
The long-term impact of COVID-19 pneumonia on pulmonary function and exercise capacity
Highlight box
Key findings
• A restrictive spirometry pattern [lower %predicted of forced vital capacity (FVC)] and lower exercise capacity (lower 6-minute walking distance) were observed in the long-term follow up of post coronavirus disease 2019 (COVID-19) pneumonia patients.
• The improvement of FVC and 6-minute walking distance were also observed from month 1 to month 12 after hospital discharge.
What is known and what is new?
• The long-term sequelae of post COVID-19 pneumonia have been widely reported.
• The sequelae of post COVID-19 pneumonia on lung function and exercise capacity were observed with short interval time during recovery.
What is the implication, and what should change now?
• Pulmonary function tests and six-minute walk test are useful tools for detection of long-term sequelae of post COVID-19 pneumonia.
• Pulmonary rehabilitation including aerobic exercise or promotion of active physical activity should be provided in post COVID-19 pneumonia.
Introduction
The sequelae of post-coronavirus disease 2019 (COVID-19) has been widely reported (1-14). They showed that the abnormal spirometry were commonly found in post COVID-19 pneumonia patients (7,13-18). Moreover, they also found that total lung capacity (TLC) and lung diffusion capacity for carbon monoxide (DLCO) were the most frequently altered parameters (13,14). However, few studies mentioned the impulse oscillometry (IOS) for measurement of small airway dysfunction (SAD) in this condition (19-21). Visconti et al. found SAD in 30.3% of post COVID-19 pneumonia (19). However, another study published by Scaramuzzo et al. found no significant difference between the IOS parameters and prevalence of SAD in post COVID-19 pneumonia with and without dyspnea at one year after the onset of the disease (20). Therefore, a long-term study regarding IOS in post COVID-19 pneumonia is still required.
Fractional exhaled nitric oxide (FeNO) is a noninvasive method that reflects airway inflammation. Two studies found that FeNO values remained within normal ranges and did not differ between severe and non-severe post COVID-19 pneumonia (12,22). Additionally, other studies noted that FeNO values did not differ between post-COVID-19 pneumonia and healthy subjects at 1–3 months from recovery (11,12). However, a long-term study regarding FeNO in post COVID-19 pneumonia is still limited.
The sequelae of post COVID-19 on exercise capacity has been reported (4,7,14,16,23,24). They showed that the prevalence of low exercise capacity defined by a 6-minute walk distance (6-MWD) lower than the predicted value ranged from 17.33–33.33% for a follow-up time of 2–6 months (4,7,16,23,24). However, a two-year longitudinal cohort study published by Zhang et al. showed that 6-MWD increased continuously after COVID-19 infection (9). But some studies found there was no improvement of 6-MWD between 6–9 and 12–18.5 months post-recovery (1,8).
As mentioned before that the sequelae of post COVID-19 has been widely reported. However, the time point of the follow-up time in the previous studies varied ranging from 3–24 months and the interval time of the follow-up time was too long (6 or 12 months). Thus, a shorter interval time during recovery for assessment of the sequelae of post COVID-19 on lung function and exercise capacity is still required. Moreover, the healthy subjects were not included for comparison between post COVID-19 pneumonia in some studies (2,15-17,23). Therefore, the objective of this study was to explore the long-term impact of COVID-19 pneumonia on pulmonary function and exercise capacity at 1-, 3-, 6-, 9-, and 12-month post-hospital discharge compared to healthy subjects. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-514/rc).
Methods
Study design
This prospective observational study was conducted on post-COVID-19 pneumonia with age more than 18 years old who were admitted at Maharaj Nakorn Chiang Mai Hospital, Chiang Mai, Thailand during April–May 2021. All subjects were diagnosed with COVID-19 pneumonia during admission based on pulmonary infiltration on chest X-rays (CXR) and confirmed by positive reverse transcription-polymerase chain reaction (RT-PCR). Subjects who were unable to understand the Thai language were excluded. Subjects with chronic respiratory diseases e.g., asthma, bronchiectasis, and chronic obstructive pulmonary disease (COPD) were also excluded. This study was conducted at the Lung Health Center, Division of Pulmonary, Critical Care and Allergy, Department of Internal Medicine, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand between May 2021 and April 2022. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Research Ethics Committee, Faculty of Medicine, Chiang Mai University (study code: MED-2564-08109, date of approval: 3 May 2021) and filed under Clinical Trials Registry (Study ID: TCTR20210827005, date of approval: 27 August 2021). Before enrollment, all participants gave written informed consent.
Baseline demographics including age, sex, body mass index (BMI), and underlying diseases were recorded. For post COVID-19 pneumonia subjects, the severity of COVID-19 pneumonia during admission was also reviewed from the patient’s medical records. Pulmonary function tests including spirometry and IOS, and FeNO were assessed at 1-, 6-, 9- and 12-month post hospital discharge when compared to healthy subjects. Exercise capacity measured by six-minute walk test (6-MWT) was also assessed at 1-, 3-, 6-, 9- and 12-month post hospital discharge when compared to healthy subjects.
Data collection
Both post COVID-19 pneumonia and healthy subjects were assessed for pre-bronchodilator (BD) spirometry and IOS. All measurements of IOS and spirometry were performed using combined spirometry and IOS equipment (MostGraph-02; Chest M.I., Co., Ltd., Tokyo, Japan). IOS value were measured using IOS machine during normal breathing for 30–40 seconds via a mouthpiece that was connected to a loudspeaker. Respiratory resistance (Rrs) reflects information about the forward pressure of the conducting airways (25). The resistance at 5 Hz (R5), the resistance at 20 Hz (R20), and the difference of the R20 from R5 (R5-R20) values represent the resistance of the total airways, large airways and small airways, respectively. The reactance reflects the capacitive and inertive properties of the airways (25). The reactance at 5 Hz (X5), resonant frequency (Fres), and reactance area (AX) represent the reactance of the airways. X5 indicates the elastic recoil of the peripheral airways, Fres indicates the frequency as Xrs crosses zero which the elastic and inertial forces are equal in magnitude but in the opposite direction, and AX indicates the area of the negative reactance between 5 Hz and Fres to the zero lines (25). Three tests were performed following the European Respiratory Society (ERS) standard (25). The average values from three IOS measurements were recorded. The predicted values of all parameters in IOS were calculated using the Thai predictive value published by Deesomchok et al. (26).
For spirometry assessment, at least three acceptable tests were performed following the guideline published by the American Thoracic Society/European Respiratory Society (ATS/ERS) (27). Forced vital capacity (FVC), forced expiratory volume in the first second (FEV1), the ratio of FEV1/FVC, and forced expiratory flow at 25–75% of FVC (FEF25–75%) were recorded. The Global Lung Function Initiative (GLI) reference equation (Southeast Asian population) was used as a prediction (28). The FeNO was also performed according to the ATS/ERS guidelines (29) using FeNO equipment (NIOX VERO® Circassia Inc., NC 27560, United States). The 6-MWT was also assessed according to the guidelines from the ATS (30).
Twenty-five healthy control subjects with no history of chronic respiratory diseases were enrolled in this study. Demographic data, IOS, spirometry, FeNO, and 6-MWD were collected for comparison with post COVID-19 pneumonia subjects.
Study size estimation
Sample size calculation was based on the mean and standard deviation (SD) of 6-MWD at one month after discharge between the non-severe post COVID-19 pneumonia and healthy controls in the previous study (12). The means and SD of 6-MWD in the non-severe post COVID-19 pneumonia and healthy controls were 451.7±78.9 meters and 525.5±36.4 meters, respectively. We need to study at least 40 subjects, 20 post COVID-19 pneumonia, and 20 healthy controls to be able to reject the null hypothesis that the population means of the post COVID-19 pneumonia and healthy control groups were equal with probability (power) of 0.95. The type I error probability associated with this test of this null hypothesis was 0.05.
Statistical analysis
Continuous data were shown as mean and SD, standard error of the mean (SEM), or median and interquartile range (IQR). An independent sample t-test was used for analyzing clinical characteristics between two groups for continuous data. For comparison of the pulmonary function data, FeNO, and exercise capacity between groups at each visit, the independent t-test was used. Paired sample t-test was used for analyzing differences in the pulmonary function data, FeNO, and 6-MWD within a group when compared to the month 1 visit. Categorical data were shown as numbers and percentages. A comparison of categorical data between groups was analyzed using Fisher’s Exact test. A P value less than 0.05 are considered statistically significant. All statistical analyses were performed using STATA version 16 (StataCorp, College Station, TX, USA).
Results
Fifty-six post COVID-19 pneumonia patients were recruited. However, five, three, eight, and two subjects lost to follow-up visits at 3 months, 6 months, 9 months, and 12 months, respectively due to various reasons including emigration, withdrawal, and loss of contact. Therefore, thirty-eight post COVID-19 pneumonia and 25 healthy controls were included in the final analysis. The baseline demographics data between post COVID-19 pneumonia and healthy controls were shown in Table 1. The mean age of post COVID-19 pneumonia and healthy controls were 41.1±14.8 and 43.0±9.6 years, respectively. There were no significant differences in age, the proportion of male sex, or underlying diseases between groups. However, BMI was significantly higher in the post COVID-19 pneumonia group. Current smokers were more frequent in the healthy controls group. Of thirty-eight post COVID-19 pneumonia group, 16 (42.1%) and 22 (57.9%) were defined as non-severe (on oxygen cannula during hospitalization) and severe [on high-flow nasal cannula (HFNC) or mechanical ventilator (MV) during hospitalization]. COVID-19 pneumonia data during admission was also shown in Table 1.
Table 1
| Variables | Post COVID-19 pneumonia (n=38) | Healthy control (n=25) | P value |
|---|---|---|---|
| Age (years) | 41.1±14.8 | 43.0±9.6 | 0.555 |
| Male gender | 20 (52.6) | 12 (48.0) | 0.961 |
| Body mass index (kg/m2) | 29.0±5.1 | 26.1±5.6 | 0.036 |
| Underlying diseases | 0.588 | ||
| Cardiovascular | 9 (23.7) | 5 (20.0) | |
| Metabolic | 1 (2.6) | 0 (0.0) | |
| Cardiovascular + metabolic | 4 (10.5) | 1 (4.0) | |
| None | 24 (63.2) | 19 (76.0) | |
| Smoking status | 0.039 | ||
| Current | 1 (2.6) | 4 (16.0) | |
| Ex-smoke | 8 (21.1) | 1 (4.0) | |
| Non-smoker | 29 (76.3) | 20 (80.0) | |
| Severity of COVID-pneumonia during hospitalization | |||
| Non-severe (on required O2 cannula) | 16 (42.1) | – | |
| Severe (on HFNC or MV) | 22 (57.9) | ||
| Laboratory results on admission | |||
| Hemoglobin (g/dL) | 13.7±1.9 | – | |
| Hematocrit (%) | 40.1±4.6 | ||
| Platelet count (×103/mm3) | 219.9±83.7 | ||
| White blood count (×103 cells/mm3), median (IQR) | 5.7 (4.7, 7.4) | ||
| Lymphocyte count (×103 cells/mm3), median (IQR) | 1.2 (0.9, 1.8) | ||
| CRP (mg/L), median (IQR) | 74.9 (38.9, 134.6) | ||
| ESR (mm/hour), median (IQR) | 40.0 (23.0, 58.0) | ||
| Chest x-ray pattern on admission | |||
| Ground glass opacities | 21 (55.3) | – | |
| Consolidation | 1 (2.6) | ||
| Mixed | 16 (42.1) | ||
| Chest x-ray distribution on admission | |||
| Multi-lobar | 5 (13.2) | – | |
| Bilateral | 33 (86.8) |
Data are presented as mean ± standard deviation, n (%), or otherwise stated. COVID-19, coronavirus disease 2019; O2, oxygen; SpO2, oxygen saturation via pulse oximeter; HFNC, high-flow nasal cannula; MV, mechanical ventilator; IQR, interquartile range; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate.
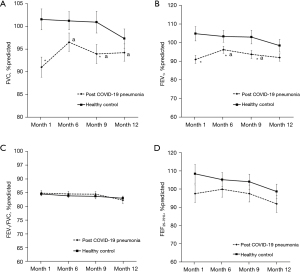
The comparison of spirometry results including FVC, FEV1, FEV1/FVC, and FEF25–75% between post COVID-19 pneumonia and healthy controls throughout the study period are shown in Figure 1. The %predicted of FVC were lower in the post COVID-19 pneumonia group than healthy controls especially at month 1 and month 9. The %predicted of FEV1 was also lower in the post COVID-19 pneumonia group throughout the study period except for month 12. The improvement of %predicted of FVC and FEV1 was observed in the post COVID-19 pneumonia group when compared to month 1 except for the %predicted of FEV1 at month 12. The %predicted of FEF25-75% were lower in the post COVID-19 pneumonia group compared to healthy controls but not significant. However, there were no significant differences between groups and within groups for the FEV1/FVC.
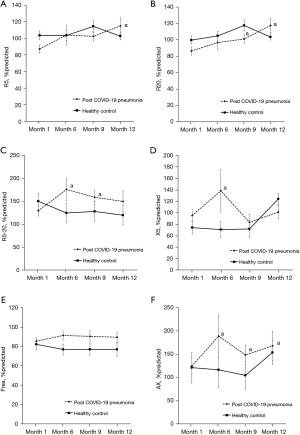
The comparison of IOS parameters including R5, R20, R5-R20, X5, Fres, and AX between post COVID-19 pneumonia and healthy controls throughout the study period are shown in Figure 2. There were no significant differences between groups for all IOS parameters. However, at month 6 the R5-R20 and AX were significantly higher and X5 was more negative when compared to month 1 in the post COVID-19 pneumonia group. At month 9, the R20, R5-R20, and AX were significantly higher when compared to month 1 in the post COVID-19 pneumonia group and at month 12 the R5, R20, and AX were also significantly higher when compared to month 1.
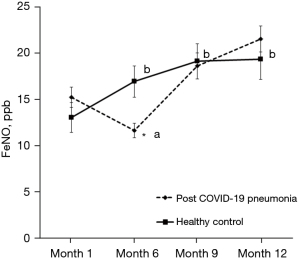
The comparison of FeNO between post COVID-19 pneumonia and healthy controls throughout the study period are shown in Figure 3. There were no significant differences between groups for FeNO, except lower FeNO in the post COVID-19 pneumonia group at month 6. Moreover, the FeNO was lower at month 6 when compared to month 1 in the post COVID-19 pneumonia group, while the FeNO was significantly higher at months 6, 9, and 12 when compared to month 1 in the healthy controls group.
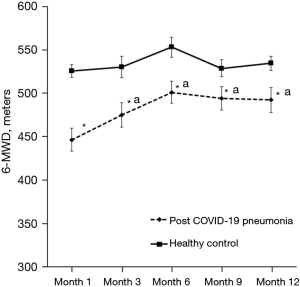
The comparison of exercise capacity measured using 6-MWT between post COVID-19 pneumonia and healthy controls throughout the study period are shown in Figure 4. The 6-MWD was significantly lower in post COVID-19 pneumonia group compared to healthy controls in all visits throughout the study period. The improvement of 6-MWD was observed in all visits in the post COVID-19 pneumonia group when compared to month 1.
Discussion
We studied the long-term impact of COVID-19 pneumonia on pulmonary function and exercise capacity from one month through one year of post-hospital discharge when compared to healthy subjects. We found the improvement of %predicted of FVC and FEV1 in the post COVID-19 pneumonia group. Moreover, we found that spirometry data including %predicted of FVC and FEV1 were significantly lower in the post COVID-19 pneumonia group compared to healthy controls at some visits. The exercise capacity was also significantly lower in the post COVID-19 pneumonia group compared to the healthy control till one year. However, the FeNO and IOS values were no significant differences between the groups.
Our study showed that lung function measured by spirometry were lower in post COVID-19 pneumonia group compared to healthy controls. Our results were supported by the previous studies indicating that the spirometric values including %predicted of FVC and FEV1 showed a marked reduction in post COVID-19 pneumonia compared to the controls after three to six months from recovery (6,11). Previous studies also showed that the prevalence of spirometric restriction defined by FVC lower than 80%predicted or lower than the limit of normal (LLN) ranged from 7.8–45.8% and 12.8–45.0% for a follow-up time of 3–6 months and 6–12 months, respectively (7,15-18). Moreover, the prevalence of FEV1 lower than 80%predicted or lower than LLN ranged from 11.8–30.5% and 13.8–34.1% for a follow-up time of 3–6 months and 6–12 months, respectively (7,15-18). For the long-term study, we serial measured the spirometry and found the improvement of lung function in post COVID-19 pneumonia. Our results were comparable to previous studies which showed that the lung function measured by spirometry was improved over time in post COVID-19 pneumonia (5,9,10,31,32). However, there was a fluctuation of the lung function data in heathy control group especially at month 9 and 12. The fluctuation of lung function results may be related to air pollution especially for particulate matter with a diameter of smaller than 2.5 microns (PM2.5) which was higher level than the World Health Organization (WHO) standard in Chiang Mai, Thailand at 9 months (January–February 2022) and 12 months (April–May 2022) after enrollment (33).
IOS, a sensitive tool for detecting small airway involvement, was also serial measured in our study and we found that there were no significant differences between post COVID-19 pneumonia and healthy controls. However, we found some degree of SAD (increase in R5–R20 and AX together with more negative of X5) at the month 6 in post COVID-19 pneumonia. These results were supported by the previous study indicating that SAD was more frequent (30.3%) in post COVID-19 pneumonia at two months after infection, and among symptomatic patients at the 12-month assessment (19). R5 and R20 in post COVID-19 pneumonia increased overtime from month 1 but not statistically significant and remained within the normal range (<120 %predicted). While X5 at 12 month seems to be comparable to at 1 month in the post COVID-19 pneumonia group which was supported by Veneroni et al. study. They found the more negative of X5 in a case study of severe COVID-19 pneumonia at the first two month post disease onset and was resolved at 6 month to one year later (21). However, this result was studied in only one case (21). The increases in airway resistance and reactance in post COVID-19 pneumonia may cause by interstitial abnormalities. A previous study showed the association between SAD and persistent tomographic abnormalities in post COVID-19 pneumonia caused by interstitial thickening (1). However, further studies on SAD in post COVID-19 when confirmed with computerized tomography (CT) are needed to better characterize the pathophysiological mechanisms and therapeutic implications. Considering the decrease in lung function measured by spirometry and increase in airway resistance and reactance measured by IOS, a restrictive type of lung disease may be developed due to COVID-19 pneumonia. Previous study suggested that pneumonia is almost completely cleared from the lung tissue within 5–6 months, and fibrous tissue is formed in this process (34).
Our study confirms that there were no significant differences in FeNO between post COVID-19 pneumonia and healthy control throughout the study period which were supported by the previous findings showing that FeNO values did not differ between post COVID-19 pneumonia and healthy subjects at one to three month from hospital discharge (11,12). Lindahl et al. suggested that eosinophilic airway inflammation might not also be the cause of airway inflammation in post COVID-19 infection (35). Although, the FeNO was lower at month 6 when compared to month 1 in the post COVID-19 pneumonia group and the FeNO values were significantly higher at months 6, 9, and 12 when compared to month 1 in the healthy controls group, all of the FeNO values in our study remained within normal ranges (<25 ppb) (36).
Improvement of exercise capacity was observed in the post COVID-19 pneumonia group but seem to be no improvement after six months. Our results were comparable to the previous studies showing that the 6-MWD increased continuously during the follow-up time (5,8,9,10,37,38). However, some studies showed no improvement between 6–9 and 12–18.5 months post-recovery (1,8). For example, Zhang et al. found that 6-MWD increased continuously (500 meters, 505 meters, and 525 meters, for 6 months, 1 year, and 2 years after COVID-19 infection, respectively) (9). We also found that the 6-MWD was significantly lower in post COVID-19 pneumonia group compared to healthy controls throughout the study period. These results are in line with the previous studies indicating that the 6-MWD in post COVID-19 pneumonia was lower when compared to healthy subjects at month 6 of follow-up time (6,38). Additionally, other previous studies also showed that the prevalence of low exercise capacity defined by 6-MWD lower than the predicted value ranged from 17.33–33.33% for a follow-up time of 2–6 months (4,7,16,23,24). The low exercise capacity could be attributed by muscle wasting and myopathy. Previous finding showed that the systemic corticosteroid therapy and hospitalization could lead to muscle wasting and physical deconditioning (39). Thus, pulmonary rehabilitation including aerobic exercise or promotion of active physical activity should be provided in these populations.
The strength of our study is a shorter interval time of follow-up time for the assessment of the sequelae of post COVID-19 pneumonia on lung function and exercise capacity. Secondly, healthy controls were enrolled for comparison with post COVID-19 pneumonia. However, this study has some limitations. Firstly, our study is a single-center study. The results may not be generalized to the other settings. Secondly, the post COVID-19 pneumonia was not separated into non-severe and severe groups for comparison with healthy controls. However, the previous studies confirmed that there was more impact on lung function and exercise capacity in severe groups when compared to the non-severe COVID-19 pneumonia or healthy subjects (6,11,12,14,38). Thirdly, the chest CT and the DLCO were not measured for confirming the association between IOS measurement and exercise capacity. Fourthly, the sample size calculation was calculated from the findings of 6-MWD in our previous publication. Therfore, the results of lung function especially for IOS may be under power due to small sample size. More sample size for IOS study in post COVID-19 pneumonia are needed. Fifthly, IOS is less utilized in clinical practice and less available in lung function testing labs. The it may not be generalized to the other settings.
Conclusions
The long term sequelae of post COVID-19 pneumonia on lung function including spirometry and impulse oscillometry and exercise capacity were observed in our study. The pulmonary function tests and six-minute walk test are useful tools for detection of long term sequelae of post COVID-19 pneumonia.
Acknowledgments
The authors would like to thank all post-COVID-19 pneumonia and healthy subjects who kindly participated in this study. The authors acknowledge the nurses of the Division of Pulmonary, Critical Care and Allergy, Department of Internal Medicine, Faculty of Medicine, Chiang Mai University for their contribution to this trial. The authors would like to thank Ruth Leatherman, Research Administration Section, Faculty of Medicine, Chiang Mai University for the native English proofreading.
Funding: This study was funded by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-514/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-514/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-514/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-514/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Research Ethics Committee, Faculty of Medicine, Chiang Mai University (study code: MED-2564-08109, date of approval: 3 May 2021), filed under Clinical Trials Registry (Study ID: TCTR20210827005, date of approval: 27 August 2021), and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Huang L, Yao Q, Gu X, et al. 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Lancet 2021;398:747-58. [Crossref] [PubMed]
- Tang YF, Han JY, Ren AM, et al. Assessment of Long-Term Effects on Pulmonary Functions Between Severe and Non-Severe Convalescent COVID-19 Patients: A Single-Center Study in China. J Inflamm Res 2022;15:4751-61. [Crossref] [PubMed]
- Lerum TV, Aaløkken TM, Brønstad E, et al. Dyspnoea, lung function and CT findings 3 months after hospital admission for COVID-19. Eur Respir J 2021;57:2003448. [Crossref] [PubMed]
- Strumiliene E, Zeleckiene I, Bliudzius R, et al. Follow-Up Analysis of Pulmonary Function, Exercise Capacity, Radiological Changes, and Quality of Life Two Months after Recovery from SARS-CoV-2 Pneumonia. Medicina (Kaunas) 2021;57:568. [Crossref] [PubMed]
- Huang L, Li X, Gu X, et al. Health outcomes in people 2 years after surviving hospitalisation with COVID-19: a longitudinal cohort study. Lancet Respir Med 2022;10:863-76. [Crossref] [PubMed]
- Sirayder U, Inal-Ince D, Kepenek-Varol B, et al. Long-Term Characteristics of Severe COVID-19: Respiratory Function, Functional Capacity, and Quality of Life. Int J Environ Res Public Health 2022;19:6304. [Crossref] [PubMed]
- Ma Y, Deng J, Liu Q, et al. Long-Term Consequences of COVID-19 at 6 Months and Above: A Systematic Review and Meta-Analysis. Int J Environ Res Public Health 2022;19:6865. [Crossref] [PubMed]
- Guo Y, Wang H, Xiao M, et al. Long-term outcomes of COVID-19 convalescents: An 18.5-month longitudinal study in Wuhan. Int J Infect Dis 2023;127:85-92. [Crossref] [PubMed]
- Zhang H, Li X, Huang L, et al. Lung-function trajectories in COVID-19 survivors after discharge: A two-year longitudinal cohort study. EClinicalMedicine 2022;54:101668. [Crossref] [PubMed]
- Eberst G, Claudé F, Laurent L, et al. Result of one-year, prospective follow-up of intensive care unit survivors after SARS-CoV-2 pneumonia. Ann Intensive Care 2022;12:23. [Crossref] [PubMed]
- Salem AM, Al Khathlan N, Alharbi AF, et al. The Long-Term Impact of COVID-19 Pneumonia on the Pulmonary Function of Survivors. Int J Gen Med 2021;14:3271-80. [Crossref] [PubMed]
- Niyatiwatchanchai N, Deesomchok A, Chaiwong W, et al. Comparative Study of Early Impacts of Post-COVID-19 Pneumonia on Clinical Manifestations, Pulmonary Function, and Chest Radiographs. Medicina (Kaunas) 2022;58:216. [Crossref] [PubMed]
- Orzes N, Pini L, Levi G, et al. A prospective evaluation of lung function at three and six months in patients with previous SARS-COV-2 pneumonia. Respir Med 2021;186:106541. [Crossref] [PubMed]
- Pini L, Montori R, Giordani J, et al. Assessment of respiratory function and exercise tolerance at 4-6 months after COVID-19 infection in patients with pneumonia of different severity. Intern Med J 2023;53:202-8. [Crossref] [PubMed]
- Fortini A, Torrigiani A, Sbaragli S, et al. COVID-19: persistence of symptoms and lung alterations after 3-6 months from hospital discharge. Infection 2021;49:1007-15. [Crossref] [PubMed]
- Bardakci MI, Ozturk EN, Ozkarafakili MA, et al. Evaluation of long-term radiological findings, pulmonary functions, and health-related quality of life in survivors of severe COVID-19. J Med Virol 2021;93:5574-81. [Crossref] [PubMed]
- Vejen M, Hansen EF, Al-Jarah BNI, et al. Hospital admission for COVID-19 pneumonitis - long-term impairment in quality of life and lung function. Eur Clin Respir J 2022;9:2024735. [Crossref] [PubMed]
- Ribeiro Carvalho CR, Lamas CA, Chate RC, et al. Long-term respiratory follow-up of ICU hospitalized COVID-19 patients: Prospective cohort study. PLoS One 2023;18:e0280567. [Crossref] [PubMed]
- Visconti NRGDR, Cailleaux-Cezar M, Capone D, et al. Long-term respiratory outcomes after COVID-19: a Brazilian cohort study. Rev Panam Salud Publica 2022;46:e187. [Crossref] [PubMed]
- Scaramuzzo G, Ronzoni L, Campo G, et al. Long-term dyspnea, regional ventilation distribution and peripheral lung function in COVID-19 survivors: a 1 year follow up study. BMC Pulm Med 2022;22:408. [Crossref] [PubMed]
- Veneroni C, Perissin R, Di Marco F, et al. Home monitoring of lung mechanics by oscillometry before, during and after severe COVID-19 disease: a case study. ERJ Open Res 2023;9:00480-2022. [Crossref] [PubMed]
- Hua-Huy T, Günther S, Lorut C, et al. Distal Lung Inflammation Assessed by Alveolar Concentration of Nitric Oxide Is an Individualised Biomarker of Severe COVID-19 Pneumonia. J Pers Med 2022;12:1631. [Crossref] [PubMed]
- Schandl A, Hedman A, Lyngå P, et al. Long-term consequences in critically ill COVID-19 patients: A prospective cohort study. Acta Anaesthesiol Scand 2021;65:1285-92. [Crossref] [PubMed]
- Kattainen S, Lindahl A, Vasankari T, et al. Lung function and exercise capacity 6 months after hospital discharge for critical COVID-19. BMC Pulm Med 2022;22:243. [Crossref] [PubMed]
- King GG, Bates J, Berger KI, et al. Technical standards for respiratory oscillometry. Eur Respir J 2020;55:1900753. [Crossref] [PubMed]
- Deesomchok A, Chaiwong W, Liwsrisakun C, et al. Reference equations of the impulse oscillatory in healthy Thai adults. J Thorac Dis 2022;14:1384-92. [Crossref] [PubMed]
- Stanojevic S, Kaminsky DA, Miller MR, et al. ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur Respir J 2022;60:2101499. [Crossref] [PubMed]
- Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J 2012;40:1324-43. [Crossref] [PubMed]
- American Thoracic Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med 2005;171:912-30. [Crossref] [PubMed]
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111-117. Correction in Am J Respir Crit Care Med 2016;193:1185. [Crossref] [PubMed]
- Lenoir A, Christe A, Ebner L, et al. Pulmonary Recovery 12 Months after Non-Severe and Severe COVID-19: The Prospective Swiss COVID-19 Lung Study. Respiration 2023;102:120-33. [Crossref] [PubMed]
- Corsi A, Caroli A, Bonaffini PA, et al. Structural and Functional Pulmonary Assessment in Severe COVID-19 Survivors at 12 Months after Discharge. Tomography 2022;8:2588-603. [Crossref] [PubMed]
- Liwsrisakun C, Chaiwong W, Bumroongkit C, et al. Influence of Particulate Matter on Asthma Control in Adult Asthma. Atmosphere 2023;14:410. [Crossref]
- Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet 2021;397:220-32. [Crossref] [PubMed]
- Lindahl A, Reijula J, Malmberg LP, et al. Small airway function in Finnish COVID-19 survivors. Respir Res 2021;22:237. [Crossref] [PubMed]
- Bjermer L, Alving K, Diamant Z, et al. Current evidence and future research needs for FeNO measurement in respiratory diseases. Respir Med 2014;108:830-41. [Crossref] [PubMed]
- Bongiovanni M, Barilaro G, Bini F. Twelve-month clinical, functional, and radiological outcomes in patients hospitalized for SARS-CoV-2 pneumonia. J Med Virol 2023;95:e28524. [Crossref] [PubMed]
- Magdy DM, Metwally A, Tawab DA, et al. Long-term COVID-19 effects on pulmonary function, exercise capacity, and health status. Ann Thorac Med 2022;17:28-36. [Crossref] [PubMed]
- Ong KC, Ng AW, Lee LS, et al. Pulmonary function and exercise capacity in survivors of severe acute respiratory syndrome. Eur Respir J 2004;24:436-42. [Crossref] [PubMed]


