary congestion in patients undergoing venoarterial extracorporeal membrane oxygenation for cardiogenic shock. Eur Heart J 2020;41:3753-61. [Crossref] [PubMed]
- Rali AS, Hall EJ, Dieter R, et al. Left Ventricular Unloading During Extracorporeal Life Support: Current Practice. J Card Fail 2022;28:1326-36. [Crossref] [PubMed]
- Schrage B, Becher PM, Bernhardt A, et al. Left Ventricular Unloading Is Associated With Lower Mortality in Patients With Cardiogenic Shock Treated With Venoarterial Extracorporeal Membrane Oxygenation: Results From an International, Multicenter Cohort Study. Circulation 2020;142:2095-106. [Crossref] [PubMed]
- Al-Fares AA, Randhawa VK, Englesakis M, et al. Optimal Strategy and Timing of Left Ventricular Venting During Veno-Arterial Extracorporeal Life Support for Adults in Cardiogenic Shock: A Systematic Review and Meta-Analysis. Circ Heart Fail 2019;12:e006486. [Crossref] [PubMed]
- Grandin EW, Nunez JI, Willar B, et al. Mechanical Left Ventricular Unloading in Patients Undergoing Venoarterial Extracorporeal Membrane Oxygenation. J Am Coll Cardiol 2022;79:1239-50. [Crossref] [PubMed]
- Fryer ML, Balsam LB. Mechanical Circulatory Support for Cardiogenic Shock in the Critically Ill. Chest 2019;156:1008-21.
- Hajjar LA, Teboul JL. Mechanical Circulatory Support Devices for Cardiogenic Shock: State of the Art. Crit Care 2019;23:76. [Crossref] [PubMed]
- McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599-726. [Crossref] [PubMed]
- Aludaat C, Dovonou E, Besnier E, et al. Upgrading extra corporeal life support to ECMELLA using Impella 5.0 in rescued INTERMACS 1 patients, lactate level matters! J Thorac Dis 2023;15:3079-88. [Crossref] [PubMed]
- Jouffroy R, Saade A, Philippe P, et al. Prognostic Value of Blood Lactate and Lactate Clearance in Refractory Cardiac Arrest Treated by Extracorporeal Life Support. Turk J Anaesthesiol Reanim 2019;47:48-54. [Crossref] [PubMed]
- Martínez-Solano J, Sousa-Casasnovas I, Bellón-Cano JM, et al. Lactate levels as a prognostic predict in cardiogenic shock under venoarterial extracorporeal membrane oxygenation support. Rev Esp Cardiol (Engl Ed) 2022;75:595-603. [Crossref] [PubMed]
- Laimoud M, Alanazi M. The clinical significance of blood lactate levels in evaluation of adult patients with veno-arterial extracorporeal membrane oxygenation. Egypt Heart J 2020;72:74. [Crossref] [PubMed]
- Ott S, Lewin D, Nersesian G, et al. Improving Survival in Cardiogenic Shock-A Propensity Score-Matched Analysis of the Impact of an Institutional Allocation Protocol to Short-Term Mechanical Circulatory Support. Life (Basel) 2022;12:1931. [Crossref] [PubMed]
- Schmidt M, Burrell A, Roberts L, et al. Predicting survival after ECMO for refractory cardiogenic shock: the survival after veno-arterial-ECMO (SAVE)-score. Eur Heart J 2015;36:2246-56. [Crossref] [PubMed]
- Slottosch I, Liakopoulos O, Kuhn E, et al. Lactate and lactate clearance as valuable tool to evaluate ECMO therapy in cardiogenic shock. J Crit Care 2017;42:35-41. [Crossref] [PubMed]
- Spiro J, Doshi SN. Use of left ventricular support devices during acute coronary syndrome and percutaneous coronary intervention. Curr Cardiol Rep 2014;16:544. [Crossref] [PubMed]


