
Intrinsic pulmonary sealing, its mechanisms and impact on validity and translational value of lung sealant studies: a pooled analysis of animal studies
Highlight box
Key findings
• Superficial lesions in healthy sheep lungs show a rapid intrinsic sealing mechanism, which invalidates these lesions for use in lung sealing studies. Sequential amputation lesions including bronchioles of >⌀1.5 mm may be a suitable alternative for acute testing of aerostatic sealant effectiveness.
What is known and what is new?
• There is no standardized in vivo model of pulmonary air leakage, and current models are heterogenous. There is some evidence for intrinsic sealing mechanisms in healthy animal lungs, which may cause a translational gap in lung sealant research.
• This study demonstrates the existence of intrinsic sealing in healthy animal lungs, offers novel insights into its mechanisms and confirms the possibility of a translational gap in preclinical studies.
What is the implication, and what should change now?
• Researchers should control for intrinsic sealing mechanisms using negative controls when investigating lung sealants in preclinical studies, to demonstrate treatment effectiveness. To this end, a standardized model of pulmonary air leakage is a prerequisite.
Introduction
Prolonged pulmonary air leak (pPAL) occurs in up to 30% of lung resections, causing increased morbidity (empyema and post-operative complications), reinterventions (4.8%), readmissions [odds ratio (OR) =2] and mortality (OR =1.9) (1-7). Preclinical animal studies with lung sealants have shown promising results, and 74% of surgeons use sealants in their high-risk patients (8-10). Results of lung sealants in preventing pPAL in clinical studies, however, are mixed and guidelines do not recommend their routine use (11-16). Recently, in a European Society of Thoracic Surgeons (ESTS) survey, the majority of the 258 responding thoracic surgeons affirmed the lack of sufficient evidence for lung sealants, and an unmet clinical need for more effective lung sealants was described (17). The marked discrepancy between the more positive preclinical studies and often unsatisfactory clinical results indicate a potential translational gap.
For effective clinical use, the performance of lung sealants should be investigated during their development in properly validated animal models. For a valid model, pulmonary air leakage (PAL) needs to be present of sufficient magnitude and without the capacity to resolve spontaneously for the study duration, ensuring accurate assessment of both acute and prolonged aerostatic efficacy of applied lung sealants. Furthermore, in contrast to patients undergoing lung resections, animals used in lung sealing experiments are healthy in 92% of cases and may poses enhanced intrinsic sealing and regenerative capacities, which can possibly invalidate positive study findings if unaccounted for (18-20). In the present literature, no standardized animal model exists that guarantees clinically significant pPAL, and negative control groups were only used in 18.7% of preclinical studies (20,21). Therefore, the validity of many previously tested lesions for pPAL in animal models is unknown (20,22,23).
Several enhancements to animal models for lung surgery research have been described, that might reduce the translational gap. First of all, the type of lesion might impact PAL, as larger lesions and lesions involving bronchioles seem more likely to result in pPAL (21,24). Secondly, disease models have been described, including models for emphysema and heparinized models (25,26). Although these disease models increase the risk of pPAL, these models may come at the cost of increased variation and decreased animal welfare. In the present study, we assessed the impact of different types of lesions on PAL from the results of negative controls (untreated lesions) in three animal experiments. These results provide insight in the impact of intrinsic sealing mechanisms on the validity and translational value of animal models for lung sealant research. We present this article in accordance with the ARRIVE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-180/rc).
Methods
Study setup
The intrinsic sealing capacities of both superficial parenchymal lesions and lesions involving bronchioles are investigated in in vivo and post-mortem ovine models, by measuring air leakage (AL) and characterizing the responsible mechanisms histopathologically. To simulate a real post-operative scenario, an ovine acute aerostasis model with both mechanical ventilation and spontaneous breathing was used, based on previous models (21,24). A bilateral thoracotomy was performed sequentially and varying standardized lung lesions were created in an explorative study design, after which AL was measured on both lungs after chest closure with a digital drainage system.
The sheep included in the analysis were used in three experiments, one post-mortem study and two in vivo studies. First, superficial parenchymal lung lesions were tested in mechanically ventilated post-mortem sheep (N=2 cadavers), to test the hypothesis that pleural apposition diminishes AL for superficial lesions after chest closure (27,28). Then, such lesions were created in a live sheep model (in vivo), testing their natural healing course in the presence of intact coagulation (N=3 sheep). Finally, based on the previous results, more extensive lesions involving macroscopically visible bronchioles were tested (N=7 sheep). Part of the lesions made in the in vivo studies were treated with a sealant. For the present study, we only pooled results for untreated lesions, as the focus of this investigation was to study the mechanisms of intrinsic sealing.
Lung lesions
Lesions were made to simulate the clinical problem of an alveolar-pleural fistula, defined as PAL arising distal to the segmental bronchus (29). Three different lung lesions were tested: superficial parenchymal, deep bowl-shaped and sequential lung amputation lesions. Superficial parenchymal lesions were made by creating n × n perpendicular cuts in a 25 mm × 25 mm square with a scalpel limited to 1–3 mm depth (Figure 1A,1B). Deep bowl-shaped lesions were made by cutting a ⌀25 mm bowl lesion using biopsy punches and scissors (Figure 1C,1D). Sequential lung amputations were made by cutting away tissue perpendicularly to the expected bronchiole branching pattern, at a width of 3–5 cm in steps of 1 cm until either bronchioles of >⌀1.5 or ⌀0.5 mm were encountered, on the ventral tips of the right middle lobe (RML) and right lower lobe (RLL) on the right, plus the left upper lobe (LUL) and the left lower lobe (LLL) on the left side (Figure 1E,1F). This method for inducing lesions involving bronchioles was found to be the most repeatable in our ex vivo investigations [Supplementary file (Appendix 1)] and is based on the model of Ranger (21). All lesions were made on a static lung with a positive end-expiratory pressure (PEEP) of 10 cmH2O. Precise lesion types per experiment are explained in Table 1.
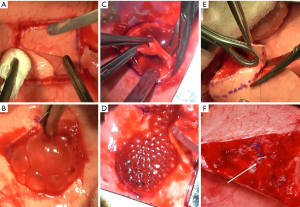
Table 1
| ID | Weight, age | Mode of ventilation | Side | Tot. (n) | Eff. (n) | Type | Eff. lobe and lesion | Drainage duration (h) |
|---|---|---|---|---|---|---|---|---|
| Post-mortem | ||||||||
| T3 | 68.5 kg, 9 y 1 mo | MV: PC, Pmax 40 cmH2O |
L | 1 | 1 | Par. | LUL: 25 mm × 25 mm × 1 mm visceral pleura removal with 10×10 incisions at 1 mm depth | 1:20 |
| R | 1 | 1 | Par. | RML: 25 mm × 25 mm × 3 mm cube excision | 4:10 | |||
| T4 | 75.5 kg, 6 y 4 mo | MV: PC, Pmax 50 cmH2O |
L | 1 | 1 | Par. | LUL: same as T3/L | 1:20 |
| R | 1 | 1 | Par. | RLL: same as T3/L | 3:30 | |||
| In vivo | ||||||||
| P1 | 73 kg, 3 y 6 mo | MV: VC, Pmax 38 cmH2O; SV: 2 h |
L | 1 | 1 | Par. | LUL: same as T3/L + additional 10×10 incisions at 3 mm depth | 3:10 |
| R | 1 | 1 | Par. | RLL: same as T3/L | 7:40 | |||
| P2 | 60.3 kg, 2 y 8 mo | MV: VC, Pmax 36 cmH2O; SV: – |
L | 2 | 1 | Par. | LUL: same as T3/L but 3 mm depth incisions | 3:30 |
| R | 2 | 1 | Br. | RLL: ⌀25 mm/1 mm depth, 2× ⌀10 mm/5 mm depth, cut bowl + 10×10 incisions at 1 mm depth | 7:40 | |||
| P4 | 50 kg, 1 y 7 mo | MV: PC, Pmax 40 cmH2O; SV: 4:30 h |
L | 5 | 1 | Par. | LUL: same as T3/L but 6×6 incisions at 3 mm depth | 5:10 |
| R | 5 | 1 | Par. | RML: same as T3/L but 6×6 incisions at 3 mm depth | 8:00 | |||
| P5 | 71 kg, 2 y 9 mo | MV: PC, Pmax 30 cmH2O; SV: 3:00 h |
L | 2 | 2 | Br. | LUL/LLL: sequential amputations until bronchioles of ⌀1.5 mm | 3:10 |
| R | 2 | 2 | Br. | RML/RLL: same as P5/L | 6:50 | |||
| P6 | 67.5 kg, 2 y 10 mo | MV: PC, Pmax 40 cmH2O; SV: – |
L | 2 | 2 | Br. | LUL/LLL: sequential amputations until bronchioles of ⌀0.5 mm | – |
| R | 2 | 2 | Br. | RML/LLL: same as P6/L | 4:40 | |||
| E2 | 82 kg, 2 y 11 mo | MV: PC, Pmax 25 cmH2O; SV: 4:09 h |
R | 2 | 2 | Br. | RML/RLL: same as P5/L | 10:09 |
| E3 | 79.8 kg, 1 y 2 mo | MV: PC, Pmax 25 cmH2O; SV: 4:07 h |
L | 2 | 2 | Br. | LUL/LLL: same as P5/L | 4:33 |
| E4 | 66.3 kg, 3 y 2 mo | MV: PC, Pmax 25 cmH2O; SV: 3:24 h |
R | 1 | 1 | Br | RLL: same as P5/L | 7:08 |
| E5 | 67 kg, 3 y 5 mo | MV: PC, Pmax 25 cmH2O; SV: 3:01 h |
L | 1 | 1 | Br | LLL: same as P5/L | 3:27 |
| E6 | 66 kg, 1 y 8 mo | MV: PC, Pmax 25 cmH2O; SV: 3:08 h |
L | 1 | 1 | Br | LLL: same as P5/L | 3:45 |
ID, identifier; Tot., total lesions; Eff., effective number of lesions leaking on one side, other created lesions were sealed before thorax closure; y, years; mo, months; kg, kilograms; MV, mechanical ventilation; PC, pressure control; Pmax, maximum pressure; h, hours; VC, volume control; SV, spontaneous ventilation; L, left; R, right; Par., superficial parenchymal type lesions; Br., deeper lesions involving bronchioles; LUL, left upper lobe; RML, right middle lobe; RLL/LLL, right/left lower lobe.
Outcome measures
In all experiments, after lesion creation and chest closure, AL was measured using a digital chest drainage system (Thopaz®, Medela, Baar, Switzerland). The time point since start of drainage when AL was <20 mL/min, was noted as time until intrinsic sealing. For lesions involving bronchioles, the AL was measured using a mechanical ventilator (SERVO-I®, Getinge, Gotenburg, Sweden), based on the inspiratory (TVi) and expiratory (TVe) tidal volumes, right before and after lesion induction. The minimal leaking pressure (MLP) was determined for these lesions by dialing down the PEEP in steps of 1 cmH2O until leakage disappeared or increasing in steps of 1 cmH2O until leakage appears. In the in vivo series, the post-mortem measurements of MLP were performed either ex-situ after lung explantation, or in-situ using selective intubation (in E4–E6, Table 1). Bronchial diameters (lumen, ⌀) were measured using a ruler with markings every 0.5 mm (Aesculap AA804R), and approximated in 0.5 mm increments. Finally, macroscopy was recorded descriptively, paying attention to mechanisms of sealing, hemostasis and atelectasis.
Animal procedures
All experiments were performed under a project license (No. AVD10300202114869) granted by national authorities (‘Centrale Commissie Dierproeven’, CCD) after review by an independent ethics board (‘Dierexperimentencommissie’, DEC) in the Netherlands, in compliance with institutional guidelines for the care and use of animals. Humane care and anesthesia were provided throughout the experiments. Experimental protocols were approved by the animal welfare body and registered internally at our institute (Nos. 2021-0012-001 and 2021-0012-002).
Adult mixed-breed female sheep (N=2) which were previously utilized for the production of antibodies in an unrelated project were euthanized using an overdose of pentobarbital, and the cadavers were directly re-used in the post-mortem pilot model. Healthy adult mixed-breed female sheep (N=10) were used in the in vivo animal model. Anesthetic protocols in vivo involved deep surgical anesthesia during surgery and lighter sedation during a spontaneous ventilation observation period [detailed in Supplementary file (Appendix 1)]. Mechanical ventilation was guided by the visual presence of atelectasis on lung surfaces and ventilation and oxygenation requirements in the live animals. Reduction of ruminal tympany was ensured throughout the procedure.
A thoracotomy was performed on both sides of in the fifth intercostal space sequentially, always beginning with the right lung. After lesion creation, hemostasis was performed if required using gauzes and diathermy, and time until hemostasis was recorded. If necessary, fibrin plugs were removed using a small tweezers from the bronchiole lesions following hemostasis. Following all measurements, a silicone drainage tube (size Ch30) was placed apically and exited the thorax ventrally on both sides. The thoracotomy was closed in layers to ensure air-tightness for accurate AL measurements. After an observation period in a back position (post-mortem model, minimum of one hour) or abdominal position (in vivo model, minimum of three hours) under mechanical or spontaneous ventilation, the live animals were euthanized using pentobarbital. The lungs were explanted through a median sternotomy for inspection, post-mortem measurements and histology.
Histology
For histological analysis, samples were taken from the created defects after MLP determination and stored in 4% formaldehyde. Subsequently, they were embedded in paraffin and 4 μm thick sections were cut and stained with hematoxylin-eosin staining. These coupes were digitalized and assessed by an experienced pathologist (Vos S).
Statistical analysis
ThopEasy + software (Medela, Baar, Switzerland) was used to import AL data, giving mean AL values (mL/min) every 10 minutes. For all lesions (post-mortem and in vivo), the mean AL was calculated over the first 80 minutes of drainage (minimum drainage time across all groups). In vivo, mean AL was calculated over the first 180 minutes of drainage, and in 30 minute intervals for the first 5 hours of drainage. For statistical comparison, drainage AL data was normalized as: , were Nleaks denotes the number of effective ALs on the drained side. Normalized values were then compared using an analysis of variance (ANOVA) with Bonferroni-Holm post-hoc test (α=0.05/3). Intraoperative AL (mL/min) based on the mechanical ventilator was calculated as: , were RR denotes respiratory rate. This AL was corrected for AL measured before the lesion was created as: . In case this calculation resulted in a negative AL, an AL of 0 mL/min was noted. MLP, time until hemostasis and AL data were compared between lesions involving bronchioles (⌀0.5 mm vs. >⌀1.5 mm lesions) using a Mann-Whitney U test (two-tailed α=0.05). IBM SPSS Statistics 27 (IBM Corp., Armonk, New York, USA) was used for statistical testing.
Results
Summary of experiment characteristics
In twelve animals, 25 untreated lesions were created and analyzed (N=4 superficial parenchyma post-mortem, N=5 superficial parenchyma in vivo, N=16 lesions involving bronchioles, N=4 ⌀0.5 mm and N=11 >⌀1.5 mm bronchioles, N=1 missing diameter). One animal in the in vivo group (P2) did not regain spontaneous ventilation and was kept on the mechanical ventilator during the observation period, another (P6) died due to acute cardiac arrest right after closure of the left thoracotomy, resulting in missing left lung drainage data and a shorter follow-up period. All experiment characteristics are displayed in Table 1.
Air leak characteristics for lesion subtypes
75% of superficial parenchymal lesions resulted in AL after thorax closure in the post-mortem model [mean ± standard deviation (SD): 760±693 mL/min, N=4]. One lesion did not exhibit AL, likely due to extensive atelectasis of the affected lobe seen at obduction. For the in vivo superficial parenchymal lesions, only minimal and rapidly decreasing post-operative AL was observed (mean ± SD: 42±33 mL/min, N=5, Figure 2A). All of these lesions stopped leaking within a median time of 20 minutes [interquartile range (IQR), 10–75 minutes, N=5]. The average AL at 80 minutes showed a trend towards statistical significance between the post-mortem and in vivo groups (P=0.055).
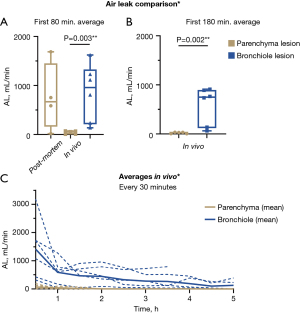
In comparison to superficial parenchymal lesions in vivo, all lesions involving bronchioles (pooled ⌀0.5 mm and >⌀1.5 mm lesions) led to significantly higher AL post-operatively (normalized flow mean ± SD: 459±221 mL/min, P=0.003, N=9, Figure 2A,2B). Despite relevant AL initially, the magnitude of the AL still decreased over the observation period (Figure 2C). At termination of the experiment, 5/9 (55.6%) of these lungs were still leaking (median drain time: 273 minutes, IQR, 207–435 minutes, N=5), and intrinsic sealing for the remaining lungs occurred within a median of 115 minutes (IQR, 52–245 minutes, N=4). The shortest time until intrinsic sealing was observed for the lung affected with a ⌀0.5 mm bronchiole lesion (50 minutes, N=1).
Bronchiole diameter and leakage capabilities
Comparing both sizes of bronchioles, >⌀1.5 mm lesions (N=11) were found to require longer time until hemostasis then ⌀0.5 mm lesions (N=4) (mean ± SD: 6.0±2.7 vs. 2.0±0 minutes, P=0.012). However, no significant difference was found between >⌀1.5 mm/⌀0.5 mm lesions for MLP (median ± IQR, 5±2 vs. 7±5 cmH2O, P=0.226) nor mechanical ventilator AL (median ± IQR, 581±1,012 vs. 140±305 mL/min, P=0.104) during the live observation. Furthermore, post-mortem MLP did not differ significantly between >⌀1.5 mm/⌀0.5 mm (median ± IQR, 6±13 vs. 10±41 cmH2O, P=0.199) A trend was seen for higher AL alive and lower MLP post-mortem for the >⌀1.5 mm lesions. Of note, N=2 lesions were excluded from MLP analysis in the >⌀1.5 mm subgroup due to blood contact during obduction, which possibly influenced the MLP (15 and 45 cmH2O).
Macroscopy and histology
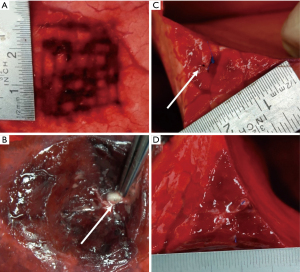
A rapid intrinsic sealing of the in vivo superficial parenchymal lesions was observed intraoperatively (illustrated in Video 1). At obduction, all superficial parenchymal lesions of the in vivo model were covered with a coagulated fibrin sealing layer (Figure 3A,3B). For the lesions involving bronchioles, the bronchioles appeared contracted or were no longer macroscopically visible (Figure 3C,3D).
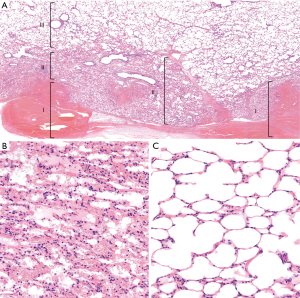
Histology for the superficial parenchymal lesions (N=2 lesions after <12 h) revealed an area of alveolar collapse surrounding the injured sites, extending beyond the coagulated parenchymal incisions. Within this area of alveolar collapse, intra-alveolar hemorrhage was observed. Going further proximally, the normal air contents of the parenchyma gradually returned. Minimal influx of immune cells was seen, but no notable immune response was noted (Figure 4). The histological response for the lesions involving bronchioles was similar to the parenchymal lesions in vivo [Supplementary file (Appendix 1), Figure S3].
Discussion
This study revealed that superficial parenchymal defects are unsuited for lung sealant testing in a healthy ovine model of PAL, due to rapid intrinsic sealing. Sequential amputation lesions involving bronchioles do result in AL in 56% of cases (median observation time: 4:33 h), but with 44% of cases sealing intrinsically in a median time of 1:55 h. Bronchioles of >⌀1.5 mm are preferable over ⌀0.5 mm, due to a trend towards a longer time to sealing. These lesions can be created in a reproducible manner and therefore seem valid for acute lung sealant testing. Nevertheless, the time to sealing is still too short for analysis of pPAL. These observed intrinsic sealing mechanisms could explain a translational gap in lung sealant research, especially in studies lacking negative control groups to demonstrate pPAL.
Comparison to literature
Presently, many clinical studies have been performed, testing numerous different sealant products. Some sealants were shown to be effective, but there were fluctuating results and no clear evidence based recommendations (16). As we suggest, disappointing results in clinical studies may be a consequence of a translational gap when negative controls are not used in the preclinical phase to ensure significant PAL. For example, effectiveness of fibrin glue was seen in an animal study that compared bursting pressures of fibrin glue with sutures in a rabbit model, without a negative control group (i.e., lesion with no treatment) (30). In contrast, the animal study by McCarthy et al., which included a negative control group to demonstrate significant PAL, showed no real effect of fibrin glue. Fifty percent of the animals in their study were free of AL after 24 h in both the fibrin glue and negative control groups (i.e. similar effectiveness to natural healing) (24). This study is in line with clinical literature, as fibrin glue has not been found significantly effective for reducing the length of hospital stay in the clinical trials by Fleisher, Wong and Mouritzen (31-33).
Our study confirms the hypothesis that it is only possible to induce PAL in healthy animal lungs when creating lesions with adequate depth, lacerating terminal bronchiole (21). Previous studies with negative control groups had similar findings, as amputations of lung lobes or deep incisions, likely resulting in bronchiolar leaks, resulted in AL complications [Supplementary file (Appendix 1), Table S1] (21,34-36). As an example, Ranger et al. produced similar bronchiolar leaks of >⌀1.5 mm and found higher AL (mean 1.4 L/min, range 0.6–3.5 L/min) in small dogs with similar drainage settings, as compared to our finding. These differences may be explained by sampling variation or lobe/species effects (21). In contrast, various superficial lesions did not lead to AL problems (18,37-39). Also in larger parenchymal lesions, AL may persist, although in small dogs and pigs (17–35 kg) it is unclear whether this is due to some degree of bronchiolar laceration at depths of ~5 mm (24,40).
Intrinsic sealing mechanisms
Pathologically, the intrinsic sealing layer is hypothesized to be more than just superficial coagulation, an extended area of alveolar collapse might be an explanation for the strong and rapid intrinsic sealing seen in this study. Similar mechanisms have previously been described, including compressed or partially aerated alveoli, subpleural alveolar edema, intra-alveolar bleeding and intra-alveolar deposition of fibrin strands (19,41-43). Based on these studies and the findings in our study, the following mechanisms are proposed to be principally responsible: first, alveolar collapse is physically initiated by air escaping from the subpleural alveolar layer, by direct impression of the surgical instrument and a lower resistance to flow of this distal AL path (41). Simultaneously, hemorrhage spreads through the pores of Kohn and channels of Lambert (43). Blood proteins and lipids are known to cause an inhibition of surfactant function, increasing alveolar surface tension (Laplace’s law, ) and thereby attenuating alveolar collapse biophysically (44-48). This results in a reduction of the common radius of the subpleural alveoli, increasing the resistance and reducing the AL by Poiseuille’s law. This attenuates coagulation mechanisms analogous to vasoconstriction in a bleeding wound (41,43). Inflammatory mechanisms were not seen in our sample (due to the short follow-up period), but may further enhance sealing through alveolar compression, due to capillary enlargement and edema as previously described (19,42,43). Pleural apposition may also reduce AL, but we hypothesize this to play a small role based on our post-mortem observations and rapid sealing seen intra-operatively (27).
These micromechanics could also provide a biophysical explanation for the lower intrinsic sealing capabilities in diseased human lungs. Pulmonary emphysema is a major risk factor for pPAL, and emphysema scores have shown to be predictive of pPAL (1,49-51). Conceptually, in emphysematous lungs, lower pressures are required to prevent alveolar collapse, due to the larger radius of emphysematous alveoli (Laplace’s law). Therefore, it is hypothesized that the proposed biophysical intrinsic sealing mechanisms will not induce the same degree of intrinsic sealing by alveolar collapse as seen in the healthy, non-emphysematous ovine lungs. Further study of intrinsic sealing mechanisms in emphysematous lungs might help us understand mechanisms of pPAL, which may improve treatment of these lesions.
The reduction in bronchiole diameter which was observed macroscopically post-mortem, may be due to sympathetic nervous system activation or bronchiole collapse due to absence of hyaline cartilage supporting structures. Especially bronchioles <1 mm lack supporting hyaline cartilage, which was also seen histologically in our study [Supplementary file (Appendix 1), Figure S3] (52). In this case, as surrounding alveolar tissues collapse due to a steal phenomenon from the leaking orifice, especially bronchioles without cartilage support could collapse and seal intrinsically (as shown in Figure 3D). A bronchiole of >⌀1.5 mm appears preferable over ⌀0.5 mm to prevent rapid and strong intrinsic sealing in the acute aerostasis model from this perspective (21).
There might be species specific considerations for sheep, which explain the strong and rapid intrinsic sealing. These include intra-vascular macrophages and an increased white blood cell count, which may cause immune mediated attenuation of intrinsic sealing mechanisms (53-55). Coagulation times are comparable to those of humans (55). High doses of intravenous propofol (dissolved in lipids) were sometimes required during surgical anesthesia, and it was hypothesized that this might result in hyperlipidemia and subsequently increased blood viscosity and coagulability. However, previous in vitro and in vivo experiments have shown no effect on blood viscosity, and even describe a reduced platelet aggregation (56).
Translational value of air leak models
The face validity of a bronchiolar AL model is reduced, as clinical pPAL mostly arises from the alveoli in superficial parenchymal injury, such as from the dissection of fissures or pleural adhesions. As an acute aerostasis model, the construct validity remains preserved. PAL >500 mL/min intraoperatively was found to predict pPAL, and AL sizes of 150–400 mL/min have been used for sealant application in a clinical trial, with positive results (57,58). Thus, the bronchiole AL seems sufficient for acute sealant testing. The model allows testing on an actively leaking lung, after thoracic closure, at least before intrinsic sealing of specific bronchiole lesion occurs. Thereby, other important mechanisms such as coagulation, immune response, pleural mechanisms and physiological breathing can be replicated, which is presently not feasible in animal-free alternatives (59,60).
Disease models may be suitable to induce pPAL, but it remains unclear how long PAL should last in a model for sealant testing. Based on the hypothesized micromechanics, emphysema may result in longer pPAL, while heparinization seems a less effective disease model, as this would not inhibit alveolar collapse, and might even enhance spreading of blood through the alveoli. One study found significantly lower bursting pressures for sealants applied to emphysematous lungs, but did not measure PAL postoperatively (26). The lower bursting pressures could be biochemically ascribed to a lower crosslink density on emphysematous lungs, which may be another explanation for a translational gap (26). When developing disease models, disease induction might be associated with an increased baseline variation, higher sample size requirement and decreased animal welfare, which needs to be weighed against the potential added benefit of these models.
Limitations
These experiments offer a valuable addition to the present preclinical literature, by confirming the possibility of a translational gap and offering novel insights into intrinsic sealing mechanisms. However, based on these experiments, it is not possible to define a global standardized model for pPAL and lung sealant research. For this, further investigations are needed. First of all, it needs to be studied how long PAL needs to be present in a model for sealant testing to ensure accurate translation. Formulated differently, the time until intrinsic sealing of human lungs when a sealant is applied needs to be studied. Disease models are hypothesized to be suitable options for developing longer leaking PAL, but remain entirely unconfirmed, and come with added costs (higher sample size, decreased animal welfare). Another option might be to create leaks in large bronchi (e.g., segmental bronchi), but this further decreases the face validity. Finally, due to small sample size and heterogenous methods in the present study, all findings described here should be interpreted with appropriate caution and need to be confirmed in a-priori statistically powered experiments.
Recommendations for further research
Clinicians and experimental researchers should be aware of the intrinsic sealing mechanisms of healthy animal lungs. Success of sealants in animal studies should therefore be interpreted cautiously. In new experimental design, negative control groups should always be considered to measure the actual treatment effect. PAL may be created in animal models by creating especially large defects or lacerating bronchioles of >⌀1.5 mm (21,24). For development of a global standardized pPAL model, further research is required into the requirements of valid pPAL models (e.g., duration of PAL), and methods for inducing longer PAL (e.g., emphysema or heparinization) (25,26). Such disease models could also be used to study mechanisms of PAL in clinical scenario’s, such PAL arising from stapler lines. The understanding of PAL sealing mechanisms is still incomplete, and the proposed mechanisms should be further investigated. With a thorough understanding hereof, a clinical solution for the problem of pPAL might be discovered, for example by making the surgical treatment better synergize with the underlying mechanisms of the specific lesion type. The conduction of animal systematic reviews and adherence to ARRIVE guidelines should be encouraged to further improve scientific rigorousness of animal studies (20,61).
Conclusions
Superficial parenchymal lesions exhibit an intrinsic sealing mechanism in a healthy ovine lung model, explained pathologically by an extended area of alveolar collapse attenuating coagulation mechanisms. These mechanisms may reduce model validity and contribute to a translational gap in lung sealant research, especially when negative control groups are not used. Experimental researchers should account for these mechanisms when designing experiments, to improve clinical applicability. One such approach for acute lung sealant testing in an ovine model, is to create deep parenchymal defects involving at least bronchioles of >⌀1.5 mm. Further study into PAL models is required to develop and validate a universal standardized acute aerostasis model.
Acknowledgments
The authors would like to thank Nicole Calon, Jort Evers, Alex Hanssen, Stefanie Schönfeld, Maikel School and Pim van Sambeeck (all are affiliated with the Radboud University Medical Center in Nijmegen, The Netherlands) for their help during the experiments, Pieter Verbost and Manon van Hulzen for their help with the experimental protocols and licenses and all other supporting personnel in the animal laboratory for their great caretaking of all laboratory animals. We want to thank the Radboudumc Department of Statistics for its help with the statistical analysis and the Department of Pathology for helping with processing the histological samples. Part of this work has previously been presented at the SEOHS 2022 conference in the Netherlands. Finally, we thank Willem de Boode for help with conceptual understanding of the intrinsic sealing mechanism.
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-180/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-180/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-180/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-180/coif). EAR is an employee of GATT Technologies B.V. and HvG was a scientific advisor for GATT Technologies B.V. until 31 December 2021, but not in relation to lung sealing technology. BPH received funding and study materials through the institution for conduction of the study from GATT Technologies B.V. The other authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Attaar A, Tam V, Nason KS. Risk Factors for Prolonged Air Leak After Pulmonary Resection: A Systematic Review and Meta-analysis. Ann Surg 2020;271:834-44. [Crossref] [PubMed]
- Attaar A, Luketich JD, Schuchert MJ, et al. Prolonged Air Leak After Pulmonary Resection Increases Risk of Noncardiac Complications, Readmission, and Delayed Hospital Discharge: A Propensity Score-adjusted Analysis. Ann Surg 2021;273:163-72. [Crossref] [PubMed]
- Brunelli A, Xiume F, Al Refai M, et al. Air leaks after lobectomy increase the risk of empyema but not of cardiopulmonary complications: a case-matched analysis. Chest 2006;130:1150-6. [Crossref] [PubMed]
- Liang S, Ivanovic J, Gilbert S, et al. Quantifying the incidence and impact of postoperative prolonged alveolar air leak after pulmonary resection. J Thorac Cardiovasc Surg 2013;145:948-54. [Crossref] [PubMed]
- Yoo A, Ghosh SK, Danker W, et al. Burden of air leak complications in thoracic surgery estimated using a national hospital billing database. Clinicoecon Outcomes Res 2017;9:373-83. [Crossref] [PubMed]
- Singhal S, Ferraris VA, Bridges CR, et al. Management of alveolar air leaks after pulmonary resection. Ann Thorac Surg 2010;89:1327-35. [Crossref] [PubMed]
- Okereke I, Murthy SC, Alster JM, et al. Characterization and importance of air leak after lobectomy. Ann Thorac Surg 2005;79:1167-73. [Crossref] [PubMed]
- Zaraca F, Brunelli A, Pipitone MD, et al. A Delphi Consensus report from the "Prolonged Air Leak: A Survey" study group on prevention and management of postoperative air leaks after minimally invasive anatomical resections. Eur J Cardiothorac Surg 2022;62:ezac211. [Crossref] [PubMed]
- Kobayashi H, Sekine T, Nakamura T, et al. In vivo evaluation of a new sealant material on a rat lung air leak model. J Biomed Mater Res 2001;58:658-65. [Crossref] [PubMed]
- Yanagihara T, Maki N, Wijesinghe AI, et al. Efficacy of Alaska Pollock Gelatin Sealant for Pulmonary Air Leakage in Porcine Models. Ann Thorac Surg 2022;113:1641-7. [Crossref] [PubMed]
- Belda-Sanchís J, Serra-Mitjans M, Iglesias Sentis M, et al. Surgical sealant for preventing air leaks after pulmonary resections in patients with lung cancer. Cochrane Database Syst Rev 2010;2010:CD003051. [Crossref] [PubMed]
- Singhal S, Shrager JB. Should buttresses and sealants be used to manage pulmonary parenchymal air leaks? J Thorac Cardiovasc Surg 2010;140:1220-5. [Crossref] [PubMed]
- Zhou J, Lyu M, Pang L, et al. Efficiency and safety of TachoSil® in the treatment of postoperative air leakage following pulmonary surgery: a meta-analysis of randomized controlled trials. Jpn J Clin Oncol 2019;49:862-9. [Crossref] [PubMed]
- McGuire AL, Yee J. Clinical outcomes of polymeric sealant use in pulmonary resection: a systematic review and meta-analysis of randomized controlled trials. J Thorac Dis 2018;10:S3728-39. [Crossref] [PubMed]
- Malapert G, Hanna HA, Pages PB, et al. Surgical sealant for the prevention of prolonged air leak after lung resection: meta-analysis. Ann Thorac Surg 2010;90:1779-85. [Crossref] [PubMed]
- Aprile V, Bacchin D, Calabrò F, et al. Intraoperative prevention and conservative management of postoperative prolonged air leak after lung resection: a systematic review. J Thorac Dis 2023;15:878-92. [Crossref] [PubMed]
- Brunelli A, Bölükbas S, Falcoz PE, et al. Exploring consensus for the optimal sealant use to prevent air leak following lung surgery: a modified Delphi survey from The European Society of Thoracic Surgeons. Eur J Cardiothorac Surg 2021;59:1265-71. [Crossref] [PubMed]
- Poticha SM, Macaladad F, Lewis FJ. The control of air leaks following subsegmental pulmonary resections. Surg Gynecol Obstet 1965;120:803-9. [PubMed]
- Kausel HW. LIndskog GE. The healing of raw lung surfaces after experimental segmental resection. J Thorac Surg 1955;29:197-211. [Crossref] [PubMed]
- Hermans BP, Poos SEM, van Dort DIM, et al. Evaluating and developing sealants for the prevention of pulmonary air leakage: A systematic review of animal models. Lab Anim 2023; Epub ahead of print. [Crossref] [PubMed]
- Ranger WR, Halpin D, Sawhney AS, et al. Pneumostasis of experimental air leaks with a new photopolymerized synthetic tissue sealant. Am Surg 1997;63:788-95. [PubMed]
- Annabi N, Zhang YN, Assmann A, et al. Engineering a highly elastic human protein-based sealant for surgical applications. Sci Transl Med 2017;9:eaai7466. [Crossref] [PubMed]
- Elvin CM, Vuocolo T, Brownlee AG, et al. A highly elastic tissue sealant based on photopolymerised gelatin. Biomaterials 2010;31:8323-31. [Crossref] [PubMed]
- McCarthy PM, Trastek VF, Bell DG, et al. The effectiveness of fibrin glue sealant for reducing experimental pulmonary ari leak. Ann Thorac Surg 1988;45:203-5. [Crossref] [PubMed]
- Balakrishnan B, Payanam U, Laurent A, et al. Efficacy evaluation of anin situforming tissue adhesive hydrogel as sealant for lung and vascular injury. Biomed Mater 2021; [Crossref] [PubMed]
- Gika M, Kawamura M, Izumi Y, et al. The short-term efficacy of fibrin glue combined with absorptive sheet material in visceral pleural defect repair. Interact Cardiovasc Thorac Surg 2007;6:12-5. [Crossref] [PubMed]
- Pedersen TB, Honge JL, Pilegaard HK, et al. Comparative study of lung sealants in a porcine ex vivo model. Ann Thorac Surg 2012;94:234-40. [Crossref] [PubMed]
- Mentzer SJ, Tsuda A, Loring SH. Pleural mechanics and the pathophysiology of air leaks. J Thorac Cardiovasc Surg 2018;155:2182-9. [Crossref] [PubMed]
- Clark JM, Cooke DT, Brown LM. Management of Complications After Lung Resection: Prolonged Air Leak and Bronchopleural Fistula. Thorac Surg Clin 2020;30:347-58. [Crossref] [PubMed]
- Bergsland J, Kalmbach T, Balu D, et al. Fibrin seal--an alternative to suture repair in experimental pulmonary surgery. J Surg Res 1986;40:340-5. [Crossref] [PubMed]
- Wong K, Goldstraw P. Effect of fibrin glue in the reduction of postthoracotomy alveolar air leak. Ann Thorac Surg 1997;64:979-81. [Crossref] [PubMed]
- Mouritzen C, Drömer M, Keinecke HO. The effect of fibrin glueing to seal bronchial and alveolar leakages after pulmonary resections and decortications. Eur J Cardiothorac Surg 1993;7:75-80. [Crossref] [PubMed]
- Fleisher AG, Evans KG, Nelems B, et al. Effect of routine fibrin glue use on the duration of air leaks after lobectomy. Ann Thorac Surg 1990;49:133-4. [Crossref] [PubMed]
- Wilder RJ, Playforth H, Bryant M, et al. The use of plastic adhesive in pulmonary surgery. J Thorac Cardiovasc Surg 1963;46:576-88. [Crossref] [PubMed]
- Nuchprayoon C, Tamayo AG, Reimann AF, et al. The use and tissue reaction of a biologic adhesive in the prevention of air leak following a transection of the lung. Dis Chest 1968;53:445-52. [Crossref] [PubMed]
- Kanzaki M, Yamato M, Yang J, et al. Dynamic sealing of lung air leaks by the transplantation of tissue engineered cell sheets. Biomaterials 2007;28:4294-302. [Crossref] [PubMed]
- Feito BA, Rath AM, Longchampt E, et al. Experimental study on the in vivo behaviour of a new collagen glue in lung surgery. Eur J Cardiothorac Surg 2000;17:8-13. [Crossref] [PubMed]
- Getman V, Devyatko E, Wolner E, et al. Fleece bound sealing prevents pleural adhesions. Interact Cardiovasc Thorac Surg 2006;5:243-6. [Crossref] [PubMed]
- Büyükkale S, Çıtak N, İşgörücü Ö, et al. The effect of sodium hyaluronate-carboxymethyl cellulose membrane in the prevention of parenchymal air leaks: an experimental and manometric study in rats. Tuberk Toraks 2017;65:265-70. [Crossref] [PubMed]
- Luh SP, Chou HH, Tsai TP, et al. Effect of Surgecel coverage with topical electrocauterization for preventing and sealing pulmonary air leakage. Int Surg 2004;89:190-4. [PubMed]
- Joannides M, Hesse AL, Joannides M Jr. Surgical wounds of the lung; the mode of healing of pulmonary tissue. J Thorac Surg 1949;18:695-706. [Crossref] [PubMed]
- Findlay CW Jr. The healing of surgical wounds of the lung with particular reference to segmental lobectomy. J Thorac Surg 1950;20:823-34. [Crossref] [PubMed]
- Wheeldon EB, Mariassy AT, McSporran KD. The pleura: a combined light microscopic and scanning and transmission electron microscopic study in the sheep. II. Response to injury. Exp Lung Res 1983;5:125-40. [Crossref] [PubMed]
- Zuo YY, Veldhuizen RA, Neumann AW, et al. Current perspectives in pulmonary surfactant--inhibition, enhancement and evaluation. Biochim Biophys Acta 2008;1778:1947-77. [Crossref] [PubMed]
- Raghavendran K, Willson D, Notter RH. Surfactant therapy for acute lung injury and acute respiratory distress syndrome. Crit Care Clin 2011;27:525-59. [Crossref] [PubMed]
- Holm BA, Keicher L, Liu MY, et al. Inhibition of pulmonary surfactant function by phospholipases. J Appl Physiol (1985) 1991;71:317-21. [Crossref] [PubMed]
- Holm BA, Wang Z, Notter RH. Multiple mechanisms of lung surfactant inhibition. Pediatr Res 1999;46:85-93. [Crossref] [PubMed]
- Walter F. Boron ELB. Medical Physiology Medical Physiology 3rd edition ed. Philadelphia: Elsevier; 2017.
- DeCamp MM, Blackstone EH, Naunheim KS, et al. Patient and surgical factors influencing air leak after lung volume reduction surgery: lessons learned from the National Emphysema Treatment Trial. Ann Thorac Surg 2006;82:197-206; discussion 206-7. [Crossref] [PubMed]
- Moon DH, Park CH, Kang DY, et al. Significance of the lobe-specific emphysema index to predict prolonged air leak after anatomical segmentectomy. PLoS One 2019;14:e0224519. [Crossref] [PubMed]
- Murakami J, Ueda K, Tanaka T, et al. Grading of Emphysema Is Indispensable for Predicting Prolonged Air Leak After Lung Lobectomy. Ann Thorac Surg 2018;105:1031-7. [Crossref] [PubMed]
- Ball M, Hossain M, Padalia D. Anatomy, Airway. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.; 2022.
- Delano ML, Mischler SA, Underwood WJ. Biology and Diseases of Ruminants: Sheep, Goats, and Cattle. Laboratory Animal Medicine 2002:519-614. doi:
10.1016/B978-012263951-7/50017-X .10.1016/B978-012263951-7/50017-X - Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol 2008;295:L379-99. [Crossref] [PubMed]
- Wilhelmi MH, Tiede A, Teebken OE, et al. Ovine blood: establishment of a list of reference values relevant for blood coagulation in sheep. ASAIO J 2012;58:79-82. [Crossref] [PubMed]
- Reinhart WH, Felix Ch. Influence of propofol on erythrocyte morphology, blood viscosity and platelet function. Clin Hemorheol Microcirc 2003;29:33-40. [PubMed]
- Brunelli A, Salati M, Pompili C, et al. Intraoperative air leak measured after lobectomy is associated with postoperative duration of air leak. Eur J Cardiothorac Surg 2017;52:963-8. [Crossref] [PubMed]
- Zaraca F, Vaccarili M, Zaccagna G, et al. Cost-effectiveness analysis of sealant impact in management of moderate intraoperative alveolar air leaks during video-assisted thoracoscopic surgery lobectomy: a multicentre randomised controlled trial. J Thorac Dis 2017;9:5230-8. [Crossref] [PubMed]
- Klassen C, Eckert CE, Wong J, et al. Ex Vivo Modeling of Perioperative Air Leaks in Porcine Lungs. IEEE Trans Biomed Eng 2018;65:2827-36. [Crossref] [PubMed]
- Horvath MA, Hu L, Mueller T, et al. An organosynthetic soft robotic respiratory simulator. APL Bioeng 2020;4:026108. [Crossref] [PubMed]
- Percie du Sert N, Hurst V, Ahluwalia A, et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol 2020;18:e3000410. [Crossref] [PubMed]


