
Is preoperative intracranial hemorrhage a surgical contraindication in infective endocarditis with stroke?
Introduction
In active infective endocarditis (IE), systemic emboli causing septic cerebral embolism, including hemorrhagic change, are not uncommon (1,2). Intracranial hemorrhage (ICH) is reported in about 5% of active IE cases (3). Septic erosion of a vessel wall or rupture of a mycotic aneurysm are known causes of ICH in IE (4). There are several reports favoring early surgery for IE, even in high-risk patients with a recent stroke (5-7).
However, the optimal timing for surgical treatment of IE in patients with severe stroke and neurologic deficits is unclear because of the risk of exacerbating the stroke or provoking intracranial hemorrhagic conversion after using cardiopulmonary bypass (CPB). The use of anticoagulants, such as heparin, increases the risk of any ICH, including hemorrhagic transformation of a previous cerebral infarction (8). The utilization of anticoagulants with artificial heart valves after open heart surgery may also increase the risk of cerebral hemorrhage.
The recent guidelines recommend delaying open heart surgery in IE until 4 weeks after the onset of an ICH (9,10). However, exceptions to these guidelines may arise in situations where emergency surgery is required and entails assuming all risks. We sought to determine the effects of open heart surgery using CPB and postoperative care on the risk of postoperative ICH.
The objective of this retrospective study was to compare the surgical outcomes between patients with ICH and without ICH accompanying recent septic cerebral embolism in IE. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-695/rc).
Methods
Study subjects
The records of 179 patients who underwent open heart surgery at Seoul St. Mary’s Hospital for active IE from February 2009 to December 2020 were retrospectively reviewed. All cases of active IE were diagnosed with the modified Duke criteria (11). We excluded 15 cases with operation to only the right side of the heart. Among 164 cases with left-sided IE, 74 patients were diagnosed with preoperative stroke. Finally, 71 cases with a period of <4 weeks from stroke onset to surgery were included for the study (Figure 1).
In all cases, indications for surgery included signs of heart failure, severe valve dysfunction, prosthetic IE, invasive paravalvular abscess or fistula, recurrent systemic embolization, large mobile vegetations, and persistent sepsis despite adequate antibiotic therapy. Factors related to complications, primarily neurologic involvement and other comorbidities, were also considered and weighed in the decision-making process. The timing of surgery was ultimately decided by the attending surgeon in each case after consultation with the cardiologist, infectious disease specialist, neurologist, and neurosurgeon and consideration of the clinical situation.
IE diagnosed after 2 days in hospital or associated with any invasive procedure performed within 6 months was defined as a nosocomial infection (12,13). Screening brain imaging including computed tomography (CT) or magnetic resonance imaging (MRI) was performed routinely except in salvage operations. Even in situations where emergency surgery is required due to worsening heart failure caused by infective endocarditis, brain imaging was performed preoperatively to assess any neurological complications. Preoperative stroke, including ICH, was diagnosed by imaging and confirmed by a radiologist and a neurologist. Preoperative ICH included intracerebral hemorrhage and subarachnoid hemorrhage, and intracerebral hemorrhage included hemorrhagic infarction and parenchymal hemorrhage. The onset of ICH was based on the occurrence of neurological symptoms. In neurologically asymptomatic patients, the time of the preoperative brain imaging was considered the time of onset of ICH.
Preoperative neurological symptoms were scored using a modified Rankin score (14) and the National Institutes of Health (NIH) stroke scale. The measurement of these two scores, used to evaluate neurological symptoms, was based on the time of stroke diagnosis in patients. Cardiac operative risk was evaluated according to the European System for Cardiac Operative Risk Evaluation (EuroSCORE) index (15) and the Validated Risk Score for Predicting 6-month Mortality in Infective Endocarditis (16).
The presence or absence of ICH in our study population did not affect the surgical method. All patients underwent median sternotomy, and received 300 units/kg of intravenous heparin for CPB. The decision for an artificial heart valve was mainly dependent on the patient’s age, comorbidity, and preference for a bioprosthetic or a mechanical valve. Neurological evaluation and the need for brain imaging were routinely discussed with all patients who had a history of stroke before undergoing surgery. Postoperative anticoagulation was started in consultation with the neurologist or the neurosurgeon. The criteria for postoperative cerebrovascular accidents (CVA) were defined as follows: based on the patient’s neurological clinical symptoms, a consultation with a neurologist or neurosurgeon was conducted, followed by a decision to perform brain imaging. Postoperative CVA was defined by radiological interpretation of brain imaging results indicating the newly occurrence of ischemic or hemorrhagic lesions. Postoperative ICH was defined as any intracerebral or subarachnoid hemorrhage occurring during the postoperative hospitalization period.
The primary outcome was defined as the occurrence of ICH during hospitalization after surgery and the secondary outcomes were defined as 30-day mortality after surgery and the one-year survival rate.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was reviewed and approved by the Institutional Review Board of Seoul St. Mary’s Hospital (IRB No. KC22RISI0866) and the requirement for informed consent was waived.
Statistical analyses
Continuous variables are presented as means with standard deviations and categorical variables are presented as counts with percentages. For comparisons between groups, continuous variables were analyzed using the independent-sample t-test or Mann-Whitney U test and categorical variables were analyzed using the chi-square or Fisher exact test. Kaplan-Meier survival curves were drawn, and logistic regression analysis was performed for the univariate analysis. All statistical analyses were performed by using R (R 4.2.1; The R Foundation for Statistical Computing, Vienna, Austria), and statistical significance was defined by P values <0.05.
Results
Records of all patients who underwent open heart surgery for active IE during the study period were examined, and 71 patients with recent (within the past 4 weeks) stroke event confirmed by preoperative brain imaging were divided into two groups according to the presence (n=22; group B) or absence (n=49; group A) of ICH. The characteristics of the included patients are described in Table 1. Among the 22 patients with preoperative ICH, 16 (72.7%) had intracerebral hemorrhage (including hemorrhagic infarction and parenchymal hemorrhage) and 12 (54.5%) had subarachnoid hemorrhage. The preoperative cerebral vascular examination revealed the presence of mycotic aneurysms, which were unruptured, in only two patients.
Table 1
| Variables | Group A (n=49) | Group B (n=22) | P value |
|---|---|---|---|
| Sex, female | 17 (34.7) | 5 (22.7) | 0.410 |
| Age, years | 56.90±15.23 | 53.91±18.79 | 0.480 |
| BMI, kg/m2 | 21.82±3.31 | 22.77±3.61 | 0.279 |
| BSA, m2 | 1.67±0.19 | 1.77±0.19 | 0.057 |
| Atrial fibrillation | 4 (8.2) | 4 (18.2) | 0.243 |
| Coronary artery disease | 11 (22.4) | 2 (9.1) | 0.319 |
| Chronic kidney disease | 7 (14.3) | 3 (13.6) | 1.000 |
| Dialysis | 2 (4.1) | 1 (4.5) | 1.000 |
| Diabetes | 12 (24.5) | 3 (13.6) | 0.363 |
| Hypertension | 18 (36.7) | 5 (22.7) | 0.285 |
| Malignancy | 7 (14.3) | 1 (4.5) | 0.420 |
| Peripheral arterial occlusive disease | 3 (6.1) | 0 (0.0) | 0.547 |
| NYHA class, III–IV | 20 (40.8) | 10 (45.5) | 0.797 |
| Ejection fraction, % | 60.09±6.31 | 58.24±6.83 | 0.269 |
| Staphylococcus aureus | 8 (16.3) | 6 (27.3) | 0.339 |
| Aortic valve involvement | 20 (40.8) | 13 (59.1) | 0.201 |
| Mitral valve involvement | 31 (63.3) | 14 (63.6) | 1.000 |
| Previous cardiac surgery | 8 (16.3) | 2 (9.1) | 0.714 |
| Prosthetic IE | 5 (10.2) | 2 (9.1) | 1.000 |
| Emergency operation | 21 (42.9) | 9 (40.9) | 1.000 |
| Stroke occurrence to surgery time, days | 4.20±5.83 | 6.82±6.31 | 0.093 |
| Nosocomial infection | 19 (38.8) | 7 (31.8) | 0.607 |
| Modified Rankin score | 1.08±1.55 | 1.91±1.66 | 0.046 |
| NIH stroke scale | 2.33±4.49 | 2.77±3.93 | 0.689 |
| EuroSCORE II, % | 12.85±14.91 | 10.27±11.31 | 0.472 |
| Predicted 6-month mortality, % | 28.82±14.87 | 28.18±12.78 | 0.860 |
Categorical variables are shown as numbers with percentages, and continuous variables are shown as mean ± standard deviation. BMI, body mass index; BSA, body surface area; NYHA, New York Heart Association; IE, infective endocarditis; NIH, National Institutes of Health; EuroSCORE, European System for Cardiac Operative Risk Evaluation.
The two groups were similar in terms of gender, age, and underlying conditions including atrial fibrillation; the proportion of female patients was lower in both groups (34.7% vs. 22.7%, P=0.410); and surgery was performed emergently (within one day of IE diagnosis) in more than 40% (42.9% vs. 40.9%, P=1.000) of the patients in both groups. The average time from stroke onset to surgery was 4.20 days in group A and 6.82 days in group B; this difference was not significant (P=0.093). The modified Rankin score was significantly higher in group B (1.08±1.55 vs. 1.91±1.66, P=0.046), but there was no significant difference in NIH scores and EuroSCORE II results (2.33±4.49 vs. 2.77±3.93, P=0.689 and 12.85%±14.91% vs. 10.27%±11.31%, P=0.472, respectively).
There were also no statistically significant differences between the two groups in terms of factors known to affect postoperative outcomes, including the incidence of Staphylococcus aureus as the causative agent of IE, the preoperative cardiac function, and the New York Heart Association (NYHA) class.
In operative data (Table 2), double valve replacement was more common among patients in group B, but not significantly (4.1% vs. 18.2%, P=0.070). In the overall surgical method, neither CPB (122.24±53.49 vs. 128.82±67.28 min, P=0.660) nor aortic cross clamp (92.73±40.86 vs. 95.73±40.33 min, P=0.775) time differed significantly between groups. There was no difference in the use of tissue valves between the two groups (30.6% vs. 50.0%, P=0.182).
Table 2
| Variables | Group A (n=49) | Group B (n=22) | P value |
|---|---|---|---|
| Aortic valve replacement | 20 (40.8) | 13 (59.1) | 0.201 |
| Mitral valve repair | 3 (6.1) | 1 (4.5) | 1.000 |
| Mitral valve replacement | 28 (57.1) | 13 (59.1) | 1.000 |
| Double valve replacement | 2 (4.1) | 4 (18.2) | 0.070 |
| Aortomitral curtain reconstruction | 2 (4.1) | 2 (9.1) | 0.583 |
| Tissue valve implantation | 15 (30.6) | 11 (50.0) | 0.182 |
| Aortic cross clamp time, minutes | 92.73±40.86 | 95.73±40.33 | 0.775 |
| Cardiopulmonary bypass time, minutes | 122.24±53.49 | 128.82±67.28 | 0.660 |
Categorical variables are shown as numbers with percentages, and continuous variables are shown as mean ± standard deviation.
The postoperative results are described in Table 3. We compared the rates of postoperative CVA, the proportion of ICH among them, and the number of patients with ICH requiring neurosurgical intervention. In addition, we summarized and compared the number of cases with acute kidney injury, postoperative bleeding requiring reoperation, pneumonia, and requirement for permanent pacemaker insertion, along with the lengths of stay in the intensive care unit (ICU) and the 30-day mortality rates. In group A, the average follow-up observation period was 1,421.61 days, while in group B, it was 1,440.05 days (P=0.956).
Table 3
| Variables | Group A (n=49) | Group B (n=22) | P value |
|---|---|---|---|
| Total CVA | 5 (10.2) | 4 (18.2) | 0.444 |
| Postoperative ICH | 5 (10.2) | 3 (13.6) | 0.696 |
| Surgery for ICH | 2 (4.1) | 0 (0.0) | 1.000 |
| Acute kidney injury | 9 (18.4) | 2 (9.1) | 0.483 |
| Postoperative bleeding | 4 (8.2) | 1 (4.5) | 1.000 |
| Pneumonia | 2 (4.1) | 0 (0.0) | 1.000 |
| Permanent pacemaker | 2 (4.1) | 1 (4.5) | 1.000 |
| ICU stay, days | 3.88±6.22 | 5.36±8.43 | 0.409 |
| 30 days mortality | 4 (8.2) | 1 (4.5) | 1.000 |
Categorical variables are shown as numbers with percentages, and continuous variables are shown as mean ± standard deviation. CVA, cerebrovascular accident; ICH, intracranial hemorrhage; ICU, intensive care unit.
Among the 49 patients in group A, 5 (10.2%) had postoperative stroke, all of which were ICH, with 2 requiring neurosurgical intervention. Among the 22 patients in group B, 4 (18.2%) had postoperative stroke, with 1 diagnosed as cerebral infarction and 3 (13.6%) diagnosed as further progression of the ICH. There was no statistically significant difference between groups in the proportion of patients who experienced postoperative ICH (10.2%, group A vs. 13.6%, group B, P=0.696). However, all 3 patients in group B who experienced postoperative ICH received conservative treatment without neurosurgical intervention. The overall incidence of postoperative CVA, including ischemic stroke, as well as the incidence of brain hemorrhage after cardiac surgery, which was the primary outcome measure, showed no statistically significant difference between the two groups (10.2% vs. 18.2%, P=0.444 and 10.2% vs. 13.6%, P=0.696, respectively). All of the cases of postoperative ICH were diagnosed within 10 days of the initial operation.
There was no statistically significant difference between the two groups in terms of other complications and length of stay in the ICU. In terms of 30-day mortality, there were 4 deaths (8.2%) in group A and 1 (4.5%) in group B (P=1.000). All patients who died within 30 days died during the hospitalization. The causes of death in 4 patients who died in group A were septic shock in 2 patients, heart failure in 1, and arrhythmia in 1. In group B, the cause of death of the only patient who died during the hospitalization was septic shock.
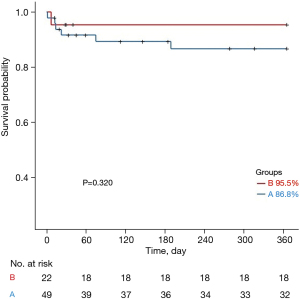
The Kaplan-Meier curves for one-year survival in groups A and B are shown in Figure 2. The one-year survival rate was 86.8% in group A and 95.5% in group B, and there was no statistically significant difference between the two groups (P=0.320).
Univariate analysis was performed and odds ratios were calculated for the total 71 patients included in the study to identify risk factors for postoperative ICH (Table 4), but no statistically significant factors were found.
Table 4
| Variables | Univariate analysis | |
|---|---|---|
| OR (95% CI) | P value | |
| Sex, female | 2.500 (0.564–11.100) | 0.228 |
| Age, years | 1.010 (0.966–1.060) | 0.624 |
| BMI, kg/m2 | 0.962 (0.766–1.210) | 0.736 |
| BSA, m2 | 0.190 (0.003–12.100) | 0.434 |
| Atrial fibrillation | 0.000 (0.000–infinity) | 0.994 |
| Coronary artery disease | 1.580 (0.2800–8.870) | 0.606 |
| Chronic kidney disease | 0.857 (0.094–7.820) | 0.891 |
| Dialysis | 4.360 (0.349–54.400) | 0.253 |
| Diabetes | 0.500 (0.057–4.410) | 0.533 |
| Hypertension | 1.290 (0.280–5.940) | 0.744 |
| Malignancy | 0.000 (0.000–infinity) | 0.994 |
| Peripheral arterial occlusive disease | 0.000 (0.000–infinity) | 0.995 |
| NYHA class, III–IV | 4.870 (0.909–26.100) | 0.064 |
| Ejection fraction, % | 1.030 (0.910–1.170) | 0.634 |
| Staphylococcus aureus | 2.840 (0.589–13.700) | 0.194 |
| Aortic valve involvement | 2.080 (0.458–9.480) | 0.342 |
| Mitral valve involvement | 0.537 (0.122–2.360) | 0.410 |
| Previous cardiac surgery | 0.857 (0.094–7.820) | 0.891 |
| Prosthetic IE | 0.000 (0.000–infinity) | 0.995 |
| Emergency operation | 2.530 (0.5550–11.600) | 0.230 |
| Stroke occurrence to surgery time, days | 0.987 (0.868–1.120) | 0.846 |
| Nosocomial infection | 1.040 (0.228–4.770) | 0.956 |
| Modified Rankin score | 1.350 (0.892–2.050) | 0.154 |
| NIH stroke scale | 1.090 (0.960–1.250) | 0.177 |
| EuroSCORE II, % | 1.000 (0.950–1.060) | 0.951 |
| Predicted 6-month mortality, % | 1.030 (0.978–1.080) | 0.290 |
| Aortic valve replacement | 2.080 (0.458–9.480) | 0.342 |
| Mitral valve repair | 2.860 (0.261–31.300) | 0.390 |
| Mitral valve replacement | 0.395 (0.087–1.800) | 0.230 |
| Double valve replacement | 0.000 (0.000–infinity) | 0.992 |
| Aortomitral curtain reconstruction | 0.000 (0.000–infinity) | 0.994 |
| Tissue valve implantation | 1.860 (0.424–8.180) | 0.410 |
| Aortic cross clamp time, minutes | 0.995 (0.975–1.020) | 0.659 |
| Cardiopulmonary bypass time, minutes | 0.996 (0.982–1.010) | 0.628 |
Categorical variables are shown as numbers with percentages, and continuous variables are shown as mean ± standard deviation. ICH, intracranial hemorrhage; OR, odds ratio; CI, confidence interval; BMI, body mass index; BSA, body surface area; NYHA, New York Heart Association; IE, infective endocarditis; NIH, National Institutes of Health; EuroSCORE, European System for Cardiac Operative Risk Evaluation.
We also performed a subgroup analyses to investigate the impact of a shorter time between preoperative stroke and open heart surgery on the postoperative outcomes, specifically comparing the effects of hemorrhagic stroke vs. ischemic stroke, and setting the time from stroke to surgery as within 2 weeks (n=66; groups C and D) and within 1 week (n=51; groups E and F), instead of within 4 weeks. The subgroups were analyzed using the same statistical methods described above. Among the 66 patients in the 2-weeks subgroup, there were 46 patients without preoperative ICH (group C) and 20 patients with preoperative ICH (group D). Among 51 patients in the 1-week subgroup, there were 39 patients without preoperative ICH (group E) and 12 patients with preoperative ICH (group F).
Preoperative characteristics, operative data, and postoperative outcomes in the subgroups are summarized in Tables S1-S3 and Figure S1 (2-week subgroups) and in Tables S4-S6 and Figure S2 (1-week subgroups). There were no significant differences in most clinical factors and no significant differences in one-year survival (Kaplan-Meier curve) between groups in either subgroup analysis.
Discussion
The safety of early surgery in patients with active IE with septic cerebral embolism has not yet been proven. Even very early surgery within 7 days after the onset of ICH is not recommended in patients with ICH (17). ICH in patients with IE is associated with a high mortality rate (approximately 50%), and is often a contraindication to surgery (18). Symptomatic ICH in IE is also known to have a negative impact on functional outcomes among the affected patients (19). Therefore, planning for open heart surgery in patients with active IE with a recent stroke requires compromises, taking all risks and benefits into consideration.
As can be seen from the data of our study, mycotic aneurysms were uncommon, and is known to be occurring in 2% of patients with IE (20). Secondary ICH is more likely in an underlying ischemic injury, and radiographic evidence of hemorrhage has been observed with 4-week serial brain imaging in approximately 43% of patients with acute ischemic stroke (21), while hemorrhagic infarction or hemorrhagic transformation of an infarct occurs in about 30% of patients with ischemic stroke (22).
In our study, none of the variables tested at univariate analysis, including age, causative pathogen, emergency surgery, time interval from stroke onset to surgery, neurological score, the use of a tissue valve, and CPB time, were associated with the worsening of postoperative ICH (all P>0.1).
Subgroup analysis was carried out based on the time from any stroke to surgery in order to thoroughly examine the preoperative characteristics, operative data, and postoperative outcomes. Patients with stroke up to two weeks and stroke up to one week preoperatively were analyzed using the same method, respectively, but no significant results were obtained.
The time intervals of one week and two weeks were chosen in the subgroup analyses because the unit of week is easily understandable. Additionally, the decision was influenced by the findings of Okita et al. (17), who indicated that surgery within one week after ICH is risky, and Yoshioka et al. (23), who suggested that there is no significant increase in risk if cardiac surgery is performed within two weeks after ICH.
One notable finding from the subgroups is that the two patients who experienced postoperative cerebral hemorrhage in group F accounted for approximately 66.7% of the three patients in group B. Additionally, the four patients who experienced postoperative cerebral hemorrhage in group E accounted for 80% of the five patients in group A. Thus, a large portion of patients with postoperative ICH had a history of stroke within one week prior to the surgery.
Although Okita et al. (17) have suggested that early surgery (within a week of stroke occurrence) in patients with ICH results in a more dangerous prognosis after surgery, this study yields a different result. Nevertheless, it is important to note that although there was no significant difference in the rates of postoperative ICH between groups in our study, a large proportion of patients with postoperative ICH had a history of stroke, including ischemic stroke, within one week before surgery. Therefore, in clinical practice, it is better to consider the possibility that ischemic stroke within one week, as well as hemorrhagic stroke, may be a potential risk factor for exacerbation postoperative ICH.
Although we described preoperative neurological symptoms using two score systems (NIH and modified Rankin score), hemorrhagic changes of preoperative septic cerebral emboli were sometimes asymptomatic. Symptomatic and asymptomatic hemorrhagic changes are reported in similar proportions (24) following administration of recombinant tissue plasminogen activator after ischemic stroke. Asymptomatic ICH not only occurs in more than 40% of patients with active IE and septic cerebral infarction, but has also been studied in the form of subarachnoid hemorrhage and subdural hemorrhage (23). Given that ICH can be asymptomatic, it is important to have brain imaging and an appropriate multidisciplinary discussion before surgery. In cases of IE accompanied by neurological complications, despite the extensive research conducted on the optimal timing for cardiac surgery, a conclusive answer is still lacking (25).
The limitations of this study include the small size of the cohort and the retrospective analysis. Postoperative asymptomatic ICH including hemorrhagic transformation or ischemic stroke could not be detected in all patients because brain imaging was performed mostly in patients with postoperative neurologic changes. We did not routinely perform brain imaging after cardiac surgery for research purposes. However, in collaboration with a multidisciplinary team, we performed brain imaging examinations after assessing the neurological condition of patients following the surgical procedure. The possibility that the prognosis could vary depending on the size or location of ICH or cerebral infarction was not considered. Since differences in results based only on the presence or absence of preoperative ICH were observed, further research on the degree or location of ICH may be necessary in the future. Also, regarding this topic, there is a need for future prospective research or multicenter studies to be conducted.
Conclusions
In patients with infectious endocarditis accompanied by a recent septic cerebral embolism, the presence or absence of preoperative ICH did not result in statistically significant differences in the occurrence or exacerbation of postoperative ICH or early mortality after cardiac surgery. This suggests that even if there is preoperative hemorrhagic transformation, there seems to be no need to delay surgery if the intervention is mandatory, taking substantial risks into consideration. Nevertheless, the patient should be carefully evaluated by neurologists or neurovascular specialists before open heart surgery.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-695/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-695/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-695/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-695/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Baddour LM, Wilson WR, Bayer AS, et al. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Scientific Statement for Healthcare Professionals From the American Heart Association. Circulation 2015;132:1435-86. [Crossref] [PubMed]
- Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015;36:3075-128. [Crossref] [PubMed]
- Pruitt AA, Rubin RH, Karchmer AW, et al. Neurologic complications of bacterial endocarditis. Medicine (Baltimore) 1978;57:329-43. [Crossref] [PubMed]
- Jones HR Jr, Siekert RG. Neurological manifestations of infective endocarditis. Review of clinical and therapeutic challenges. Brain 1989;112:1295-315. [Crossref] [PubMed]
- Liang F, Song B, Liu R, et al. Optimal timing for early surgery in infective endocarditis: a meta-analysis. Interact Cardiovasc Thorac Surg 2016;22:336-45. [Crossref] [PubMed]
- Kang DH, Lee S, Kim YJ, et al. Long-Term Results of Early Surgery versus Conventional Treatment for Infective Endocarditis Trial. Korean Circ J 2016;46:846-50. [Crossref] [PubMed]
- Barsic B, Dickerman S, Krajinovic V, et al. Influence of the timing of cardiac surgery on the outcome of patients with infective endocarditis and stroke. Clin Infect Dis 2013;56:209-17. [Crossref] [PubMed]
- Pettersson GB, Coselli JS, Hussain ST, et al. 2016 American Association for Thoracic Surgery (AATS) Consensus Guidelines: Surgical Treatment of Infective Endocarditis. J Thorac Cardiovasc Surg 2017;153:1241-58. [Crossref] [PubMed]
- Salgado AV, Furlan AJ, Keys TF, et al. Neurologic complications of endocarditis: a 12-year experience. Neurology 1989;39:173-8. [Crossref] [PubMed]
- Angstwurm K, Borges AC, Halle E, et al. Timing the valve replacement in infective endocarditis involving the brain. J Neurol 2004;251:1220-6. [Crossref] [PubMed]
- Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000;30:633-8. [Crossref] [PubMed]
- Lomas JM, Martínez-Marcos FJ, Plata A, et al. Healthcare-associated infective endocarditis: an undesirable effect of healthcare universalization. Clin Microbiol Infect 2010;16:1683-90. [Crossref] [PubMed]
- Fernández-Hidalgo N, Almirante B, Tornos P, et al. Contemporary epidemiology and prognosis of health care-associated infective endocarditis. Clin Infect Dis 2008;47:1287-97. [Crossref] [PubMed]
- Banks JL, Marotta CA. Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke 2007;38:1091-6. [Crossref] [PubMed]
- Roques F, Nashef SA, Michel P, et al. Risk factors and outcome in European cardiac surgery: analysis of the EuroSCORE multinational database of 19030 patients. Eur J Cardiothorac Surg 1999;15:816-22; discussion 822-3. [Crossref] [PubMed]
- Park LP, Chu VH, Peterson G, et al. Validated Risk Score for Predicting 6-Month Mortality in Infective Endocarditis. J Am Heart Assoc 2016;5:e003016. [Crossref] [PubMed]
- Okita Y, Minakata K, Yasuno S, et al. Optimal timing of surgery for active infective endocarditis with cerebral complications: a Japanese multicentre study. Eur J Cardiothorac Surg 2016;50:374-82. [Crossref] [PubMed]
- Derex L, Bonnefoy E, Delahaye F. Impact of stroke on therapeutic decision making in infective endocarditis. J Neurol 2010;257:315-21. [Crossref] [PubMed]
- Yaghi S, Boehme AK, Dibu J, et al. Treatment and Outcome of Thrombolysis-Related Hemorrhage: A Multicenter Retrospective Study. JAMA Neurol 2015;72:1451-7. [Crossref] [PubMed]
- Salaun E, Touil A, Hubert S, et al. Intracranial haemorrhage in infective endocarditis. Arch Cardiovasc Dis 2018;111:712-21. [Crossref] [PubMed]
- Hornig CR, Dorndorf W, Agnoli AL. Hemorrhagic cerebral infarction--a prospective study. Stroke 1986;17:179-85. [Crossref] [PubMed]
- Hart RG, Easton JD. Hemorrhagic infarcts. Stroke 1986;17:586-9. [Crossref] [PubMed]
- Yoshioka D, Toda K, Sakaguchi T, et al. Valve surgery in active endocarditis patients complicated by intracranial haemorrhage: the influence of the timing of surgery on neurological outcomes. Eur J Cardiothorac Surg 2014;45:1082-8. [Crossref] [PubMed]
- Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995;333:1581-7. [Crossref] [PubMed]
- Siquier-Padilla J, Cuervo G, Urra X, et al. Optimal Timing for Cardiac Surgery in Infective Endocarditis with Neurological Complications: A Narrative Review. J Clin Med 2022;11:5275. [Crossref] [PubMed]



