
Comparison of self- and balloon-expandable valves in patients with dilatated ascending aorta undergoing transcatheter aortic valve replacement
Highlight box
Key findings
• In patients with dilatated ascending aorta (AA) who underwent transcatheter aortic valve replacement (TAVR), the type of valve was not a risk factor for procedural device failure.
What is known and what is new?
• Early clinical trials regarding TAVR have excluded patients with significant AA dilatation.
• Both self- and balloon-expandable valves (SEVs and BEVs) had satisfactory performance in patients with dilatated AA, although BEVs had slightly higher post-procedural aortic expansion rate.
What is the implication, and what should change now?
• Randomized trials are needed to compare the performance of SEV and BEV in patients with dilatated AA.
Introduction
Ascending aortic (AA) dilatation is a common feature in patients with aortic stenosis (AS), especially in those with bicuspid aortic valve (BAV) (1,2). For patients undergoing surgical aortic valve replacement (SAVR), current guidelines recommend concomitant aortic repair or replacement if the diameter of AA exceeds 45 mm to avoid aortic dissection or rupture (3).
Transcatheter aortic valve replacement (TAVR) has profoundly changed the clinical management of AS patients who cannot tolerate SAVR (4,5). For patients who are candidates for TAVR, simultaneous repair of a dilatated AA can be technically difficult. The safety and feasibility of the procedure and the fate of AA after the procedure in these patients remain unclear. Moreover, there are limited data comparing the performance of self-expandable valves (SEVs) versus BEVs in these patients. The aim of the present study is to evaluate the impact of type of transcatheter heart valves (THVs) on intra-procedural device success and post-procedural AA progression in patients with dilatated AA (≥40 mm) undergoing TAVR. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-364/rc).
Methods
Study population
We retrospectively evaluated all patients who underwent transfemoral TAVR from January 2016 to May 2021 at Beijing Fuwai Hospital. Exclusion criteria were dominant aortic regurgitation, a history of SAVR or TAVR, a history of AA surgery, unavailable preoperative aortic computed tomography (CT), and preoperative maximal AA diameter <40 mm, as shown in Figure 1. A total of 207 patients were finally identified. These patients were divided into two groups according to the type of THVs (SEV vs. BEV). Electronic medical records were reviewed to obtain baseline characteristics, procedural details, clinical outcomes, and follow-up data. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board of Fuwai Hospital (approval No. 2022-1829), and informed consent was obtained from all patients.
CT measurements
All preoperative aortic CTs were electrocardiogram-gated with contrast enhancement. The maximal AA diameter was measured at the broadest level of AA by inner-edge to inner-edge method, perpendicular to the axis of blood flow. The AA diameter was calculated as: (maximal diameter + minimal diameter)/2. Dilatation of the AA was defined as a maximal AA diameter of ≥40 mm, in accordance with previous studies (1,6,7). The post-procedural AA expansion rate was calculated as the change of AA diameters (before the procedure and at the latest follow-up) divided by the follow-up period.
Surgical procedure
All transfemoral TAVR procedures were conducted in accordance with guidelines using standard techniques. In SEV group, the types of THV included Venus-A (Venus MedTech, Hangzhou, China), Taurus One (Peijia Medical, Suzhou, China), and VitaFlow (MicroPort, Shanghai, China). In BEV group, Edwards Sapien XT and Sapien 3 (Edwards Lifesciences, Irvine, CA, USA) were used.
Follow-up
Follow-up data were collected from the electronic medical record and telephone interview with patients or their family members. The primary endpoint was the device success as defined by Valve Academic Research Consortium-3 criteria (VARC-3) (8). Secondary endpoints included all-cause mortality and the occurrence of aortic dissection and/or rupture during the follow-up.
Statistical analysis
Categorical variables were expressed as frequencies and percentages, and were tested by the chi-square test or Fisher exact test. Normally distributed continuous variables were expressed as means ± standard deviations and non-normally distributed variables as median (interquartile range), and were compared using Student t-test or the Mann-Whitney U test. Overall survival was estimated using Kaplan-Meier methods and compared with the log-rank test.
All baseline variables were examined in a univariable logistic regression model to identify the risk factors for intra-procedural device failure. Patients were divided into two groups (device success and device failure). Baseline variables that were found to be different in univariable analyses with a P value of <0.1 were identified and included in the multivariable analyses. A backward method was used to leave covariates with P values <0.10 in the final multivariable model.
Aortic expansion rates were determined from a subset of patients who had follow-up aortic CTs ≥12-month intervals, and expressed as millimeter per year. Preoperative AA diameters and follow-up AA diameters were compared with paired Student t-test.
SPSS software version 23.0 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism version 9.3.1 (GraphPad Software, San Diego, CA, USA) were used for data analyses and visualization. All reported P values were 2-sided, and a value of P<0.05 was considered statistically significant.
Results
Baseline characteristics
A total of 207 patients with AA dilatation (≥40 mm) who underwent transfemoral TAVR were identified (Figure 1). Baseline characteristics are summarized in Table 1. The majority of procedures were performed using SEVs (88.4%), while BEVs accounted for 11.6%. Patients in SEV group had higher prevalence of BAV (56.8% vs. 33.3%, P=0.030). The rates of peripheral artery disease and moderate-to-severe aortic regurgitation were higher in BEV group (P<0.05).
Table 1
| Variables | SEV (n=183) | BEV (n=24) | P value |
|---|---|---|---|
| Age, years | 74 [69–77] | 73 [69–77] | 0.816 |
| Female gender | 68 (37.2) | 9 (37.5) | 0.974 |
| Body surface area, m2 | 1.71 [1.56–1.85] | 1.66 [1.53–1.81] | 0.452 |
| STS score, % | 4.0 [3.5–5.0] | 4.2 [3.5–4.5] | 0.997 |
| Smoking | 64 (35.0) | 9 (37.5) | 0.808 |
| Serum creatinine, mg/dL | 1.0 [0.9–1.3] | 1.0 [0.9–1.3] | 0.765 |
| Hypertension | 94 (51.4) | 11 (45.8) | 0.610 |
| Diabetes mellitus | 33 (18.0) | 6 (25.0) | 0.410 |
| History of coronary artery disease | 72 (39.3) | 10 (41.7) | 0.827 |
| History of cerebrovascular disease | 28 (15.3) | 4 (16.7) | 0.771 |
| Peripheral artery disease | 42 (23.0) | 10 (41.7) | 0.047 |
| Prior coronary artery intervention | 12 (6.6) | 4 (16.7) | 0.097 |
| Prior coronary artery bypass grafting | 0 (0.0) | 1 (4.2) | 0.116 |
| Atrial fibrillation | 29 (15.8) | 2 (8.3) | 0.524 |
| Bicuspid aortic valve | 104 (56.8) | 8 (33.3) | 0.030 |
| Left ventricular ejection fraction <40% | 40 (21.9) | 3 (12.5) | 0.288 |
| Mean aortic valve gradient, mmHg | 58 [46–70] | 57 [49–62] | 0.467 |
| Moderate-to-severe aortic regurgitation | 47 (25.7) | 11 (45.8) | 0.039 |
| Preoperative AA diameter, mm | 44 [41–48] | 43 [41–45] | 0.233 |
Values are presented as n (%) or median [interquartile range]. SEV, self-expandable valve; BEV, balloon-expandable valve; STS, Society of Thoracic Surgeons; AA, ascending aorta.
Perioperative results
Procedural details are shown in Table 2. The rates of mild or moderate-to-severe paravalvular regurgitation were higher in SEV group (P=0.006). Implantation of second valve was observed in 9.3% of patients in SEV group, while none occurred in BEV group (P=0.229). Although not statistically significant, the overall device success was lower in SEV group compared with SEV group (84.2% vs. 95.8%, P=0.213). In univariable logistic regression analysis, three variables (age, gender, and BAV) had P values <0.10. In the multivariable model, only BAV was found to be an independent risk factor for device failure (OR: 2.632, CI: 1.107–6.257, P=0.029) (Table 3). There was no significant difference in 30-day complications between two groups.
Table 2
| Variables | SEV (n=183) | BEV (n=24) | P value |
|---|---|---|---|
| Implanted valve type | NA | ||
| VenusA | 141 (77.1) | NA | |
| VitaFlow | 33 (18.0) | NA | |
| TaurusOne | 9 (4.9) | NA | |
| Sapien XT | NA | 7 (29.2) | |
| Sapien 3 | NA | 17 (70.8) | |
| Implanted valve size, mm | 0.364 | ||
| 23 | 49 (26.8) | 9 (37.5) | |
| 24 | 13 (7.1) | 0 | |
| 26 | 76 (41.5) | 11 (45.8) | |
| 27 | 18 (9.8) | 0 | |
| 29 | 20 (10.9) | 4 (16.7) | |
| 30 | 2 (1.1) | 0 | |
| 32 | 5 (2.7) | 0 | |
| Conversion to open surgery | 5 (2.8) | 1 (4.2) | 0.527 |
| Implantation of second valve | 17 (9.3) | 0 | 0.229 |
| Device success | 154 (84.2) | 23 (95.8) | 0.213 |
| Pre-dilation | 176 (96.7) | 21 (87.5) | 0.073 |
| Post-dilation | 50 (27.5) | 4 (16.7) | 0.258 |
| Paravalvular regurgitation* | 0.006 | ||
| None or trace | 81 (44.8) | 19 (79.2) | |
| Mild | 94 (51.9) | 5 (20.8) | |
| Moderate to severe | 6 (3.3) | 0 | |
| Post-procedural mean aortic valve gradient, mmHg | 13 [9–18] | 14.0 [11–19] | 0.440 |
| 30-day outcome | |||
| Mortality | 3 (1.6) | 0 | 1.000 |
| Stroke | 1 (0.5) | 0 | 1.000 |
| Permanent pacemaker | 12 (6.6) | 1 (4.2) | 1.000 |
| Myocardial infarction | 0 | 0 | NA |
| Major vascular complication | 3 (1.6) | 0 | 1.000 |
| New requirement for dialysis | 1 (0.5) | 0 | 1.000 |
Values are presented as n (%) or median [interquartile range]. *, available in 205 patients. SEV, self-expandable valve; BEV, balloon-expandable valve; NA, not applicable.
Table 3
| Variables | Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|---|
| Odds ratio | Confidence interval | P value | Odds ratio | Confidence interval | P value | ||
| Age, years | 0.960 | 0.919–1.003 | 0.067 | ||||
| Female gender | 0.465 | 0.190–1.142 | 0.095 | ||||
| Body surface area, m2 | 4.077 | 0.575–28.921 | 0.160 | ||||
| STS score, % | 0.924 | 0.696–1.226 | 0.583 | ||||
| Smoking | 1.491 | 0.679–3.274 | 0.319 | ||||
| Serum creatinine, mg/dL | 0.980 | 0.258–3.721 | 0.976 | ||||
| Hypertension | 1.323 | 0.606–2.885 | 0.482 | ||||
| Diabetes mellitus | 0.841 | 0.300–2.357 | 0.742 | ||||
| History of coronary artery disease | 0.864 | 0.388–1.926 | 0.721 | ||||
| History of cerebrovascular disease | 1.850 | 0.719–4.765 | 0.202 | ||||
| Peripheral artery disease | 0.893 | 0.359–2.221 | 0.807 | ||||
| Prior coronary artery intervention | 0.832 | 0.179–3.859 | 0.814 | ||||
| Prior coronary artery bypass grafting | NA | NA | NA | ||||
| Atrial fibrillation | 0.855 | 0.276–2.645 | 0.785 | ||||
| Bicuspid aortic valve | 2.658 | 1.124–6.289 | 0.026 | 2.632 | 1.107–6.257 | 0.029 | |
| Left ventricular ejection fraction <40% | 1.803 | 0.758–4.284 | 0.182 | ||||
| Mean aortic valve gradient, mmHg | 1.001 | 0.980–1.021 | 0.944 | ||||
| Moderate-to-severe aortic regurgitation | 1.120 | 0.480–2.613 | 0.794 | ||||
| Preoperative AA diameter, mm | 1.003 | 0.920–1.092 | 0.951 | ||||
| SEV | 4.331 | 0.563–33.344 | 0.159 | ||||
STS, Society of Thoracic Surgeons; AA, ascending aorta; SEV, self-expandable valve; NA, not applicable.
Follow-up outcomes
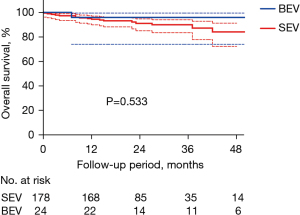
Follow-up was completed in 98.6% (204 of 207) of individuals. The median follow-up was 21 [15–34] months in SEV group and 26 [13–44] months in BEV group (P=0.579). No statistical difference was found between two groups regarding the overall survival (83.1%±4.7% vs. 95.8%±4.1%, P=0.533) (Figure 2). No definite aortic dissection or rupture was found during the follow-up period, although there were 2 sudden deaths with unknown reasons. In subgroup analyses, we classified patients according to the type of aortic valve [BAV vs. tricuspid aortic valve (TAV)], degree of preoperative aortic regurgitation (AR) (≥ moderate AR vs. < moderate AR), and whether preoperative AA diameter ≥45 mm or not (AA ≥45 vs. <45 mm). No statistical differences were found regarding the overall survival (Figures S1-S3).
Postoperative AA progression
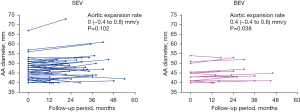
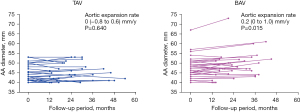
Follow-up CT assessments beyond 12 months from the procedure were available for 68 patients (32.9%), including 51 in SEV group and 17 in BEV group. The median CT follow-up time was 22 [16–34] months in SEV group and 24 [16–33] months in BEV group (P=0.793). In this subset of patients, the AA diameter appeared to remain stable in SEV group with an aortic expansion rate of 0 (−0.4 to 0.8) mm/y (P=0.102), while it slightly enlarged in BEV group with an aortic expansion rate of 0.4 (−0.4 to 0.6) mm/y (P=0.038) (Figure 3). When classifying these patients into BAV group and TAV group, the AA diameter slightly enlarged in BAV group with an aortic expansion rate of 0.2 (0 to 1.0) mm/y (P=0.015), while it remained stable in TAV group with an aortic expansion rate of 0 (−0.8 to 0.6) mm/y (P=0.640) (Figure 4).
Discussion
In patients with AS, AA dilatation is a common aortopathy with an incidence rate of 20–25% (9,10). The results of our study reveal a higher incidence (42.5%), which might be explained by a high prevalence of BAV in the Chinese population (11). Patients with BAV present more frequently with AA dilatation because both intrinsic disease of the vascular media and modified flow patterns through the stenotic valve contribute to the AA dilatation (12), unlike patients with TAV whose post-stenotic AA dilatation is more related to the hemodynamic disturbance (13,14).
A previous meta-analysis study regarding the comparison of SEVs and BEVs found no differences on all-cause and cardiovascular mortality, although BEVs were associated with a reduced risk of permanent pacemaker implantation and paravalvular leak (15). However, the impact of AA dilatation on device success following TAVR has not been systemically described before. Early PARTNER trials have excluded patients with significant AA dilatation (≥50 mm) (3,4). Manufacturer specifications for CoreValve required that proximal AA diameter should not exceed 40–43 mm for the 3 valve sizes (16,17). As for BEVs, a previous study by Rylski et al. reported that Edwards Sapien valves can be safely used in patients with AA dilatation (40–50 mm) without adding intraprocedural risk of adverse aortic events (18).
In a SEV, the long stent frame extends beyond the sinotubular junction into the AA. Although the inflow portion of the stent frame exerts high radial force for anchoring, the outflow portion also helps secure the SEV in the AA and orients the valve to the blood flow (19,20). In the setting of dilatated AA, the reduced anchoring of the outflow portion may raise the concern for unstable device position (21). On the other hand, a BEV has a short stent frame which does not extend beyond the aortic sinus. The study by Rylski et al. suggested that the intra-annular implantation of a BEV might be a safe choice in patients with dilatated AA (18). However, in the present study, the use of SEVs was not an independent risk factor for device failure. The fact that device success rate was slightly lower in SEV group might be explained by the higher prevalence of BAV in SEV group, which is the only significant risk factor for device failure in the present study.
In addition to the perioperative outcomes, it is also important to explore the AA progression and the risk of adverse aortic events after the procedure. In the present study, no aortic dissection or rupture was found in both groups, and the overall survival was not affected by the type of THV during a median follow-up of 21 months. The AA diameter appeared to slightly grow in BEV group, while it remained stable in SEV group despite larger baseline AA diameters and higher rate of BAV. Previous studies demonstrated that SEV offered a better hemodynamic profile compared with BEV (22-25), which might play a role in AA progression. However, these hypotheses need to be confirmed in further studies.
It should be noted that among patients whose follow-up CTs were available, three had baseline AA diameter exceeding 55 mm (all in SEV group). Although perioperative device success was achieved, postoperative AA enlarged rapidly in all of them (4–6 mm). Therefore, indications of TAVR should be evaluated very cautiously in patients with extremely dilatated AA. Both acute procedural success and post-procedural AA progression should be taken into account. In these patients, other strategies and accesses, such as concomitant TAVR and wrapping of AA through mini-sternotomy, might be considered. Postoperative follow-ups are important to evaluate the AA progression. If rapid AA expansion (3–5 mm/y) is noted, further intervention (surgical or endovascular treatment) might be needed. As the indications for TAVR have expanded to low-risk and young patients, AA dilatation should be considered as a criterion to refine risk stratification.
Several limitations should be acknowledged. First, the SEVs used in the present study are locally manufactured valves that have received Chinese regulatory approval. Other widely used SEVs, such as Medtronic CoreValves, are not available in China until very recently. Although studies regarding domestic THVs are limited, several previous reports have verified the safety and feasibility of these THVs (26-28). The BEVs (Edward Sapien XT and Sapien 3) were also not available in the early clinical practice, therefore the sample size of SEV group was drastically larger than that of BEV group. This might affect the external validity of the results. Further studies with larger sample size are required. Second, discrepancies in measurements of the AA may impair clinical assessment (29). In the present study, to minimize the errors, the measurements of the preoperative AA diameters were performed by the same standard: contrast-enhanced, electrocardiogram-gated, and inner-edge to inner-edge. In addition, 75% of the follow-up CTs were performed at our center by the same standard, making the comparison at the same plane and level possible. Third, the calculation of the aortic expansion rate, defined by the changes of AA diameter (preoperative AA diameter- latest follow-up) divided by follow-up period, might not reflect the variations during the follow-up period, although this method has been used in the previous studies (30,31). Finally, the present study represents a single-center experience. The small sample size and the retrospective nature limit generalizability of the findings. Randomized studies would be necessary to compare the performance of SEV and BEV in patients with dilatated AA.
Conclusions
In patients with dilatated AA who underwent TAVR, the type of THVs did not affect the procedural device success. BAV appeared to be a risk factor for device failure and higher aortic expansion rate in these patients.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-364/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-364/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-364/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-364/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board of Fuwai Hospital (approval No. 2022-1829), and informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kerneis C, Pasi N, Arangalage D, et al. Ascending aorta dilatation rates in patients with tricuspid and bicuspid aortic stenosis: the COFRASA/GENERAC study. Eur Heart J Cardiovasc Imaging 2018;19:792-9. [Crossref] [PubMed]
- Verma S, Siu SC. Aortic dilatation in patients with bicuspid aortic valve. N Engl J Med 2014;370:1920-9. [Crossref] [PubMed]
- Vahanian A, Beyersdorf F, Praz F, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2022;43:561-632. [Crossref] [PubMed]
- Siontis GCM, Overtchouk P, Cahill TJ, et al. Transcatheter aortic valve implantation vs. surgical aortic valve replacement for treatment of symptomatic severe aortic stenosis: an updated meta-analysis. Eur Heart J 2019;40:3143-53. [Crossref] [PubMed]
- Mack MJ, Leon MB, Smith CR, et al. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet 2015;385:2477-84. [Crossref] [PubMed]
- Kim JB, Spotnitz M, Lindsay ME, et al. Risk of Aortic Dissection in the Moderately Dilated Ascending Aorta. J Am Coll Cardiol 2016;68:1209-19. [Crossref] [PubMed]
- Ochiai T, Yoon SH, Sharma R, et al. Prevalence and Prognostic Impact of Ascending Aortic Dilatation in Patients Undergoing TAVR. JACC Cardiovasc Imaging 2020;13:175-7. [Crossref] [PubMed]
- Généreux P, Piazza N, Alu MC, et al. Valve Academic Research Consortium 3: Updated Endpoint Definitions for Aortic Valve Clinical Research. J Am Coll Cardiol 2021;77:2717-46. [Crossref] [PubMed]
- Son JY, Ko SM, Choi JW, et al. Measurement of the ascending aorta diameter in patients with severe bicuspid and tricuspid aortic valve stenosis using dual-source computed tomography coronary angiography. Int J Cardiovasc Imaging 2011;27:61-71. [Crossref] [PubMed]
- Crawford MH, Roldan CA. Prevalence of aortic root dilatation and small aortic roots in valvular aortic stenosis. Am J Cardiol 2001;87:1311-3. [Crossref] [PubMed]
- Giannini F, Baldetti L, Gallone G, et al. Transcatheter Valve Replacement in Asia Pacific: Current Practice and Perspectives. J Am Coll Cardiol 2018;72:3189-99. [Crossref] [PubMed]
- Guzzardi DG, Verma S, Fedak PWM. Bicuspid aortic valve aortopathy. Current Opinion in Cardiology 2017;32:111-6. [Crossref] [PubMed]
- Gaudino M, Anselmi A, Morelli M, et al. Aortic expansion rate in patients with dilated post-stenotic ascending aorta submitted only to aortic valve replacement long-term follow-up. J Am Coll Cardiol 2011;58:581-4. [Crossref] [PubMed]
- Yasuda H, Nakatani S, Stugaard M, et al. Failure to prevent progressive dilation of ascending aorta by aortic valve replacement in patients with bicuspid aortic valve: comparison with tricuspid aortic valve. Circulation 2003;108:II291-4. [Crossref] [PubMed]
- D'Ascenzo F, Bruno F, Baldetti L, et al. Aortic valve replacement vs. balloon-expandable and self-expandable transcatheter implantation: A network meta-analysis. Int J Cardiol 2021;337:90-8. [Crossref] [PubMed]
- Leipsic J, Gurvitch R, Labounty TM, et al. Multidetector computed tomography in transcatheter aortic valve implantation. JACC Cardiovasc Imaging 2011;4:416-29. [Crossref] [PubMed]
- Achenbach S, Delgado V, Hausleiter J, et al. SCCT expert consensus document on computed tomography imaging before transcatheter aortic valve implantation (TAVI)/transcatheter aortic valve replacement (TAVR). J Cardiovasc Comput Tomogr 2012;6:366-80. [Crossref] [PubMed]
- Rylski B, Szeto WY, Bavaria JE, et al. Transcatheter aortic valve implantation in patients with ascending aortic dilatation: safety of the procedure and mid-term follow-up dagger. Eur J Cardiothorac Surg 2014;46:228-33; discussion 233. [Crossref] [PubMed]
- Hellhammer K, Piayda K, Afzal S, et al. The Latest Evolution of the Medtronic CoreValve System in the Era of Transcatheter Aortic Valve Replacement: Matched Comparison of the Evolut PRO and Evolut R. JACC Cardiovasc Interv 2018;11:2314-22. [Crossref] [PubMed]
- Costa G, Criscione E, Reddavid C, et al. Balloon-expandable versus self-expanding transcatheter aortic valve replacement: a comparison and evaluation of current findings. Expert Rev Cardiovasc Ther 2020;18:697-708. [Crossref] [PubMed]
- Maeno Y, Yoon SH, Abramowitz Y, et al. Effect of ascending aortic dimension on acute procedural success following self-expanding transcatheter aortic valve replacement: A multicenter retrospective analysis. Int J Cardiol 2017;244:100-5. [Crossref] [PubMed]
- Abdel-Wahab M, Landt M, Neumann FJ, et al. 5-Year Outcomes After TAVR With Balloon-Expandable Versus Self-Expanding Valves: Results From the CHOICE Randomized Clinical Trial. JACC Cardiovasc Interv 2020;13:1071-82. [Crossref] [PubMed]
- Thiele H, Kurz T, Feistritzer HJ, et al. Comparison of newer generation self-expandable vs. balloon-expandable valves in transcatheter aortic valve implantation: the randomized SOLVE-TAVI trial. Eur Heart J 2020;41:1890-9. [Crossref] [PubMed]
- Hatoum H, Yousefi A, Lilly S, et al. An in vitro evaluation of turbulence after transcatheter aortic valve implantation. J Thorac Cardiovasc Surg 2018;156:1837-48. [Crossref] [PubMed]
- Hatoum H, Samaee M, Sathananthan J, et al. Comparison of performance of self-expanding and balloon-expandable transcatheter aortic valves. JTCVS Open 2022;10:128-39. [Crossref] [PubMed]
- Liao YB, Zhao ZG, Wei X, et al. Transcatheter aortic valve implantation with the self-expandable venus A-Valve and CoreValve devices: Preliminary Experiences in China. Catheter Cardiovasc Interv 2017;89:528-33. [Crossref] [PubMed]
- Hong N, Pan W, Chen S, et al. Transcatheter Aortic Valve Replacement in Low-Risk Patients With Severe Aortic Valve Stenosis in Chinese Patients. JACC Asia 2022;2:210-2. [Crossref] [PubMed]
- Zhou D, Pan W, Wang J, et al. VitaFlow™ transcatheter valve system in the treatment of severe aortic stenosis: One-year results of a multicenter study. Catheter Cardiovasc Interv 2020;95:332-8. [Crossref] [PubMed]
- Elefteriades JA, Mukherjee SK, Mojibian H. Discrepancies in Measurement of the Thoracic Aorta: JACC Review Topic of the Week. J Am Coll Cardiol 2020;76:201-17. [Crossref] [PubMed]
- Lee SH, Kim JB, Kim DH, et al. Management of dilated ascending aorta during aortic valve replacement: valve replacement alone versus aorta wrapping versus aorta replacement. J Thorac Cardiovasc Surg 2013;146:802-9. [Crossref] [PubMed]
- Song SW, Chang BC, Cho BK, et al. Effects of partial thrombosis on distal aorta after repair of acute DeBakey type I aortic dissection. J Thorac Cardiovasc Surg 2010;139:841-7.e1; discussion 847. [Crossref] [PubMed]



