
Simultaneous surgical treatment of aortic coarctation and aortic root disease: a report of case series
Highlight box
Key findings
• This study found simultaneous surgical treatment with ascending-to-abdominal aorta bypass grafting and aortic root replacement is a feasible surgical approach for patients with aortic coarctation (CoA) and aortic root disease.
What is known and what is new?
• CoA is often accompanied by aortic root disease. The staged surgery is more painful and economically costly for these patients.
• This simultaneous surgical treatment with ascending-to-abdominal aorta bypass grafting and aortic root replacement solved the patien’s lesions in one go, avoiding two separate operations for aortic root correction and transcatheter aortic valve replacement.
What is the implication, and what should change now?
• This simultaneous surgical treatment with ascending-to-abdominal aorta bypass grafting and aortic root replacement should be more considered for patients with CoA and aortic root disease.
Introduction
Aortic coarctation (CoA) is a common congenital aortic disease, accounting for 5–8% of neonates with congenital heart diseases (1,2). The incidence of CoA has been reported to be 1/2,500 in births (1,3). CoA is currently classified into three types based on the positional relationship between the CoA and ductus arteriosus: preductal, periductal, and postductal. The common type of CoA in adults is the postductal type (4,5). Many patients with CoA are complicated by bicuspid aortic valves (BAV), and some develop aortic root aneurysms. What is the appropriate surgical treatment for patients with CoA and aortic root aneurysms? Herein, we describe our experience with these cases. Our study aimed to explore the feasibility and prognosis of patients with CoA and aortic root disease undergoing simultaneous surgical treatment with aortic root replacement and ascending-abdominal aortic bypass grafting. We present this article in accordance with the AME Case Series reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-384/rc).
Case presentation
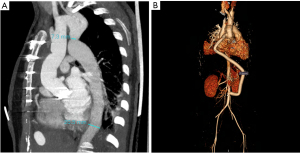
From June 2014 to December 2019, nine adult patients (seven male and two females) with an average age of 32±10 years were diagnosed with CoA with aortic root aneurysm and underwent surgical treatment at the Department of Cardiovascular Surgery, Beijing Anzhen Hospital, Capital Medical University. Patients’ eligibility was assessed based on the following inclusion criteria: (I) post-ductal CoA with pressure gradient >50 mmHg or mean differential pressure >20 mmHg as detected by echocardiography and aortic computed tomographic angiography (CTA); (II) echocardiography and aortic CTA examination showing moderate or more stenosis or regurgitation of the aortic valve, and the diameter of the ascending aorta or aortic sinus >45 mm; and (III) patient and family consent for simultaneous surgical treatment. The exclusion criteria were as follows: (I) patients who have undergone previous surgery for aortic root or coarctation of the aorta; (II) inflammatory arterial disease; (III) infective endocarditis or infectious aortic disease; (IV) surgical contraindications. All patients underwent preoperative examinations, including laboratory examinations, cardiac ultrasonography, and aortic CTA (Figure 1), and blood pressure was measured in four limbs. Among the nine patients, two had severe aortic valve stenosis, seven had severe aortic valve insufficiency, two had moderate mitral insufficiency, one had patent ductus arteriosus (PDA), and one had descending aortic dissection (Table 1, Table 2). All procedures performed in this study were in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this case series and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
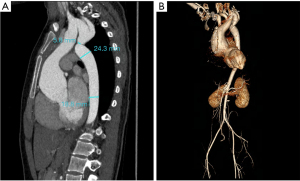
Table 1
| Patient number | Age | Sex | Height (cm) | Weight (kg) | Past history | The diameter of CoA (mm) | Aortic root diameter (mm) | Aortic valve | Other situations |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 40 | M | 170 | 68 | Hypertension; dizzy | 3.2 | 51 | AI | – |
| 2 | 39 | M | 168 | 61 | Hypertension | 4.0 | 56 | AS | – |
| 3 | 25 | M | 174 | 70 | Hypertension | 7.0 | 45 | AS | – |
| 4 | 50 | M | 171 | 73 | Hypertension; dizzy | 2.0 | 51 | AI | AD |
| 5 | 32 | M | 177 | 72 | Hypertension | 5.3 | 49 | BAV, AI | – |
| 6 | 31 | M | 171 | 70 | Hypertension; cerebral hemorrhage | 4.6 | 53 | AI | – |
| 7 | 19 | M | 165 | 65 | – | 6.6 | 47 | AI, AS | MI |
| 8 | 33 | F | 161 | 58 | Chest tightness | 11.0 | 53 | AI | MI |
| 9 | 17 | F | 156 | 52 | Lower limb weakness | 5.3 | 57 | BAV, AI | PDA |
CoA, aortic coarctation; M, male; F, female; AI, aortic insufficiency; AS, aortic stenosis; AD, aortic dissection; BAV, bicuspid aortic valve; MI, mitral insufficiency; PDA, patent ductus arteriosus.
Table 2
| Patient number | ALT (U/L) | AST (U/L) | Cr (µmol/L) | UREA (mmol/L) | CK (U/L) | Hb (g/L) | PLT (G/L) |
|---|---|---|---|---|---|---|---|
| 1 | 30 | 22 | 89.0 | 9.3 | 56 | 167 | 184 |
| 2 | 19 | 15 | 62.1 | 3.3 | 50 | 135 | 178 |
| 3 | 13 | 22 | 71.0 | 6.5 | 67 | 143 | 88 |
| 4 | 19 | 14 | 84.9 | 6.1 | 35 | 122 | 259 |
| 5 | 11 | 18 | 77.2 | 5.6 | 57 | 143 | 173 |
| 6 | 20 | 19 | 93.0 | 9.2 | 106 | 148 | 130 |
| 7 | 26 | 24 | 61.5 | 4.3 | 107 | 142 | 226 |
| 8 | 11 | 20 | 59.2 | 6.0 | 60 | 132 | 186 |
| 9 | 16 | 16 | 50.9 | 4.1 | 68 | 122 | 153 |
ALT, alanine transaminase; AST, aspartate amino transferase; Cr, serum creatinine; UREA, urea nitrogen; CK, creatine kinase; Hb, hemoglobin; PLT, platelet.
Definition of endpoints and follow-up
The primary endpoint was one-year survival after surgery. The secondary endpoints included surgical time, cardiopulmonary bypass time, aortic cross-clamp time, duration of ventilation support, postoperative complications (including respiratory failure, abdominal organ-related complications, and paraplegia), and changes in differential pressure before and after surgery. All patients were followed for at least 12 months after surgery. Follow-up information was collected through telephone interviews, electronic medical records or outpatient visits.
Statistical analysis
Statistical analyses were performed using SPSS 16.0. The Kolmogorov-Smirov testing method was used to assess whether continuous data followed a normal distribution. Continuous data with a normal distribution were presented as mean ± standard deviation, while categorical data were presented as counts and percentages. Differential pressure changes were analyzed using paired t-tests. Statistical significance was set at P<0.05.
Operative technique
Under general anesthesia, the patient was placed in the supine position. A midline incision was made in the sternum to expose the heart. The abdominal cavities were opened along the midline to expose the abdominal aorta below the renal artery. The artificial vessels were selected based on the diameter of the abdominal aorta. The abdominal aorta was blocked with sidewall forceps, and the artificial vessel was sutured to the abdominal aorta (end-to-side anastomosis). Cardiopulmonary bypass was established through the distal ascending aorta, and artificial vascular catheterization was used to perform aortic root replacement. The pathological aortic root was then replaced with a valved (mechanical valve) artificial vessel (St. Jude Medical, St. Paul, MN, USA). After the heart regained normal beating, heart-beating and cardiopulmonary bypass were performed simultaneously. The course of the artificial vessel (Dacron prostheses, InterGard, Maquet Carvatical, La Ciotat, France or Vascutek, Inchinnan, UK) transversed the mesocolon, and the root segment of the omentum passed anteriorly the liver and was then scheduled to head upward through a hole cut in the diaphragm muscle to reach the ascending aorta. The ascending aortic artificial vessel was blocked using sidewall forceps, and the bypass artificial vessel was sutured to the ascending aortic artificial vessel (end-to-side anastomosis). The aortic root was covered with an aneurysm wall and pericardium, which served as a distributary of the right atrium. Then cardiopulmonary bypass was stopped and withdrawn. Finally, the incision was sutured. All patients were required to take warfarin throughout their lives. All patients were followed for at least one year.
Caution should be exercised regarding the following details: (I) the length of the artificial blood vessel should be appropriate to avoid compression of abdominal organs or bending. (II) Stanching bleeding should be carefully performed to avoid blood stimulation of the pancreas and other organs.
Patient characteristics and outcomes
The clinical characteristics and outcomes of all patients are summarized in Table 3.
Table 3
| Patient number | Cardiopulmonary bypass time (min) | Occlusion time (min) | Operation time (h) | Blood loss (mL) | Ventilator supporting time (h) |
|---|---|---|---|---|---|
| 1 | 128 | 87 | 6 | 1,400 | 14 |
| 2 | 109 | 78 | 4 | 2,650 | 13 |
| 3 | 162 | 107 | 11 | 3,910 | 46 |
| 4 | 115 | 83 | 5 | 2,030 | 12 |
| 5 | 146 | 101 | 6 | 2,360 | 16.5 |
| 6 | 137 | 65 | 7 | 1,270 | 37 |
| 7 | 105 | 72 | 6 | 1,400 | 15 |
| 8 | 142 | 103 | 5 | 1,500 | 15 |
| 9 | 129 | 72 | 6 | 1,100 | 14 |
The mean cardiopulmonary bypass time was 130±17 min. The mean ascending aortic occlusion time was 85±14 min. The mean operation time was 6.2±1.9 hours. The mean blood loss during and after surgery was 1,958±849 mL. The mean ventilator supporting time after operation was 20.3±11.6 hours. Operative mortality was not observed. None of patients experienced complications, such as cerebral infarction, cerebral hemorrhage, renal failure, respiratory failure, paraplegia, or wound infection after surgery. The arterial pressure gradient in the upper and lower limbs was significantly improved (P=0.003 for maximum differential pressure changes and P=0.001 for average differential pressure changes) (Table 4).
Table 4
| Patient number | Preoperative (mmHg) | Postoperative (mmHg) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Upper | Lower | Max | Average | Upper | Lower | Max | Average | ||
| 1 | 187/70 | 89/45 | 98 | 50 | 135/60 | 106/55 | 29 | 13 | |
| 2 | 160/86 | 97/63 | 63 | 36 | 125/45 | 110/40 | 14 | 7 | |
| 3 | 170/95 | 110/78 | 60 | 32 | 135/55 | 105/49 | 30 | 14 | |
| 4 | 172/95 | 122/73 | 50 | 32 | 115/60 | 75/50 | 40 | 20 | |
| 5 | 160/85 | 90/55 | 70 | 44 | 115/78 | 95/67 | 20 | 14 | |
| 6 | 190/110 | 95/65 | 95 | 61 | 135/65 | 110/70 | 25 | 5 | |
| 7 | 108/53 | 69/42 | 39 | 20 | 110/50 | 80/55 | 30 | 7 | |
| 8 | 107/44 | 55/35 | 52 | 24 | 124/78 | 108/71 | 16 | 10 | |
| 9 | 119/53 | 77/35 | 42 | 26 | 137/82 | 98/76 | 39 | 17 | |
| Mean ± SD | 63±20 | 36±13 | 27±9 | 12±5 | |||||
| P* | – | – | 0.003 | 0.001 | |||||
P*, preoperative blood pressure as a reference. Upper, upper limb; Lower, lower limb; Max, maximum differential pressure; Average, average pressure difference; SD, standard deviation.
Recent computer-enhanced transvenous angiograms showed that the grafts were open with fluent flow (Figure 2). A diagram of the aorta pre- and post-operation is shown in Figure 3. The patients were followed up for 24±7 months. During the follow-up period, only one patient had elevated serum amylase levels without abdominal pain, which returned to normal one week later. No other gastrointestinal complications or adverse events were noted.
Discussion
CoA is a common congenital cardiovascular malformation, with increased blood pressure in the proximal coarctation region being the main cause of hemodynamic changes. Such changes cause an increase in left ventricular (LV) afterload, which induces LV hypertrophy and strain, leading to congestive heart failure (CHF). Persistent hypertension in cerebral blood vessels may result in arteriosclerosis and cerebral hemorrhage. Reduced blood flow and increased flow rate in distal coarctation induce dilatation of the descending aorta and poor perfusion of distal organs (6,7). Simple CoA can be surgically treated using anatomical and non-anatomical correction. The anatomical correction includes coarctation resection with end-to-end anastomosis, artificial blood vessel transplantation, and artificial patch aortoplasty. The non-anatomical correction mainly includes extra-anatomical artificial blood vessel bypass grafting (8,9).
Complex CoA combined with intracardiac malformations has a low incidence (less than 1%) (10). In complex CoA, common intracardiac malformations primarily include BAV with dilatation of the ascending aorta, atrial septal defect (ASD), ventricular septal defect (VSD), and PDA (11). Additionally, many patients did not receive surgical treatment during childhood due to late detection. The risk of heart diseases, such as coronary heart disease (CHD) and heart valve degeneration, also increases significantly with age. Among the aforementioned malformations, CoA with aortic root lesions is relatively rare, and most cases present with aortic dilatation caused by BAV.
However, the optimal regimen for the treatment of complex CoA complicated with aortic root lesions remains unclear. Currently, staged anatomical correction and simultaneous extra-anatomical anatomical correction are two available therapeutic regimens.
Conventional anatomical correction requires staged surgery, including median thoracotomy to complete aortic root replacement at stage I and lateral thoracotomy (left posterolateral incision) to complete anatomical correction, such as thoracic aorta replacement, at stage II. During conventional anatomical correction, the aorta is widely dissociated, and the dilated collateral artery is freely sutured. Consequently, the aorta is prone to hemorrhage, laryngeal nerve and lung tissue injury, spinal cord injury, and even paraplegia in severe cases (12). After aortic root replacement at stage I, the LV afterload caused by the CoA does not decrease, which may induce perioperative CHF. Additionally, the administration of anticoagulants after replacement may also significantly increase the risk of intraoperative and postoperative hemorrhage at stage II. It is worth noting that staged surgery is more painful for patients and incurs higher economic costs.
Simultaneous extra-anatomical correction requires a median thoracoabdominal incision. Through such incisions, aortic root replacement and extra-anatomical artificial blood vessel bypass grafting can be performed to mitigate hypertension in the upper body and improve blood supply to the lower body. Extra-anatomical correction was reported by Rosseĭkin et al. (13). This involved performing ascending-to-descending bypass grafting of the aorta, along with prosthetic repair of the ascending portion of the aorta and/or correction of cardiac pathology. These procedures were carried out through the median sternotomy approach. According to Wang et al. (14), extra-anatomic aortic bypass can be conducted through either median sternotomy or median sternotomy-laparotomy. This approach has been found to have a low rate of morbidity and mortality in patients with complex coarctation and concurrent cardiovascular disorders. Gelpi et al. (15) reported a single-stage procedure for the patient with an ascending aorta aneurysm and a BAV with moderate insufficiency. The procedure involved replacing the ascending aorta, repairing the aortic valve, and bypassing the coarctation using an extra-anatomic graft. Furthermore, international research has shown that the distal end of artificial blood vessels is anastomosed to the abdominal aorta below the diaphragm and above the celiac trunk. Such bypass procedures are performed with the assistance of cardiopulmonary bypass. Despite being applicable for the simultaneous surgical treatment of patients with intracardiac and ascending aortic vascular diseases, the duration of cardiopulmonary bypass and myocardial blood supply occlusion is prolonged. Simultaneously, it is difficult to expose the anastomosis between the distal descending aorta and the abdominal aorta artificial blood vessels above the celiac trunk through the median incision after cardiac recovery. These conditions have a higher incidence among obese patients, making it difficult to prevent hemorrhage, and secondary thoracotomy can easily occur after surgery (16,17).
In the present study, ascending-to-infrarenal abdominal aorta artificial blood vessel bypass was performed simultaneously. Cardiopulmonary bypass was established by intubating the proximal aortic arch at the distal end of the ascending aorta and artificial blood vessels in the abdominal aorta to ensure effective perfusion of all organs before and after coarctation. There were no deaths or serious complications among the nine patients during the follow-up. This surgical approach has several advantages. First, aortic root replacement can be completed simultaneously under one anesthesia, and the abdominal aorta under the kidneys can be feasibly exposed, avoiding cutting off the pectoral and dorsal muscles and intercostal nerves, which has little effect on pulmonary function. This simultaneous surgical treatment solved the patient’s lesions in one go, avoiding two separate operations for aortic root correction and transcatheter aortic valve replacement (TAVR). Second, the coarctation part does not need to be dissociated, which prevents local tissue damage. Third, anastomosis does not need to be performed on the affected aortic wall near the coarctation segment, reducing the risk of restenosis and pseudoaneurysm formation. Fourth, aortic lateral wall forceps can be used to complete the anastomosis, and there is no ischemia time in the intercostal artery and organs, which reduces the corresponding complications caused by ischemia in the spinal cord and abdominal organs. Furthermore, the duration of cardiopulmonary bypass and myocardial blood supply occlusions did not increase. Moreover, the proximal end of the bypass blood vessel was anastomosed with the artificial blood vessel of the ascending aorta and wrapped in the bypass lumen of the ascending aorta, reducing the risk of hemorrhage. This surgical method can significantly reduce the difference in blood pressure between the upper and lower limbs. However, improper operation may also cause serious complications, such as abdominal organ injuries and mediastinal and abdominal infections (14,18,19). The described surgical method mainly includes the following limitations: (I) CoA patients complicated with severe dilation of the descending aorta may still suffer from rupture of the descending aorta after surgery (20). (II) Patients who have previously undergone laparotomy or have a definitive diagnosis of abdominal infections and abdominal adhesions may develop artificial blood vessel infections and abdominal organ injuries. (III) Excessively short or thin artificial blood vessels, mismatched conditions in the long-term, or even pseudoaneurysms may occur in patients in the stage of rapid growth and development before puberty (21). Such patients may undergo the surgical treatment described above after a detailed assessment. (IV) Because of the low incidence of CoA and aortic root diseases, fewer cases were included in the study, and this was a single-center study, which needs to be confirmed by more multicenter studies.
Conclusions
In conclusion, aortic root replacement combined with extra-anatomical artificial blood vessel bypass is a safe and feasible therapeutic regimen for patients with complex CoA complicated by aortic root lesions.
Acknowledgments
We thank Dr. Sheng Wu (ICON Biotech Solutions) for her help with the language modification in this paper.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the AME Case Series reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-384/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-384/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-384/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this case series and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cangussú LR, Lopes MR, Barbosa RHA. The importance of the early diagnosis of aorta coarctation. Rev Assoc Med Bras (1992) 2019;65:240-5. [Crossref] [PubMed]
- Lee MG, d'Udekem Y. Coarctation of the aorta can no longer be considered a benign condition. Heart Lung Circ 2014;23:297-8. [Crossref] [PubMed]
- Kenny D, Hijazi ZM. Coarctation of the aorta: from fetal life to adulthood. Cardiol J 2011;18:487-95. [Crossref] [PubMed]
- Yokoyama U, Ichikawa Y, Minamisawa S, et al. Pathology and molecular mechanisms of coarctation of the aorta and its association with the ductus arteriosus. J Physiol Sci 2017;67:259-70. [Crossref] [PubMed]
- Doshi AR, Chikkabyrappa S. Coarctation of Aorta in Children. Cureus 2018;10:e3690. [Crossref] [PubMed]
- Kim YY, Andrade L, Cook SC. Aortic Coarctation. Cardiol Clin 2020;38:337-51. [Crossref] [PubMed]
- Hager A. Hypertension in aortic coarctation. Minerva Cardioangiol 2009;57:733-42.
- Sadeghi R, Tomka B, Khodaei S, et al. Impact of extra-anatomical bypass on coarctation fluid dynamics using patient-specific lumped parameter and Lattice Boltzmann modeling. Sci Rep 2022;12:9718. [Crossref] [PubMed]
- Cardoso G, Abecasis M, Anjos R, et al. Aortic coarctation repair in the adult. J Card Surg 2014;29:512-8. [Crossref] [PubMed]
- Kumar S, Raghuram A, Kumar S. Complex coarctation of aorta. Asian Cardiovasc Thorac Ann 2016;24:112. [Crossref] [PubMed]
- Hu X, Yuan Z. Adult-type Aortic Coarctation with Multiple Cardiovascular Anomalies. Radiology 2023;307:e221882. [Crossref] [PubMed]
- Ungerleider RM, Pasquali SK, Welke KF, et al. Contemporary patterns of surgery and outcomes for aortic coarctation: an analysis of the Society of Thoracic Surgeons Congenital Heart Surgery Database. J Thorac Cardiovasc Surg 2013;145:150-7; discussion 157-8. [Crossref] [PubMed]
- Rosseĭkin EV, Evdokimov ME, Bazylev VV, et al. Single-stage correction of aortic coarctation combined with an aneurysm of the ascending portion of the aorta and/or intracardiac pathology in adults. Angiol Sosud Khir 2013;19:101-6.
- Wang R, Sun LZ, Hu XP, et al. Treatment of complex coarctation and coarctation with cardiac lesions using extra-anatomic aortic bypass. J Vasc Surg 2010;51:1203-8. [Crossref] [PubMed]
- Gelpi G, Lemma M, Pettinari M, et al. One-stage repair of aortic coarctation and ascending aortic aneurysm by extra-anatomic graft. J Cardiovasc Med (Hagerstown) 2009;10:554-6. [Crossref] [PubMed]
- Reents W, Froehner S, Diegeler A, et al. Ascending-to-descending bypass for simultaneous surgery of aortic coarctation with other cardiac pathologies. Thorac Cardiovasc Surg 2012;60:210-4. [Crossref] [PubMed]
- Cabasa AS, Bower TC, Pochettino AB. Arch reconstruction after a previous ascending-to-descending aortic bypass for coarctation of the aorta. J Thorac Cardiovasc Surg 2016;151:1760-3. [Crossref] [PubMed]
- Brink J, Lee MG, Konstantinov IE, et al. Complications of extra-anatomic aortic bypass for complex coarctation and aortic arch hypoplasia. Ann Thorac Surg 2013;95:676-81. [Crossref] [PubMed]
- Bozzani A, Arici V, Rodolico G, et al. Endovascular Exclusion of Aortobronchial Fistula and Distal Anastomotic Aneurysm after Extra-Anatomic Bypass for Aortic Coarctation. Tex Heart Inst J 2017;44:55-7. [Crossref] [PubMed]
- Clark JB. Commentary: Aortic anatomy late after coarctation repair: Size matters. J Thorac Cardiovasc Surg 2021;162:193-4. [Crossref] [PubMed]
- Alnasser SA, Vunnamadala KC, Preventza OA, et al. Endovascular Repair of a Pseudoaneurysm After Multiple Open Repairs of Aortic Coarctation. Tex Heart Inst J 2020;47:149-51. [Crossref] [PubMed]



