
Assessment of a collaborative treatment model for trimodal management of esophageal cancer
Highlight box
Key findings
• A collaborative treatment model—chemoradiotherapy (CRT) at local center, followed by esophagectomy at regional center—is associated with a reduction in travel, but similar 90-day and 5-year survival compared to treatment at a single institution.
What is known and what is new?
• Concentration of complex surgeries at high-volume centers improves outcomes, but can place a significant travel burden on patients.
• Delivery of neoadjuvant CRT at local centers can reduce this burden, with minimal impact on the quality of trimodality therapy or long-term survival.
What is the implication, and what should change now?
• A collaborative treatment model is a common and reasonable approach to delivery of trimodality therapy for patients with resectable esophageal cancer that should be offered to patients to alleviate travel burden.
Introduction
Multimodal therapy is the standard of care in patients with loco-regional esophageal adenocarcinoma and squamous cell carcinoma. The combination of neoadjuvant chemoradiotherapy (CRT) and surgical resection increases R0 resection rates, decreases local and systemic recurrence, and improves disease-specific and overall survival (1). At the same time, high-volume highly-specialized centers have consistently been associated with improved operative outcomes for complex oncologic resections, including esophagectomy (2,3).
While regionalization and evolving neoadjuvant treatment regimens have led to a nearly 50% five-year survival in patients with resectable disease, they can represent a major burden to patients and their caregivers in terms of travel, time, and cost (4,5). One strategy to reduce the burden on patients and their families is to consider a collaborative (multi-institutional) treatment model—neoadjuvant CRT at a local center and operative resection at a regional high-volume center.
The effect of collaborative treatment on the quality of CRT and long-term survival are unknown. In this study, we sought to understand treatment practices and their association with short and long-term outcomes using the National Cancer Database (NCDB). In this analysis, we compared compliance with guideline recommendations for multiagent chemotherapy, receipt of 41.4–50.4 Gy of radiation, R0 resection, pathologic complete response (pCR), and survival in patients receiving single institution or multi-institutional collaborative treatment (6). We hypothesized that patients who received collaborative treatment would have a decreased rate of guideline compliant CRT compared to patients receiving single institution treatment. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-346/rc).
Methods
Data source
Retrospective cohort study using the NCDB, a robust hospital-based tumor registry maintained by the Commission on Cancer of the American College of Surgeons and the American Cancer Society (7). The Commission on Cancer of the American College of Surgeons and the American Cancer Society have not verified and are not responsible for the statistical validity of the data. Given the use of the NCDB this study was exempted from further approval by the institutional review board. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Inclusion criteria and exclusion criteria
The database was queried for patients diagnosed with esophageal cancer between 2012 and 2017. Patients were included if they were over the age of 18, had an invasive malignancy (as opposed to carcinoma in situ), received neoadjuvant treatment (chemotherapy and radiotherapy), and underwent an esophagectomy. Patients with incomplete clinical staging, incomplete pathologic staging, and known stage IV disease at time of esophagectomy were excluded. In addition, patients with missing follow-up or mortality data or unknown site of CRT were excluded.
Treatment exposure, covariates, and outcomes
All analysis was performed at the patient level. Patients who received neoadjuvant treatment and esophagectomy at a single NCDB reporting institution and associated outpatient clinics (single institutional treatment) were compared to those that received at least part of their CRT at an institution separate from the site of their esophagectomy (collaborative treatment). Outside institutions were identified based on the NCDB variables “RAD_LOCATION_OF_RX”, “RX_SUMM_CHEMO”, and “RX_HOSP_CHEMO” and defined as another commission on cancer accredited facility that independently reports to the NCDB.
Independent variables included sociodemographics, Charlson-Deyo comorbidity index, tumor histology, clinical stage, operative approach, facility type (academic vs. non-academic), geographic location, and year of diagnosis. Travel distance to the site of esophagectomy was included and categorized into tertiles. Based on work by Metzger et al., an annual esophagectomy volume of 20 was used to identify high-volume centers (8). Primary outcomes included compliance with guideline recommendations for multiagent chemotherapy (vs. single agent), receipt of 41.4–50.4 Gy of radiation (vs. out of recommended range), pCR, R0 resection, 90-day mortality, and 5-year survival (6). Subgroup analysis was performed stratifying patients by clinical stage.
Statistical analysis
Descriptive analysis of the distribution of patient characteristics and outcomes was performed by treatment model (single institution vs. collaborative). Statistically significant differences in these distributions were identified using the χ2 test or Fisher exact test for categorical variables, and the t-test or Mann-Whitney U test for continuous variables. An inclusive multivariable logistic regression model was used to assess factors associated with receiving collaborative treatment and expressed in odds ratios (ORs) with 95% confidence intervals (95% CIs). The missing rate for radiation doses was 17.8%. A survey performed to identify patterns in the missing data found that these data appeared to be missing at random. A complete case analysis was performed.
To provide estimates for the overall survival of patients who did and did not receive collaborative treatment, Kaplan-Meier estimates were generated. The survival benefit associated with multi-institutional treatment was analyzed in a Cox proportional hazard models stratified by stage and expressed as a hazard ratio (HR). These models included age as a continuous variable, race, ethnicity, insurance status, median income quartile based on the zip code of the patient’s residence, facility type and location, patient area of residence, year of diagnosis, Charlson-Deyo comorbidity index, and travel distance as categorical variables. In addition, a hospital-specific random effect to account for clustering at the hospital level was included. A sensitivity analysis including only patients receiving guideline concordant CRT was performed to determine any effect on survival. All statistical tests were 2-sided with a P value of 0.05 as the threshold for statistical significance. Analyses were performed using SAS, version 9.4 (SAS Institute Inc., Cary, NC, USA) and Stata software, version 15.1 (StataCorp, College Station, TX, USA).
Results
Patient characteristics
Among the 8,396 patients who met inclusion criteria, 3,276 (39%) received single-institution treatment, while 5,113 (61%) received collaborative treatment (Figure 1). Patients who received collaborative treatment were more likely to be older, White, and to have Medicare insurance; however, none of these variables differed by more than 5% (Table 1). Similarly, Charlson-Deyo comorbidity index, clinical T status, and clinical N status all differed by less than 3% between the two treatment groups. Patients who received collaborative treatment were more likely to originate from a non-academic center (40.7% vs. 36.5%; P<0.001), and to live in the Southern or Western United States. The percentage of patients receiving collaborative treatment increased from 56.7% in 2012 to 60.2% in 2017. Slightly more patients in the single-institution group received an esophagectomy at a high-volume center (72.5% vs. 69.7%; P=0.01).
Table 1
| Characteristics | Treatment at single institution (n=3,276) | Treatment at multiple institutions (n=5,113) |
P value |
|---|---|---|---|
| Age, median [IQR] | 63 [57, 69] | 64 [57, 69] | 0.03 |
| Male, n (%) | 2,735 (83.5) | 4,308 (84.3) | 0.34 |
| Travel distance†, miles, median (IQR) | 15.1 (6.5, 38.1) | 30.5 (11.7, 70.2) | <0.001 |
| <12.5 miles, n (%) | 1,293 (44.3) | 1,164 (26.4) | <0.001 |
| 12.6–42.6 miles, n (%) | 964 (33.0) | 1,466 (33.3) | |
| >42.6 miles, n (%) | 661 (22.7) | 1,774 (40.3) | |
| Race, n (%) | 0.34 | ||
| White | 3,023 (92.3) | 4,784 (93.5) | |
| Black | 147 (4.5) | 166 (3.3) | |
| Asian | 62 (1.9) | 85 (1.7) | |
| Other | 24 (0.7) | 46 (0.9) | |
| Unknown | 20 (0.6) | 32 (0.64) | |
| Hispanic, n (%) | 104 (3.2) | 141 (2.8) | 0.53 |
| Insurance, n (%) | <0.001 | ||
| Private | 1,527 (46.6) | 2,271 (44.4) | |
| Medicaid | 207 (6.3) | 290 (5.7) | |
| Medicare | 1,372 (41.9) | 2,298 (44.9) | |
| Other government insurance | 44 (1.3) | 121 (2.4) | |
| Uninsured | 75 (2.3) | 79 (1.5) | |
| Unknown | 51 (1.6) | 54 (1.1) | |
| Income quartiles‡, n (%) | <0.001 | ||
| <$40,227 | 441 (13.5) | 664 (13.0) | |
| $40,227–$50,353 | 612 (18.7) | 1,038 (20.4) | |
| $50,354–$63,332 | 709 (21.6) | 1,090 (21.3) | |
| ≥$63,333 | 1,121 (34.2) | 1,568 (30.7) | |
| Unknown | 393 (1.6) | 753 (14.7) | |
| Population density§, n (%) | <0.001 | ||
| Metropolitan | 2,570 (78.5) | 3,817 (74.7) | |
| Urban | 474 (14.5) | 937 (18.3) | |
| Rural | 52 (1.6) | 103 (2.01) | |
| Unknown | 180 (5.4) | 256 (5.0) | |
| Facility type, n (%) | <0.001 | ||
| Non-academic | 1,194 (36.5) | 2,082 (40.7) | |
| Academic | 2,029 (61.9) | 2,978 (58.2) | |
| Unknown | 53 (1.6) | 53 (1.0) | |
| Esophagectomy at high-volume center¶, n (%) | 2,375 (72.5) | 3,565 (69.7) | 0.01 |
| Facility location, n (%) | <0.001 | ||
| Northeast | 911 (27.8) | 1063 (20.8) | |
| Midwest | 1,040 (31.7) | 1,552 (30.3) | |
| South | 856 (26.1) | 1,697 (33.2) | |
| West | 416 (12.7) | 748 (14.6) | |
| Unknown | 53 (1.6) | 53 (1.0) | |
| Year of diagnosis, n (%) | <0.001 | ||
| 2012 | 514 (15.7) | 672 (13.2) | |
| 2013 | 549 (16.8) | 794 (15.5) | |
| 2014 | 524 (16.0) | 892 (17.5) | |
| 2015 | 555 (17.0) | 935 (18.3) | |
| 2016 | 529 (16.1) | 904 (17.7) | |
| 2017 | 606 (18.5) | 916 (17.9) | |
| Charlson-Deyo comorbidity index, n (%) | 0.03 | ||
| 0 | 2,315 (70.7) | 3,498 (68.4) | |
| 1 | 712 (21.8) | 1,151 (22.5) | |
| ≥2 | 249 (7.6) | 464 (9.1) | |
| Squamous cell carcinoma, n (%) | 517 (15.8) | 731 (14.3) | 0.14 |
| Clinical stage, n (%) | 0.09 | ||
| I | 248 (7.6) | 438 (8.6) | |
| II | 1,209 (36.9) | 1,946 (38.1) | |
| III | 1,819 (55.5) | 2,729 (53.4) |
†, from hospital at which esophagectomy is performed; ‡, average income in patient’s residing zip code; §, metropolitan defined at population >20,000 within residing county, rural defined at population <2,500 within residing county; ¶, greater than or equal to 20 esophagectomies per year. Facility refers to the institution in which the esophagectomy was performed. IQR, interquartile range.
The greatest relative difference between the two groups was in travel distance to the primary treatment center: those receiving collaborative treatment traveled twice the median distance to where they received their esophagectomy (30.5 vs. 15.1 miles; P<0.001) compared to single institution treatment. When categorized in tertiles, the lowest, middle, and highest groups traveled a median of 6.1, 23.2, and 84.3 miles respectively. In the collaborative treatment model, 40.3% of patients were in the highest tertile as opposed to 22.7% of patients in the single institution treatment model (P<0.001).
Multivariable logistic regression
Variables associated with a collaborative treatment model were assessed in a multivariable logistic regression (Table 2). Esophagectomy at a non-academic center was associated with a collaborative treatment model. Esophagectomy at a high-volume center trended toward significance but did not cross the predetermined threshold for significance. Increasing travel distance from the site of esophagectomy was associated with a stepwise increase in the receipt of multi-institutional treatment. In contrast, residence within urban areas and rural (as opposed to more densely population metropolitan areas) was associated with single institutional treatment. In a sensitivity analysis this trend was reversed if travel distance was not included in the model (Table S1). Similarly, when controlling for travel distance, geographic location lost its significance.
Table 2
| Variables | OR (95% CI) | P value |
|---|---|---|
| Age | 1.00 (0.99–1.00) | 0.42 |
| Female | 1.00 (0.87–1.16) | 0.96 |
| Median travel distance in miles† | ||
| ≤12.5 | Ref. | |
| 12.6–42.6 | 1.96 (1.66–2.31) | <0.001 |
| ≥42.7 | 4.81 (3.43–6.74) | <0.001 |
| Race | ||
| White | Ref. | |
| Black | 0.90 (0.65–1.13) | 0.27 |
| Asian | 1.27 (0.82–1.97) | 0.29 |
| Other | 1.14 (0.68–1.95) | 0.61 |
| Unknown | 1.25 (0.60–2.62) | 0.55 |
| Hispanic | 0.89 (0.62–1.30) | 0.57 |
| Insurance status | ||
| Private | Ref. | |
| Medicaid | 1.00 (0.78–1.29) | 0.97 |
| Medicare | 1.11 (0.96–1.29) | 0.15 |
| Other government insurance | 1.52 (0.93–2.50) | 0.10 |
| Uninsured | 0.61 (0.42–1.01) | 0.05 |
| Unknown | 0.52 (0.23–1.15) | 0.11 |
| Income quartiles‡ | ||
| ≥$63,333 | Ref. | |
| <$40,227 | 0.78 (0.65–1.18) | 0.07 |
| $40,227–$50,353 | 0.90 (0.76–1.21) | 0.33 |
| $50,354–$63,332 | 0.94 (0.77–1.15) | 0.54 |
| Unknown income | 0.70 (0.44–1.11) | 0.13 |
| Population density§ | ||
| Metropolitan | Ref. | |
| Urban | 0.66 (0.50–0.83) | <0.01 |
| Rural | 0.52 (0.35–0.77) | <0.01 |
| Unknown | 0.89 (0.71–1.12) | 0.13 |
| Facility type | ||
| Academic | Ref. | |
| Non-academic | 1.11 (1.05–1.22) | 0.01 |
| Unknown | 0.68 (0.49–1.03) | 0.08 |
| Esophagectomy at high-volume center¶ | 0.76 (0.58–1.00) | 0.05 |
| Facility location | ||
| Northeast | Ref. | |
| Midwest | 1.16 (0.67–1.99) | 0.59 |
| South | 1.60 (0.98–2.61) | 0.06 |
| West | 1.49 (0.82–2.67) | 0.19 |
| Year of diagnosis | ||
| 2012 | Ref. | |
| 2013 | 1.11 (0.92–1.35) | 0.22 |
| 2014 | 1.28 (1.04–1.52) | 0.02 |
| 2015 | 1.24 (0.96–1.49) | 0.08 |
| 2016 | 1.22 (0.98–1.50) | 0.10 |
| 2017 | 1.11 (0.82–1.30) | 0.85 |
| Charlson-Deyo comorbidity index | ||
| 0 | Ref. | |
| 1 | 1.09 (0.92–1.30) | 0.67 |
| ≥2 | 1.27 (0.99–1.60) | 0.05 |
| Squamous cell carcinoma | 0.98 (0.83–1.15) | 0.81 |
| Clinical stage | ||
| Stage I | Ref. | |
| Stage II | 0.88 (0.72–1.07) | 0.20 |
| Stage III | 0.84 (0.68–1.04) | 0.11 |
†, from hospital at which esophagectomy is performed; ‡, average income in patient’s residing zip code; §, metropolitan defined at population >20,000 within residing county, rural defined at population <2,500 within residing county; ¶, greater than or equal to 20 esophagectomies per year. Facility refers to the institution in which the esophagectomy was performed. OR, odds ratio; CI, confidence interval.
Multimodal therapy, R0 resection, pCR, and 90-day mortality
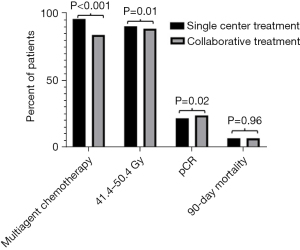
Patients in the collaborative treatment model were less likely to receive dual agent chemotherapy (84.6% vs. 96.5%; P<0.001) compared to patients who received single-institution treatment (Figure 2). In addition, patients who received collaborative treatment were less likely to receive guideline recommended 41.4–50.4 Gy of radiation (89.3% vs. 91.1%; P=0.01). Patients who received collaborative treatment were slightly more likely to have a pCR at time of esophagectomy (24.3% vs. 22.0%; P=0.02). However, there was no significant difference in R0 resection between patients treated in a collaborative treatment and single-institution treatment (94.4% vs. 93.7%; P=0.17). Lastly, there were no statistically or clinically significant differences in 90-day mortality between the two groups (7.1% vs. 7.1%; P=0.96). When stratified by clinical stage these effects were maintained, with the exception of pCR and R0 resection, which lost their significance in patients with clinical stage I and stage II disease (Table S2). Other quality markers including days from diagnosis to initiation of chemotherapy, radiotherapy, and operative resection were evaluated. For these events, the median difference in timing between the two groups was less than or equal to 5 days (Table S3).
5-year survival and cox-proportional hazard model
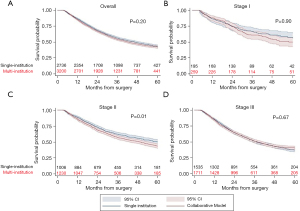
Overall, 5-year survival was 42.6% and did not differ significantly between patients in the single institution or collaborative treatment groups (Figure 3A). This relationship was preserved in the Cox proportional hazard model. Variables associated with worse survival included older age, male sex, lack of insurance, increasing Charlson-Deyo comorbidity index, esophagectomy at a low-volume center, and increasing clinical stage (Table 3). When stratified by clinical stage, there was no difference in 5-year survival in patients with stage I and stage III disease who received multi-modal treatment at a single institution or multiple institutions. However, there was a 5-year survival advantage in patients with clinical stage II disease who received treatment at a single institution (49.2% vs. 44.1%; P=0.01) (Figure 3B-3D). This effect was maintained in the Cox-proportional hazard model (HR =1.23; 95% CI: 1.09–1.34; P<0.01) (Tables S4-S6). Finally, in a sensitivity analysis including only patients receiving guideline concordant CRT, there was no qualitative difference in survival compared to the overall cohort (Figure 4).
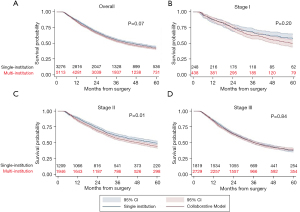
Table 3
| Variables | HR (95% CI) | P value |
|---|---|---|
| Collaborative (multi-institutional) treatment | 1.04 (0.98–1.17) | 0.09 |
| Age | 1.02 (1.01–1.02) | <0.001 |
| Female | 0.81 (0.73–0.89) | <0.001 |
| Travel distance in miles† | ||
| ≤12.5 | Ref. | |
| 12.6–42.6 | 0.98 (0.91–1.09) | 0.87 |
| ≥42.7 | 1.00 (0.89–1.13) | 0.89 |
| Race | ||
| White | Ref. | |
| Black | 0.94 (0.80–1.13) | 0.54 |
| Asian | 0.97 (0.76–1.24) | 0.64 |
| Other | 1.07 (0.72–1.39) | 0.96 |
| Unknown | 0.92 (0.66–1.48) | 0.94 |
| Hispanic | 0.87 (0.71–1.08) | 0.20 |
| Insurance status | ||
| Private | Ref | |
| Medicaid | 1.14 (0.98–1.31) | 0.07 |
| Medicare | 1.01 (0.98–1.32) | 0.77 |
| Other government insurance | 1.01 (0.93–1.11) | 0.78 |
| Uninsured | 1.30 (1.01–1.66) | 0.04 |
| Unknown | 1.07 (0.81–1.40) | 0.62 |
| Income quartiles‡ | ||
| ≥$63,333 | Ref. | |
| <$40,227 | 1.18 (1.03–1.27) | 0.01 |
| $40,227–$50,353 | 1.06 (0.94–1.13) | 0.28 |
| $50,354–$63,332 | 1.13 (1.04–1.22) | 0.01 |
| Unknown | 1.16 (0.83–1.64) | 0.38 |
| Facility type | ||
| Academic | Ref. | |
| Non-Academic | 1.05 (0.94–1.16) | 0.48 |
| Unknown | 1.03 (0.42–1.81) | 0.70 |
| Esophagectomy at high-volume center§ | 0.93 (0.85–0.99) | 0.04 |
| Facility location | ||
| Midwest | Ref. | |
| Northeast | 1.05 (0.91–1.21) | 0.50 |
| South | 1.03 (0.95–1.12) | 0.45 |
| West | 0.84 (0.74–0.96) | 0.01 |
| Population density¶, n (%) | ||
| Metropolitan | Ref. | |
| Urban | 1.14 (1.04–1.24) | 0.003 |
| Rural | 1.16 (0.93–1.44) | 0.18 |
| Unknown | 0.66 (0.56–0.77) | <0.001 |
| Year of diagnosis | ||
| 2012 | Ref. | |
| 2013 | 1.03 (0.89–1.08) | 0.60 |
| 2014 | 0.96 (0.81–0.98) | 0.03 |
| 2015 | 0.99 (0.88–1.07) | 0.48 |
| 2016 | 0.89 (0.82–1.00) | 0.05 |
| 2017 | 0.78 (0.74–0.96) | 0.01 |
| Charlson-Deyo comorbidity index | ||
| 0 | Ref. | |
| 1 | 1.08 (1.01–1.15) | 0.04 |
| ≥2 | 1.25 (1.15–1.41) | <0.001 |
| Squamous cell carcinoma | 0.94 (0.85–1.06) | 0.26 |
| Clinical stage | ||
| Stage I | Ref. | |
| Stage II | 1.23 (1.04–1.45) | <0.01 |
| Stage III | 1.54 (1.31–1.81) | <0.001 |
†, from hospital at which esophagectomy is performed; ‡, average income in patient’s residing zip code; §, greater than or equal to 20 esophagectomies per year. Facility refers to the institution in which the esophagectomy was performed; ¶, metropolitan defined at population >20,000 within residing county, rural defined at population <2,500 within residing county. HR, hazard ratio; CI, confidence interval.
Discussion
In this study of more than 8,000 patients receiving trimodal treatment for esophageal cancer, we found that 61% of patients received part of their care at a different institution than where they ultimately underwent esophagectomy. A collaborative treatment model across multiple institutions was associated with slightly decreased rates of guideline concordant CRT but was not associated with a decrease in pCR, 90-day survival, or 5-year survival. This finding is important because it demonstrates CRT can be effectively coordinated for patients receiving complex oncologic care across multiple institutions with only a modest effect on quality.
As popularized by Birkmeyer et al. in 2002, concentration of complex operations such as esophagectomy among high-volume, high-quality centers has the potential to improve operative morbidity and mortality (9). The association between improved surgical quality and higher volume specialty cancer centers has been demonstrated in multiple studies over the past 20 years and regionalization of complex surgery, including esophagectomy, has been advocated for by leaders in the field (10-13). Recent studies focused on esophageal cancer have shown that both short-term and long-term outcomes are improved by regionalization of care to high-volume centers (14,15). However, the downside of regional care includes increased cost and travel burden on patients, which can represent significant barriers (5,16-18). These barriers help explain a lack spontaneous regionalization found specifically in high-risk patients with comorbidities who might otherwise benefit the most from treatment at high-volume centers (19).
In our study, the most striking difference between patients who received collaborative treatment compared to those who received single-institutional treatment was the travel distance to the site of their esophagectomy. On average, patients who received collaborative treatment traveled almost twice as far as single institution patients. Nearly 1 in 5 patients in the collaborate treatment model traveled a median of 80 miles to the site of esophagectomy. By receiving CRT at local institutions, these patients are potentially spared a significant travel burden without a demonstratable impact on pCR or overall survival. Given that neoadjuvant CRT requires multiple encounters at an infusion/radiation treatment center, this represents a major benefit for patients and their caregivers.
Recently, Rhodin et al. demonstrated the efficacy of multi-institutional treatment of esophageal cancer in the NCDB (20). We build on that work by including more recent data and focusing on patients receiving standard neoadjuvant regiments of CRT rather than just chemotherapy. Our study shows that even when accounting for the added complexity of coordinating radiotherapy long-term survival is maintained. Equally important, our study uniquely describes the quality of neoadjuvant therapy and the performance of guideline concordant treatments. Additionally, by categorizing travel distance we show a powerful association between travel distance and collaborative treatment that has not previously been recognized. Prior associations with geographic location (e.g., Northeast, South, etc.) become insignificant when controlling for travel distance in this manner.
While we demonstrated that complex oncologic care can be coordinated among multiple centers without a significant decrease in overall 90-day or 5-year survival; we did note there was a slight decrease in the rate of appropriate radiotherapy dosing (89% vs. 91%) among patients who received collaborative treatment. In addition, there was a more significant drop in the rate of multiagent chemotherapy treatment (85% vs. 97%). There are several explanations for this finding. First, patients who received collaborative treatment had a higher co-morbidity burden as demonstrated by Charlson-Deyo comorbidity index and this may have limited their ability to tolerate multimodal therapy. Second, these patients tended to be older than those receiving all their care at a single institution, and this may have further limited their ability to tolerate more toxic neoadjuvant regimens. Interestingly, despite this relative decrease in standard-of-care CRT, the population that received collaborative care had a small increase in cPR (24% vs. 22%). This rate of cPR was similar to that reported in the CROSS trial and other studies evaluating the utility of neoadjuvant multimodal therapy (21,22). It is surprising the patients in the collaborative treatment model had a greater rate cPR despite slightly lower rates of guideline concordant CRT. This result may reflect slight differences in the population in each treatment arm; however, the clinical impact of this ~2% difference in cPR is unclear. More importantly, the 90-day or 5-year survival did not differ between the two treatment groups.
In subgroup analysis, patients with clinical stage II disease had a decreased 5-year survival in the collaborative treatment model (44% vs. 49%). This may be attributed to differences in age or comorbidities, although it remained significant when controlling for these in multivariable analysis. It is also possible that it became more difficult to care for these patients in the event of late complications. Several authors have shown increased care burden and cost for patients who present to non-index hospitals after a surgical procedure (23,24). We expect that the collaborative treatment group would be more likely to present to an alternative hospital given that on average they lived twice as far away from the site of their esophagectomy. More concerningly, the drop in long-term survival for the stage II patients may be related to the relative decrease in appropriate multimodal therapy. However, in sensitivity analysis including only patients who received guideline concordant CRT these differences persisted. Furthermore, differences in the quality of CRT delivery persisted across all stages.
An additional contributor may be attributed to staging error. Relative to other cancers esophageal cancer is difficult to stage and this is especially true in stage II disease (25). Given the potential challenges in coordinating care, patients treated in the collaborate care model may have been more likely to be under staged, thus resulting in long-term outcomes that appear worse. Regardless, the ~5% decrease in 5-year survival in patients with clinical stage II disease should be considered in the context of their disease and the burdens associated with single institutional care.
There is enormous benefit to neoadjuvant CRT in the treatment of resectable esophageal cancer, but it is associated with significant decreases in quality of life (26). Fortunately, quality of life does improve after completion of neoadjuvant treatment, and post-esophagectomy quality of life is similar between patients who do and do not receive neoadjuvant CRT (27). Getting patients safely through their neoadjuvant treatment and surgery is a priority of the multidisciplinary cancer team. In the treatment of pancreatic adenocarcinoma—a similar cancer requiring coordination of neoadjuvant therapy and surgical resection—sociodemographic barriers are a significant hurdle to patients receiving guideline recommended treatment, most pronounced in under-represented minorities and those of lower socioeconomic status (28,29). These factors may also contribute to distrust for specific healthcare networks and influence a patient’s choice to receive single-institution or collaborative treatment. Indeed, we found that patients who received collaborative treatment were more likely to originate from non-academic centers. Further qualitative analysis will be necessary to develop an understanding behind these treatment-decision and will be the focus of ongoing studies. Despite the need for additional studies, decreasing travel time is expected to help increase the number of patients who are able to complete multimodal therapy. The results described above show that collaborative, multi-institutional, treatment can be performed effectively, but will require diligent monitoring and additional study, especially in patients with clinical stage II disease.
Limitations
Limitations of this study include those commonly associated with large retrospective observational studies. We are unable to account for differences in treatment regimens or why certain patients did or did not receive a given therapy. Most importantly, we are unable to accurately identify patients who may have started neoadjuvant treatment but then failed to proceed to surgery. We do not know if these events occurred due to treatment complications, patient preference, or a decision to defer surgery in favor of clinical monitoring. We also do not know the reasons a patient elected for treatment in a single-institution or a collaborative model, or how this may have affected their trust and satisfaction with a given healthcare network. Differences in staging modality and accuracy between patients in the collaborative and single-institution treatment groups could have further biased this study. Unfortunately, the NCDB lacks the granularity to satisfactorily answer these questions, and this will the subject of focused qualitative and quantitative studies.
Likewise, the treatment of esophageal cancer is evolving and increasingly includes immunotherapy. Given the timing and irregular use of immunotherapy during the study time-period, we are unable to accurately assess its role and if this contributed to any difference in outcomes between the study groups. As with other studies using the NCDB, we cannot comment on specific comorbidities, performance, status, nutritional status, and smoking history, which may have influenced neoadjuvant CRT regimens and contributed to long-term survival. There is also a significant amount of missing clinical and pathologic staging data. Exclusion of patients with missing data may bias these results and reduce their generalizability. We do not know the quality of staging, staging modality, or location at which cancer was first diagnosed. These factors may have differed between the study groups and biased the study. Similarly, we were unable to assess cancer specific survival and recurrence rates, which may have provided insight into competing mortality risks (7,30). We are unable to account for care that may have occurred outside of a NCDB reporting center and was not captured in the database. Among patients excluded due to care outside of an NCDB center, we did see a similar ratio of patients receiving single-institution and collaborative treatment. We are also reliant on the NCDB classification of individual centers and their associated outpatient clinics. We cannot make assessments regarding in network versus out of network care or if certain independent institutions have preexisting relationships that enable coordination of complex oncologic treatment.
Conclusions
Multiple institutions commonly collaborate to deliver trimodality treatment to patients with esophageal cancer without an overall reduction in R0 resection, pCR, 90-day survival, or long-term survival. Collaborative care is a reasonable treatment model that can allow patients to receive CRT at local centers and alleviate barriers to accessing guideline recommended care. Collaborative deliver of trimodality treatment should be encouraged as it may allow more patients with esophageal cancer to access and benefit from guideline recommended therapy.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-346/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-346/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-346/coif). DJB was paid a stipend from Iovance to attend a panel discussion on cell-based therapy that was unrelated to this work. The other authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Greally M, Ilson DH. Neoadjuvant therapy for esophageal cancer: Who, when, and what? Cancer 2018;124:4276-8. [Crossref] [PubMed]
- Hillner BE, Smith TJ, Desch CE. Hospital and physician volume or specialization and outcomes in cancer treatment: importance in quality of cancer care. J Clin Oncol 2000;18:2327-40. [Crossref] [PubMed]
- Jacobs RC, Groth S, Farjah F, et al. Potential Impact of "Take the Volume Pledge" on Access and Outcomes for Gastrointestinal Cancer Surgery. Ann Surg 2019;270:1079-89. [Crossref] [PubMed]
- Eyck BM, van Lanschot JJB, Hulshof MCCM, et al. Ten-Year Outcome of Neoadjuvant Chemoradiotherapy Plus Surgery for Esophageal Cancer: The Randomized Controlled CROSS Trial. J Clin Oncol 2021;39:1995-2004. [Crossref] [PubMed]
- Fong ZV, Hashimoto DA, Jin G, et al. Simulated Volume-Based Regionalization of Complex Procedures: Impact on Spatial Access to Care. Ann Surg 2021;274:312-8. [Crossref] [PubMed]
- NCCN. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): esophageal and esophagogastric junction cancers. J Natl Compr Canc Netw Published online March 9, 2021. Available online: https://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf
- Boffa DJ, Rosen JE, Mallin K, et al. Using the National Cancer Database for Outcomes Research: A Review. JAMA Oncol 2017;3:1722-8. [Crossref] [PubMed]
- Metzger R, Bollschweiler E, Vallböhmer D, et al. High volume centers for esophagectomy: what is the number needed to achieve low postoperative mortality? Dis Esophagus 2004;17:310-4. [Crossref] [PubMed]
- Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med 2002;346:1128-37. [Crossref] [PubMed]
- Hoag JR, Resio BJ, Monsalve AF, et al. Differential Safety Between Top-Ranked Cancer Hospitals and Their Affiliates for Complex Cancer Surgery. JAMA Netw Open 2019;2:e191912. [Crossref] [PubMed]
- Schlottmann F, Strassle PD, Charles AG, et al. Esophageal Cancer Surgery: Spontaneous Centralization in the US Contributed to Reduce Mortality Without Causing Health Disparities. Ann Surg Oncol 2018;25:1580-7. [Crossref] [PubMed]
- Urbach DR. Pledging to Eliminate Low-Volume Surgery. N Engl J Med 2015;373:1388-90. [Crossref] [PubMed]
- Rice TW, Blackstone EH. Esophagectomy volume threshold as a criterion for centers of excellence: causation or cause, strategy or strategem? J Thorac Cardiovasc Surg 2009;137:10-2. [Crossref] [PubMed]
- Ely S, Alabaster A, Ashiku SK, et al. Regionalization of thoracic surgery improves short-term cancer esophagectomy outcomes. J Thorac Dis 2019;11:1867-78. [Crossref] [PubMed]
- Ely S, Alabaster A, Dominguez DA, et al. Effect of Thoracic Surgery Regionalization on 1- and 3-Year Survival after Cancer Esophagectomy. Ann Surg 2023;277:e305-12. [Crossref] [PubMed]
- Resio BJ, Chiu AS, Hoag JR, et al. Motivators, Barriers, and Facilitators to Traveling to the Safest Hospitals in the United States for Complex Cancer Surgery. JAMA Netw Open 2018;1:e184595. [Crossref] [PubMed]
- Fong ZV, Lim PW, Hendrix R, et al. Patient and Caregiver Considerations and Priorities When Selecting Hospitals for Complex Cancer Care. Ann Surg Oncol 2021;28:4183-92. [Crossref] [PubMed]
- Clark JM, Boffa DJ, Meguid RA, et al. Regionalization of esophagectomy: where are we now? J Thorac Dis 2019;11:S1633-42. [Crossref] [PubMed]
- Arnold BN, Chiu AS, Hoag JR, et al. Spontaneous regionalization of esophageal cancer surgery: an analysis of the National Cancer Database. J Thorac Dis 2018;10:1721-31. [Crossref] [PubMed]
- Rhodin KE, Raman V, Jensen CW, et al. Multi-institutional Care in Clinical Stage II and III Esophageal Cancer. Ann Thorac Surg 2023;115:370-7. [Crossref] [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Xi M, Yang Y, Zhang L, et al. Multi-institutional Analysis of Recurrence and Survival After Neoadjuvant Chemoradiotherapy of Esophageal Cancer: Impact of Histology on Recurrence Patterns and Outcomes. Ann Surg 2019;269:663-70. [Crossref] [PubMed]
- Stitzenberg KB, Chang Y, Smith AB, et al. Exploring the burden of inpatient readmissions after major cancer surgery. J Clin Oncol 2015;33:455-64. [Crossref] [PubMed]
- Zheng C, Habermann EB, Shara NM, et al. Fragmentation of Care after Surgical Discharge: Non-Index Readmission after Major Cancer Surgery. J Am Coll Surg 2016;222:780-789.e2. [Crossref] [PubMed]
- da Costa WL Jr, Gu X, Farjah F, et al. Staging Concordance and Guideline-Concordant Treatment for Esophageal Adenocarcinoma. Ann Thorac Surg 2022;113:279-85. [Crossref] [PubMed]
- Blazeby JM, Sanford E, Falk SJ, et al. Health-related quality of life during neoadjuvant treatment and surgery for localized esophageal carcinoma. Cancer 2005;103:1791-9. [Crossref] [PubMed]
- Noordman BJ, Verdam MGE, Lagarde SM, et al. Effect of Neoadjuvant Chemoradiotherapy on Health-Related Quality of Life in Esophageal or Junctional Cancer: Results From the Randomized CROSS Trial. J Clin Oncol 2018;36:268-75. [Crossref] [PubMed]
- Cloyd JM, Shen C, Santry H, et al. Disparities in the Use of Neoadjuvant Therapy for Resectable Pancreatic Ductal Adenocarcinoma. J Natl Compr Canc Netw 2020;18:556-63. [Crossref] [PubMed]
- Cloyd JM, Hyman S, Huwig T, et al. Patient experience and quality of life during neoadjuvant therapy for pancreatic cancer: a systematic review and study protocol. Support Care Cancer 2021;29:3009-16. [Crossref] [PubMed]
- Pathak R, Goldberg SB, Canavan M, et al. Association of Survival With Adjuvant Chemotherapy Among Patients With Early-Stage Non-Small Cell Lung Cancer With vs Without High-Risk Clinicopathologic Features. JAMA Oncol 2020;6:1741-50. [Crossref] [PubMed]



