
Prognostic significance of limited resection in pathologic stage I lung adenocarcinoma with spread through air spaces
Highlight box
Key findings
• Limited resection was associated with worse survival than lobectomy in stage I spread through air spaces (STAS)-positive patients, but not in stage IA STAS-positive patients.
What is known and what is new?
• Limited resection can achieve comparable outcomes to lobectomy for radiologic noninvasive peripheral early-stage lung cancer.
• Limited resection was also a safe and effective option for STAS-positive patients with pathologic IA stage disease, but not for those with stage IB disease.
What is the implication, and what should change now?
• This study suggests that limited resection is an available option for patients with STAS-positive stage IA adenocarcinoma, but larger tumors still require lobectomy to ensure adequate surgical margins.
Introduction
Globally, lung cancer remains the leading cause of cancer death, and cases are rising (1). Surgery is the most effective treatment for early-stage non-small cell lung cancer (NSCLC). Lobectomy has been reputed to be the standard of care for the management of early-stage NSCLC since 1995 (2). With the development of computed tomography (CT) and findings of radiologic noninvasiveness patterns for NSCLC, a wedge resection with negative margins or a segmentectomy is also performed for these lesions as a parenchyma-sparing alternative with a fast recovery (3). Although some studies have reported similar recurrence-free survival (RFS) and overall survival (OS) outcomes of limited resection and lobectomy for early-stage lung cancer and recommended segmentectomy as the standard surgical treatment (4), others have found worse prognosis with segmentectomy. The suitability of limited resection (including wedge resection and segmentectomy) for early-stage NSCLC remains under investigation.
Spread through air spaces (STAS) is recognized as an additional invasive pattern of lung adenocarcinoma (ADC) beyond the nonlepidic pattern, stroma infiltration and invasion into adjacent structures and was first named by Kadota and colleagues in 2015 (5). It is defined as micropapillary clusters, solid nests, or single cells within the air spaces in the lung parenchyma beyond the edge of the main tumor. Studies have shown that STAS is associated with key clinical variables and is a significant prognostic factor for poor OS and RFS in stage I ADC (6-8), even in stage II and III ADC (9,10). However, there have been conflicting findings regarding whether limited resection could be performed when STAS is present. Several studies showed that limited resection was associated with worse OS and RFS of resected stage I lung ADC with STAS (11-13). Others found that the prognosis after limited resection was comparable with that of after lobectomy in lung ADC with STAS (14). However, these findings have not yet been validated in large cohorts, and subgroup analysis still needs to be completed. Therefore, we conducted a retrospective analysis of 1,566 patients with resected pathologic stage I lung ADC to examine the association of limited resection with recurrence and survival. Using the propensity score matching (PSM), we also compared survival outcomes in subgroups stratified by pathologic stage and histological grade and assessed the effect of adjuvant chemotherapy (ACT) in stage IB patients who underwent different resection types. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-552/rc).
Methods
Patient selection
The records of patients with pathologic stage I lung ADC who underwent limited resection or lobectomy at the Shanghai Chest Hospital between May 2017 and October 2020 were reviewed. Patients with pathologically confirmed stage I lung ADC who received surgical treatment and had complete follow-up data were included in this retrospective study. Patients received neoadjuvant therapy with multiple nodules, underwent pneumonectomy or bilobectomy, underwent other lung cancer surgery within the 2 years following the surgery, experienced progression of other concurrent diseases or had positive surgical margins were excluded from the study cohort. We identified a total of 1,348 patients according to these criteria. We categorized any type of sublobar resection, including wedge resections and anatomic segmentectomies, as limited resections. Lobectomy is defined as the surgical removal of certain lobes of the lung along with radical lymph node dissection. Segmentectomy was performed through an anterolateral or limited lateral thoracotomy with complete dissection of hilar and mediastinal nodes using sentinel node identification. Limited resection is usually indicated for patients who are not candidates for lobectomy due to poor lung function or other comorbidities. The clinical features, including age at surgery, gender, smoking history, and Eastern Cooperative Oncology Group performance status (ECOG PS), were examined. Figure S1 shows the patient selection scheme and flowchart of this study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This retrospective study was approved by the Institutional Review Board of Shanghai Chest Hospital, China (No. IS22024), and informed consent requirement was waived due to the retrospective nature of the research design. The data analyzed and reported in our study were obtained from existing medical records, ensuring patient anonymity and privacy.
Pathological evaluation
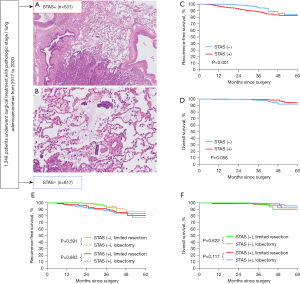
After removing the tumor specimen, all soft tissues and lymph nodes around the specimen were immediately fixed in formalin, sectioned and stained with hematoxylin and eosin. The sections were then analyzed by two pathologists using a microscope with a standard 22-mm diameter eyepiece. ADC and other lung tumors were identified in accordance with the 2015 World Health Organization classification criteria (15). Then, the tumors were staged according to the eighth edition of the tumor-node-metastasis (TNM) classification for lung cancer (16) and classified into lepidic, mucous, acinar, papillary, micropapillary and solid subtypes based on their predominant pattern. In addition, we divided the tumors into three grades using the International Association for the Study of Lung Cancer (IASLC), American Thoracic Society (ATS), and European Respiratory Society (ERS) classification of 2011 (17), as follows: low grade, lepidic predominant; intermediate grade, acinar or papillary predominant; and high grade, solid or micropapillary predominant. The morphological definition of STAS was consistent with that of Kadota et al. (5) i.e., the presence of micropapillary structures in the papillary structures without central fibrovascular cores, solid nests or tumor islands of solid collections of tumor cells filling the air spaces and scattered discohesive single cells (Figure 1A,1B). The edge of the main tumor was defined as the smooth surface of the tumor, which was easily recognizable grossly or by low-power field examination. The presence of visceral pleural, lymphovascular invasion was also recorded.
Patient follow-up
Patients were regularly followed up in the outpatient department every 3 months for the first year after surgery and with a 6-month interval thereafter. For patients who were examined at local health facilities, survival status and clinical information were collected by telephone. OS was defined as the time from the date of surgery until the last follow-up or death, while RFS was defined as the length of time from surgery to recurrence or the last follow-up. Recurrence type, including local recurrence and distant metastasis, was assessed by CT, magnetic resonance imaging (MRI), bone scintigraphy, ultrasonography, and positron emission tomography (PET)-CT, if feasible. Local recurrence was defined as evidence of a tumor identified within the diseased hemithorax, ipsilateral lung, or ipsilateral mediastinal or hilar lymph nodes. Distant metastasis was defined as recurrence in any site outside the ipsilateral hemithorax, including the contralateral lung and hilum.
Statistical analysis
Categorical variables were compared using the chi-square test. Student’s t-test was used to compare continuous variables between two groups. RFS and OS were estimated using the Kaplan-Meier method, and nonparametric group comparisons were performed using the log-rank test. Survival curves were plotted using the Kaplan-Meier method and drawn by GraphPad Prism for Windows (version 9.0.0; San Diego, CA, USA). Multivariate analyses were performed using the Cox proportional hazards regression model to assess the prognostic value of each factor found to be significant in univariate analysis. To match the STAS-positive patients who underwent limited resection and lobectomy, we used 1:2 PSM without replacement, adjusting for patient age, sex, smoking history, ECOG PS, pathologic stage, pleural invasion, lymphovascular invasion and histological grade. We performed the PSM in RStudio (version 4.2.1, The R Foundation, Vienna, Austria) with the R packages “MatchIt” version 4.4.0 and “foreign” version 0.8–82, and applied the nearest neighbor matching method with a caliper of 0.01. All statistical tests were two-sided, with a 5% significance level. Statistical analyses were conducted using SPSS Statistics for Windows (version 26.0; IBM Corporation, Armonk, NY, USA).
Results
Patient characteristics
A total of 1,348 patients with pathologic stage I lung ADC who underwent radical resection were included, and 531 of them were STAS-positive. With respect to the entire cohort, the majority of patients (1,066/1,348) underwent lobectomy, and 282 underwent limited resection. The distributions of general characteristics were compared between the lobectomy group and the limited resection group before and after PSM in the STAS-positive cohort, as shown in Table 1. Before PSM, the lobectomy group had a significantly higher proportion of patients with higher pathologic TNM stage (stage IA3 for 26.6% and stage IB for 34.8%) than those in the limited resection group (P<0.001). Patients who underwent lobectomy were more likely to have solid nodule on CT, pleural invasion and receive ACT than those who underwent limited resection (all P<0.05). After PSM, most of the clinicopathologic characteristics showed no significant difference between the two group.
Table 1
| Variables | Original cohort (n=531) | Matched cohort (n=309) | |||||
|---|---|---|---|---|---|---|---|
| Limited resection (n=103) | Lobectomy (n=428) | P | Limited resection (n=103) | Lobectomy (n=206) | P | ||
| Age at surgery (years) | 0.099 | 0.196 | |||||
| ≤65 | 41 (39.8) | 209 (48.8) | 41 (39.8) | 98 (47.6) | |||
| >65 | 62 (60.2) | 219 (51.2) | 62 (60.2) | 108 (52.4) | |||
| Sex | 0.778 | 0.629 | |||||
| Male | 54 (52.4) | 231 (54.0) | 54 (52.4) | 102 (49.5) | |||
| Female | 49 (47.6) | 197 (46.0) | 49 (47.6) | 104 (50.5) | |||
| Smoking history | 0.209 | 0.214 | |||||
| Never | 69 (67.0) | 267 (62.4) | 69 (67.0) | 123 (59.7) | |||
| Ever | 34 (33.0) | 161 (37.6) | 34 (33.0) | 83 (40.3) | |||
| ECOG PS | 0.338 | 0.282 | |||||
| 0–1 | 89 (86.4) | 353 (82.5) | 89 (86.4) | 168 (81.6) | |||
| ≥2 | 14 (13.6) | 75 (17.5) | 14 (13.6) | 38 (18.4) | |||
| Pathologic stage | <0.001* | 0.136 | |||||
| Stage IA1 | 27 (26.2) | 33 (7.7) | 27 (26.2) | 33 (16.0) | |||
| Stage IA2 | 36 (35.0) | 132 (30.8) | 36 (35.0) | 94 (45.6) | |||
| Stage IA3 | 15 (14.6) | 114 (26.6) | 15 (14.6) | 29 (14.1) | |||
| Stage IB | 25 (24.3) | 149 (34.8) | 25 (24.3) | 50 (24.3) | |||
| Nodal dissection | <0.001* | <0.001* | |||||
| No dissection | 17 (16.5) | 0 (0.0) | 17 (16.5) | 0 (0.0) | |||
| Hilar | 62 (60.2) | 25 (5.8) | 62 (60.2) | 16 (7.8) | |||
| Mediastinal, systematic or selective | 24 (23.3) | 403 (94.2) | 24 (23.3) | 190 (92.2) | |||
| Pleural invasion | 0.045* | 0.691 | |||||
| Absent | 83 (80.6) | 303 (70.8) | 83 (80.6) | 162 (78.6) | |||
| Present | 20 (19.4) | 125 (29.2) | 20 (19.4) | 44 (21.4) | |||
| Lymphovascular invasion | 0.139 | 0.322 | |||||
| Absent | 88 (85.4) | 338 (79.0) | 88 (85.4) | 184 (89.3) | |||
| Present | 15 (14.6) | 90 (21.0) | 15 (14.6) | 22 (10.7) | |||
| Architectural histological grade | 0.204 | 0.264 | |||||
| Low grade | 5 (4.9) | 10 (2.3) | 5 (4.9) | 4 (1.9) | |||
| Intermediate grade | 80 (77.7) | 319 (74.5) | 80 (77.7) | 157 (76.2) | |||
| High grade | 18 (17.5) | 99 (23.1) | 18 (17.5) | 45 (21.8) | |||
| CEA level (ng/mL) | 2.7 (1.8–4.6) | 2.8 (1.8–4.4) | 0.975 | 2.7 (1.8–4.6) | 2.7 (1.8–4.5) | 0.211 | |
| Nodule type on CT | <0.001* | <0.001* | |||||
| Part solid | 52 (50.5) | 92 (21.5) | 52 (50.5) | 49 (23.8) | |||
| Solid | 51 (49.5) | 336 (78.5) | 51 (49.5) | 157 (76.2) | |||
| ACT | <0.001* | 0.004* | |||||
| Absent | 71 (68.9) | 191 (44.6) | 71 (68.9) | 107 (51.9) | |||
| Present | 32 (31.1) | 237 (55.4) | 32 (31.1) | 99 (48.1) | |||
Values are median (interquartile range) or n (%). *, significant P values. STAS, spread through air spaces; ECOG PS, Eastern Cooperative Oncology Group Performance Status; CEA, carcinoembryonic antigen; CT, computed tomography; ACT, adjuvant chemotherapy.
Survival analysis stratified by STAS and resection type
The 3-year OS and 3-year RFS of this study’s pathological stage I ADC were 98.7% and 91.9%, respectively, with a median follow-up time of 34 months. Recurrence occurred in 33 STAS-negative patients and 77 STAS-positive patients, with local recurrence in 16 and 48 of them, respectively. RFS was significantly different between the STAS-positive and STAS-negative groups (Figure 1C, 3-year RFS: 88.2% vs. 94.3%, log-rank test P<0.001), but OS was not (Figure 1D, 3-year OS: 99.2% vs. 98.0%, P=0.056). All enrolled cases were classified into two groups according to resection type. Before PSM, limited resection showed no significant difference in survival between the two groups. Figure 1E,1F indicate no significant difference for limited resection or lobectomy in RFS and OS in patients without STAS (P=0.591, P=0.622, respectively) and with STAS (P=0.883, P=0.117, respectively).
Survival analysis in the STAS-positive cohort
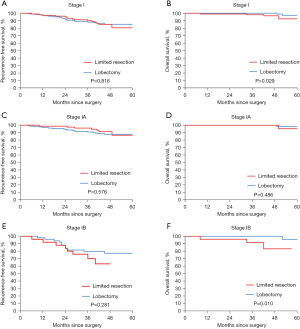
The matched cohort had a median follow-up of 47 months after PSM. The recurrence rates were comparable between the limited resection group (14.6%, n=15) and the lobectomy group (14.1%, n=29). The frequency of distant recurrence was also similar in both groups (n=9, 8.7% for limited resection and n=21, 10.2% for lobectomy). Lung cancer-related mortality was slightly higher in the limited resection group (3.9%, n=4) than in the lobectomy group (1.0%, n=2). We found comparable RFS (P=0.816) between limited resection and lobectomy group but worse OS (P=0.029) in the limited resection group (Figure 2A,2B). We also investigated the effect of the type of surgery on survival in stage IA and IB STAS-positive patients in the matched cohort. Kaplan-Meier analysis revealed that the 3-year RFS and 3-year OS for patients who underwent limited resection were not significantly different from those for patients who underwent lobectomy in stage IA tumors (3-year RFS, 96.2% vs. 90.9%; P=0.576; 3-year OS, 100.0% vs. 100.0%; P=0.486, respectively) (Figure 2C,2D). The RFS at 3 years for patients who received limited resection was not significantly different from that for lobectomy patients with stage IB tumors (3-year RFS, 70.2% vs. 79.5%; P=0.281) (Figure 2E). However, patients who received limited resection in this cohort had a significantly lower OS at 3 years compared to those who received lobectomy (3-year OS, 91.6% vs. 100.0%; P=0.010) (Figure 2F). Furthermore, we observed that among the patients who underwent limited resection, 43 had wedge resection and 59 had segmentectomy, and most of them were stage IA patients (75.5%). Survival analysis comparing the outcomes of these two subgroups of patients revealed no significant difference (Figure S2).
Univariable and multivariable analysis
The results of univariable analyses for all survival endpoints of interest are shown in Table 2. Poor RFS was significantly associated with solid nodules on CT, ACT, advanced pathologic TNM stage and high-grade histological subtype (all with P values less than 0.05). Factors significantly associated with a higher risk of recurrence also included pleural invasion (P=0.001). However, those factors were not independent prognostic factors for a worse RFS in the multivariate Cox proportional hazards model except for the high-grade histological subtype [hazard ratio (HR): 1.982, 95% confidence interval (CI): 1.047–3.753, P=0.001] (Table 2). Univariate analysis showed that only high-grade histological subtype was found to be significantly associated with poor OS (HR: 6.463, 95% CI: 1.176–35.516, P=0.032); thus, multivariate analysis was waived. Limited resection was not an independent prognostic factor of OS or RFS.
Table 2
| Variables | RFS | OS | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | ||||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P Value | |||
| Age (>65 vs. ≤65 years) | 1.113 (0.613–2.021) | 0.725 | 5.547 (0.075–40.931) | 0.233 | ||||
| Sex (female vs. male) | 0.856 (0.473–1.550) | 0.608 | 0.240 (0.028–2.059) | 0.193 | ||||
| Smoking history (ever vs. never) | 0.983 (0.536–1.805) | 0.957 | 7.312 (0.853–62.659) | 0.070 | ||||
| ECOG PS (≥2 vs. 0–1) | 0.942 (0.420–2.115) | 0.886 | 0.039 (0.001–81.600) | 0.523 | ||||
| Pathologic stage | 0.010* | 0.272 | 0.474 | |||||
| Stage IA1 | Ref. | Ref. | Ref. | |||||
| Stage IA2 | 1.591 (0.524–4.834) | 0.413 | 1.598 (0.514–4.971) | 0.418 | – | 0.957 | ||
| Stage IA3 | 2.433 (0.712–8.312) | 0.156 | 1.970 (0.557–6.973) | 0.293 | – | 0.953 | ||
| Stage IB | 4.200 (1.429–12.349) | 0.009* | 2.565 (0.598–11.002) | 0.205 | – | 0.950 | ||
| Nodal dissection | 0.742 | 0.346 | ||||||
| No dissection | Ref. | Ref. | Ref. | |||||
| Hilar | 1.365 (0.308–6.048) | 0.682 | 0.173 (0.011–2.785) | 0.216 | ||||
| Mediastinal, systematic or selective | 1.062 (0.253–4.456) | 0.934 | 0.211 (0.023–1.931) | 0.168 | ||||
| Pleural invasion (present vs. absent) |
2.732 (1.488–5.015) | 0.001* | 1.349 (0.443–4.104) | 0.598 | 3.070 (0.613–15.372) | 0.172 | ||
| Lymphovascular invasion (present vs. absent) |
1.584 (0.706–3.555) | 0.265 | 1.588 (0.185–13.609) | 0.673 | ||||
| Histological subtype grade (high vs. intermediate) |
2.274 (1.209–4.275) | 0.011* | 1.982 (1.047–3.753) | 0.001* | 6.463 (1.176–35.516) | 0.032* | ||
| CEA (>5 vs. ≤5 ng/mL) | 1.180 (0.583–2.388) | 0.646 | 1.749 (0.320–9.571) | 0.519 | ||||
| Nodule type on CT (solid vs. part solid) |
2.374 (1.103–5.109) | 0.027* | 1.937 (0.865–4.336) | 0.108 | 0.828 (0.151–4.552) | 0.829 | ||
| ACT (present vs. absent) | 1.972 (1.085–3.582) | 0.026* | 1.524 (0.813–2.857) | 0.189 | 1.274 (0.257–6.323) | 0.767 | ||
| Surgical procedure (lobectomy vs. limited resection) |
0.928 (0.497–1.735) | 0.816 | 0.182 (0.033–1.005) | 0.051 | ||||
*, significant P values. RFS, recurrence-free survival; OS, overall survival; STAS, spread through air spaces; HR, hazard ratio; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group Performance Status; Ref., reference; CEA, carcinoembryonic antigen; CT, computed tomography; ACT, adjuvant chemotherapy.
Subgroup analysis
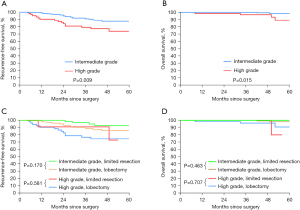
To further identify the feasibility of limited resection for STAS-positive ADC, we divided the matched STAS-positive patients into groups based on histological grade. According to the grading system, we identified 9 patients with low-grade disease, 237 with intermediate-grade disease and 63 with high-grade disease. Kaplan-Meier analysis revealed that those with a high histological grade had significantly worse RFS and OS than those with an intermediate grade (3-year RFS, 79.3% vs. 91.0%; P=0.009; 3-year OS, 96.8% vs. 100.0%; P=0.015, respectively) (Figure 3A,3B). Limited resection was not associated with a significantly shorter RFS or OS than lobectomy in the intermediate-grade group (P=0.170, P=0.463, respectively) or the high-grade group (P=0.581, P=0.707, respectively) (Figure 3C,3D).
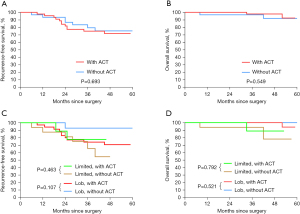
To evaluate the possible effects of postoperative ACT on the survival of stage IB STAS-positive patients, we further stratified the matched STAS-positive patients by stage IB and ACT status. Our results demonstrate that patients who underwent ACT had a higher rate of lobectomy and mediastinal nodal dissection than those who did not. Furthermore, they showed a higher prevalence of elevated CEA level (Table S1). Surprisingly, we found that ACT did not confer any survival benefit (with ACT vs. without ACT: 3-year RFS, 74.9% vs. 79.4%; P=0.693; 3-year OS, 97.8% vs. 96.7%; P=0.549; respectively) (Figure 4A,4B). Moreover, there was no significant difference in RFS and OS between patients who underwent limited resection and those who underwent lobectomy, regardless of whether they received ACT or not (Figure 4C,4D). Among the patients who received ACT (n=45), the most frequently used regimen was cisplatin plus vinorelbine (n=25, 55.6%), followed by cisplatin plus pemetrexed (n=15, 33.3%).
Discussion
The definition of STAS implies that it represents an unconventional concept of airspace invasion, which may be considered on par with other established invasive patterns, such as lymphovascular or pleural invasion, signifying that its presence indicates a more aggressive biological potential that is important for pathologists to recognize. Many studies have shown that STAS is associated with aggressive tumor behavior, such as lymph node metastasis, higher pathological stage, vascular invasion, and high histological grade (18-20), and is an important predictor of worse clinical outcomes (recurrence and survival) in patients with early-stage lung ADC who have undergone limited surgical resection (6,14).
However, the necessity of performing lobectomy for early-stage STAS-positive ADC, especially pathologic stage I ADC, remains controversial. While data have shown that the prognosis of patients with STAS-positive lung ADC after segmentectomy is comparable with that of patients who underwent lobectomy (14,21), some investigators hold a contrary opinion that STAS is actually ex vivo artifactual (22), as the coexistence of STAS with widely known poor prognostic factors might explain why tumors with STAS have a poor outcome.
Our study demonstrated that STAS was a significant prognostic factor for recurrence in stage I ADC patients, in agreement with most of the existing literature (11,23). Moreover, after adjusting confounding factor, we confirmed the previous findings (13,24) that STAS-positive patients who received limited resection had, though comparable RFS, inferior survival outcomes compared to those who received other surgical modalities in stage I ADC, particularly in stage IB ADC. Limited resection for NSCLC exceeding 3 cm is generally not indicated. Worse survival in the limited group could be attributed to the advanced-stage tumors and limited resection ordinarily performed in patients with declined lung function. Another potential factor is the surgical margin distance in the limited resection group, defined as the distance between the surgical staple margin and the nearest primary tumor edge. Wider surgical margins in lobectomy may prevent the spread of tumor cells, as data have shown that there was no local recurrence in stage I non-small cell lung carcinoma tumors with a surgical margin distance greater than 2.0 cm, irrespective of the presence or absence of STAS (25). A recent study by Kagimoto et al. showed that the 5-year RFS and OS rates of segmentectomy patients with a mean margin distance of 10 mm were comparable to those of lobectomy patients (14). However, our study confirmed negative margins with proper distances on frozen sections intraoperatively to avoid postoperative staging migration.
Interestingly, for patients with stage IA STAS-negative ADC, a margin-to-tumor ratio of 1 or higher was a favorable factor associated with a significantly lower risk of recurrence than a margin-to-tumor ratio less than 1; however, the risk of recurrence was high in STAS-positive patients regardless of the margin-to-tumor ratio (13). This finding suggests that the surgical margin distance and margin-to-tumor ratio might affect the prognosis of patients with different STAS statuses. A previous study suggested that the grading system based on architectural subtypes has been associated with a better prognosis for stage I lung ADC, with high-grade tumors reflecting the aggressive nature of ADC and having a significantly higher risk for recurrence (26). In the present study, we found that the 3-year RFS rate in the high-grade group was lower than that after lobectomy, indicating that a larger surgical margin may be warranted in high-grade ADC.
Although STAS is a prognostic factor, it is difficult to recognize preoperatively. Toyokawa and colleagues (7) retrospectively investigated lung cancer on CT to identify features of STAS-positive ADC and found that it was significantly larger and usually presented spiculated signs, nodular notches, vascular convergence, and pleural indentation. In our study, we found that solid nodules were an unfavorable prognostic factor for RFS in ADC, and while studies have already identified solid nodules as a poor prognostic factor regardless of STAS status, the percentage of solid components and the maximum standardized uptake value on PET-CT have also been independently associated with STAS in lung ADC (19,27). Therefore, it is necessary to build a precise prognostic model for limited resection that includes preoperative CT and PET-CT findings. It is difficult to predict STAS intraoperatively as well. Frozen sections were inadequate for predicting STAS, with an overall sensitivity and specificity of 71% (95% CI: 63–78%) and 92% (95% CI: 84–96%), respectively, as calculated across the five pathologists using a generalized estimating equation logistic regression model (13,28). Thus, STAS has little influence on determining treatment strategies for lung ADC.
Previous study reported that the administration of ACT was a favorable predictor for prognosis in stage IB STAS-positive patients and stage IA STAS-positive patients who underwent segmentectomy (29). Our study showed that ACT did not provide additional survival benefits in stage IB patients, even when they underwent limited resection. However, we propose that it may be advisable to consider the use of ACT because the recurrence sites of 6 out of 7 patients who had recurrence in the ACT-negative group were distant from the surgical site, and five of them underwent limited resection. Moreover, risk factors such as pleural invasion, lymphovascular invasion, and high-grade subtype, could effectively predict recurrence and mortality in a prognostic model developed for invasive ADC by Liu et al. (30). This suggests that the consensus on the use of ACT in stage IB ADC patients should be based on a combination of the postoperative pathological examination results, tumor markers, and benefits of ACT on different histological subtypes of STAS-positive ADC in future studies.
There are several limitations in this study, including the possibility of type II error due to the retrospective nature of a single-center study. Additionally, we only took into account of the presence or absence of STAS without performing quantitative analysis, including for the extent or number of affected tumors, which may have an effect on survival in STAS-positive ADC. Long-term prospective multicenter studies are needed to confirm the acceptable outcomes of limited resection in this population, and such studies should include analyses of subgroups stratified by other clinicopathological factors. Finally, our study was limited by preoperative patient selection, inclusion criteria, incomplete clinical information (e.g., ACT modality), and lack of stratification, which could have introduced bias between different subgroups. These limitations require clarification directly in a prospective study, although our results suggest that stage IB STAS-positive ADC may not benefit from ACT.
Conclusions
In conclusion, our study indicates that lobectomy should remain the standard treatment for STAS-positive stage I ADC and limited resection may be an available option for patients with stage IA ADC. This finding supports the growing interest in personalized medicine and highlights the potential benefits of minimal lung resection for selected patients. Our analysis also suggests that ACT may not provide an additional prognostic advantage in stage IB ADC patients with STAS who undergo lobectomy or limited resection. Nonetheless, our conclusions require further validation through large-scale, prospective, multicenter studies.
Acknowledgments
Funding: This work was supported by a grant from
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-552/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-552/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-552/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-552/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This retrospective study was approved by the Institutional Review Board of Shanghai Chest Hospital, China (No. IS22024), and informed consent requirement was waived due to the retrospective nature of the research design.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bade BC, Dela Cruz CS. Lung Cancer 2020: Epidemiology, Etiology, and Prevention. Clin Chest Med 2020;41:1-24. [Crossref] [PubMed]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [Crossref] [PubMed]
- Wolf AS, Richards WG, Jaklitsch MT, et al. Lobectomy versus sublobar resection for small (2 cm or less) non-small cell lung cancers. Ann Thorac Surg 2011;92:1819-23; discussion 1824-5. [Crossref] [PubMed]
- Saji H, Okada M, Tsuboi M, et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022;399:1607-17. [Crossref] [PubMed]
- Kadota K, Nitadori JI, Sima CS, et al. Tumor Spread through Air Spaces is an Important Pattern of Invasion and Impacts the Frequency and Location of Recurrences after Limited Resection for Small Stage I Lung Adenocarcinomas. J Thorac Oncol 2015;10:806-14. [Crossref] [PubMed]
- Yi E, Lee JH, Jung Y, et al. Clinical implication of tumour spread through air spaces in pathological stage I lung adenocarcinoma treated with lobectomy. Interact Cardiovasc Thorac Surg 2021;32:64-72. [Crossref] [PubMed]
- Toyokawa G, Yamada Y, Tagawa T, et al. Significance of spread through air spaces in early-stage lung adenocarcinomas undergoing limited resection. Thorac Cancer 2018;9:1255-61. [Crossref] [PubMed]
- Dai C, Xie H, Su H, et al. Tumor Spread through Air Spaces Affects the Recurrence and Overall Survival in Patients with Lung Adenocarcinoma >2 to 3 cm. J Thorac Oncol 2017;12:1052-60. [Crossref] [PubMed]
- Khalil HA, Shi W, Mazzola E, et al. Analysis of recurrence in lung adenocarcinoma with spread through air spaces. J Thorac Cardiovasc Surg 2023; Epub ahead of print. [Crossref] [PubMed]
- Terada Y, Takahashi T, Morita S, et al. Spread through air spaces is an independent predictor of recurrence in stage III (N2) lung adenocarcinoma. Interact Cardiovasc Thorac Surg 2019;29:442-8. [Crossref] [PubMed]
- Kadota K, Kushida Y, Kagawa S, et al. Limited Resection Is Associated With a Higher Risk of Locoregional Recurrence than Lobectomy in Stage I Lung Adenocarcinoma With Tumor Spread Through Air Spaces. Am J Surg Pathol 2019;43:1033-41. [Crossref] [PubMed]
- Shiono S, Endo M, Suzuki K, et al. Spread Through Air Spaces Is a Prognostic Factor in Sublobar Resection of Non-Small Cell Lung Cancer. Ann Thorac Surg 2018;106:354-60. [Crossref] [PubMed]
- Eguchi T, Kameda K, Lu S, et al. Lobectomy Is Associated with Better Outcomes than Sublobar Resection in Spread through Air Spaces (STAS)-Positive T1 Lung Adenocarcinoma: A Propensity Score-Matched Analysis. J Thorac Oncol 2019;14:87-98. [Crossref] [PubMed]
- Kagimoto A, Tsutani Y, Kushitani K, et al. Segmentectomy vs Lobectomy for Clinical Stage IA Lung Adenocarcinoma With Spread Through Air Spaces. Ann Thorac Surg 2021;112:935-43. [Crossref] [PubMed]
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- Detterbeck FC, Boffa DJ, Kim AW, et al. The Eighth Edition Lung Cancer Stage Classification. Chest 2017;151:193-203.
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [Crossref] [PubMed]
- Onozato ML, Kovach AE, Yeap BY, et al. Tumor islands in resected early-stage lung adenocarcinomas are associated with unique clinicopathologic and molecular characteristics and worse prognosis. Am J Surg Pathol 2013;37:287-94. [Crossref] [PubMed]
- Toyokawa G, Yamada Y, Tagawa T, et al. Significance of Spread Through Air Spaces in Resected Pathological Stage I Lung Adenocarcinoma. Ann Thorac Surg 2018;105:1655-63. [Crossref] [PubMed]
- Aly RG, Rekhtman N, Li X, et al. Spread Through Air Spaces (STAS) Is Prognostic in Atypical Carcinoid, Large Cell Neuroendocrine Carcinoma, and Small Cell Carcinoma of the Lung. J Thorac Oncol 2019;14:1583-93. [Crossref] [PubMed]
- Kamigaichi A, Tsutani Y, Kagimoto A, et al. Comparing Segmentectomy and Lobectomy for Clinical Stage IA Solid-dominant Lung Cancer Measuring 2.1 to 3 cm. Clin Lung Cancer 2020;21:e528-38. [Crossref] [PubMed]
- Thunnissen E, Blaauwgeers HJ, de Cuba EM, et al. Ex Vivo Artifacts and Histopathologic Pitfalls in the Lung. Arch Pathol Lab Med 2016;140:212-20. [Crossref] [PubMed]
- Yokoyama S, Murakami T, Tao H, et al. Tumor Spread Through Air Spaces Identifies a Distinct Subgroup With Poor Prognosis in Surgically Resected Lung Pleomorphic Carcinoma. Chest 2018;154:838-47. [Crossref] [PubMed]
- Shiono S, Endo M, Suzuki K, et al. Spread through air spaces in lung cancer patients is a risk factor for pulmonary metastasis after surgery. J Thorac Dis 2019;11:177-87. [Crossref] [PubMed]
- Masai K, Sakurai H, Sukeda A, et al. Prognostic Impact of Margin Distance and Tumor Spread Through Air Spaces in Limited Resection for Primary Lung Cancer. J Thorac Oncol 2017;12:1788-97. [Crossref] [PubMed]
- Hung JJ, Jeng WJ, Chou TY, et al. Prognostic value of the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society lung adenocarcinoma classification on death and recurrence in completely resected stage I lung adenocarcinoma. Ann Surg 2013;258:1079-86. [Crossref] [PubMed]
- Kim SK, Kim TJ, Chung MJ, et al. Lung Adenocarcinoma: CT Features Associated with Spread through Air Spaces. Radiology 2018;289:831-40. [Crossref] [PubMed]
- Walts AE, Marchevsky AM. Current Evidence Does Not Warrant Frozen Section Evaluation for the Presence of Tumor Spread Through Alveolar Spaces. Arch Pathol Lab Med 2018;142:59-63. [Crossref] [PubMed]
- Chen D, Wang X, Zhang F, et al. Could tumor spread through air spaces benefit from adjuvant chemotherapy in stage I lung adenocarcinoma? A multi-institutional study. Ther Adv Med Oncol 2020;12:1758835920978147. [Crossref] [PubMed]
- Liu A, Hou F, Qin Y, et al. Predictive value of a prognostic model based on pathologic features in lung invasive adenocarcinoma. Lung Cancer 2019;131:14-22. [Crossref] [PubMed]


