
Nadir oxygen delivery during cardiopulmonary bypass in acute type A aortic dissection repair
Highlight box
Key findings
• The application of a goal-directed perfusion (GDP) strategy to maintain DO2 ≥280 mL/(min·m2) during cardiopulmonary bypass (CPB) can significantly decrease 90-day postoperative mortality of acute type A aortic dissection (ATAAD) patients.
What is known and what is new?
• Recent studies indicated that the application of a GDP strategy to maintain DO2 ≥280 mL/(min·m2) during CPB could ensure organ perfusion effectively and decrease postoperative morbidity rate.
• We found that maintaining DO2 ≥280 mL/(min·m2) during CPB was associated with decreased 90-day postoperative mortality of ATAAD patients.
What is the implication, and what should change now?
• The application of a GDP strategy to maintain DO2 ≥280 mL/(min·m2) during CPB might be effective in reducing the 90-day mortality of ATAAD patients. Consequently, it is suggested to increase CPB flow and hematocrit (HCT) level to maintain DO2 ≥280 mL/(min·m2) during CPB in ATAAD repair.
Introduction
Acute type A aortic dissection (ATAAD) is a catastrophic disease with high mortality that involves many organs (1). Consequently, emergent aortic repair remains the best therapeutic option for ATAAD. Despite the improvements in diagnosis and surgical techniques of ATTAD, the aortic repair is still associated with high mortality and morbidity rates, including massive hemorrhage, delirium, hyperlactatemia, acute kidney injury (AKI), and acute lung injury (2-6). Recent studies indicated that the application of a goal-directed perfusion (GDP) strategy to maintain DO2 ≥280 mL/(min·m2) during cardiopulmonary bypass (CPB) could ensure organ perfusion effectively and decrease postoperative morbidity rate (7,8). However, the included patients in these studies were those who underwent simple congenital heart disease correction or adult valve replacement or repair, or coronary artery bypass grafting. The minimum threshold of intraoperative DO2 for ATAAD patients had not been determined. Moreover, the relationship between intraoperative DO2 and the clinical prognosis of ATAAD patients was rarely reported (9). Therefore, the current study was performed to investigate the relationship between maintaining intraoperative DO2 ≥280 mL/(min·m2) and the 90-day postoperative mortality of ATAAD patients, so as to provide a theoretical basis for the clinical implementation of the GDP strategy in ATAAD repair. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-561/rc).
Methods
Study design
This prospective observational cohort study was carried out in the Department of Cardiovascular Surgery, Nanfang Hospital, Southern Medical University, China, from January 2018 to July 2022. Clinical data were collected from the electronic medical record database. The included patients were divided into hypoxic group [DO2 <280 mL/(min·m2); n=23] and normoxic group [DO2 ≥280 mL/(min·m2); n=155]. The preoperative, intraoperative, postoperative and survival data of the patients were compared between both groups. Additionally, the relationship between maintenance of DO2 ≥280 mL/(min·m2) during CPB and 90-day postoperative mortality rate of ATAAD patients was studied. The study was approved by the Biomedical Research Ethics Committee of Nanfang Hospital of Southern Medical University (No. NFEC-2022-521) and was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All patients provided written informed consent to participate before enrolling in the study.
Patient population
One hundred seventy-eight patients who met the ATAAD diagnostic criteria in 2022 ACC/AHA Guidelines for the Diagnosis and Management of Aortic Diseases (10) and underwent emergent ATAAD repair (Sun’s procedure) under CPB were enrolled in this study. Furthermore, the patients who died 24 hours postoperatively or had preoperative abdominal organ malperfusion were excluded in the study (11). The specific process of screening patients is shown in Figure 1.
Anesthesia, surgery, and CPB management
General anesthesia was induced with etomidate 0.2 mg/kg, vecuronium bromide 0.1 mg/kg, sufentanil 0.5 to 1 µg/kg, and propofol 2 to 2.5 mg/kg. The trachea was intubated, and mechanical ventilation was then started.
All the operation was conducted through median sternotomy. After systemic heparinization, cannulas were inserted into the right axillary artery, the left common carotid artery, the right atrium, and the right superior pulmonary vein, respectively. CPB was started when the activated clotting time of whole blood was more than 480 s. Surgery was performed under a targeted pump flow (PF) rate between 2.2 and 3.4 L/(min·m2) with mean arterial pressure (MAP) of 40 to 80 mmHg, hematocrit (HCT) of 20 to 28%, partial pressure of arterial oxygen (PaO2) of 200 to 300 mmHg, arterial oxygen saturation (SaO2) above 95% and mixed venous oxygen saturation (SvO2) of 65 to 85%. The blood monitoring unit (BMU40, Maquet, Germany) was applied to achieve GDP strategy and gain some relevant information dynamically (including the value of SvO2, HCT and DO2, etc.) every five minutes. Dubois Formula was applied to calculate the body surface area. The remaining formulas are as follows: oxygen delivery: DO2 [mL/(min·m2)] = PF [L/(min·m2)] × [HCT (%)/3 × 1.36 × SaO2 (%) + [PaO2 × 0.003)]} ×10; oxygen consumption: VO2 [mL/(min·m2)] = PF [L/(min·m2)] × {[HCT (%)/3 × 1.36 × (SaO2 − SvO2) (%)] + [PaO2 (mmHg) ×0.003)]} ×10; oxygen delivery/oxygen consumption ratio =DO2/VO2.
Aortic proximal manipulations, including aortic valve repair, sinus of valsalva reconstruction, and composite valved graft replacement, were performed after the heart stopped beating. CPB was discontinued and bilateral cerebral perfusion was conducted when the nasopharyngeal temperature reached 26 ℃ and the bladder temperature reached 28 ℃ approximately, and then total aortic arch replacement combined with frozen elephant trunk implantation was performed. Rewarming was conducted after the end of deep hypothermia circulatory arrest (DHCA). Modified ultrafiltration (MUF) was performed in some patients, and all the CPB cannulas were removed. All the patients were transported intubated to the intensive care unit to recover.
Data collection and definitions
The clinical data of the patients were retrieved and collected through the hospital information system. Preoperative variables were age, sex, height, weight, European cardiac operative risk evaluation (EuroScore II), and preoperative left ventricular ejection fraction. Operative variables were type of surgery, CPB time, aortic cross-clamping time, DHCA time, ultrafiltration method, type of cardioplegic solution, mode of cardiac rebeating, MAP during CPB, minimum nasopharyngeal and bladder temperature, PF, HCT, SvO2, and DO2. Systolic blood pressure, diastolic blood pressure, central venous pressure, and blood lactate acid when CPB was terminated were also included.
Postoperative variables were 24-h thoracic fluid content, thoracotomy hemostasis surgery, RBC infusion rate, anesthesia time, mechanical ventilation time, the application rate of CRRT, the occurrence rate of cerebral apoplexy, postoperative length of stay, and perioperative mortality. Follow-up date and the patient’s survival situation would be directly inquired in the electronic medical record system or the follow-up with the patient or his family members.
Statistical analysis
Normally distributed variables were presented as mean ± standard deviation with the application of Student’s t-test for the comparison between groups. Non-normally distributed variables were expressed as a median and interquartile range [M (P25, P75)] with the application of Mann-Whitney U test for comparison between groups. Categorical variables were presented as frequency (percentage) with the application of Chi-square test or Fisher’s exact test for comparison between groups. Kaplan-Meier method was used to analyze the survival data, and then the survival curves were drawn. Multivariable regression analyses were conducted to explore relationships between perioperative parameters and the postoperative survival rate of the ATAAD patients using binary logistic modeling techniques. The differences were considered statistically significant if P<0.05. Statistical analyses were performed using the statistical software SPSS 19.0 (IBM SPSS, Armonk, NY, United States).
Results
Preoperative and intraoperative data characteristics
Among the 178 patients, 158 (88.8%) of them were men, 20 (11.2%) of them were women. Moreover, the average age of the patients in the hypoxic group and the hyperoxic group was 50.9±12.5 and 51.0±13.1 years old, respectively. None of patients in both groups have preoperative shock, preoperative intubation, and preoperative inotropic use. Compared with the hypoxic group, in the normoxic group, the maximum and average perfusion pressure during CPB were significantly lower, while the application rate of MUF (57.4%), the HCT at three different time points (the moment when the patients entered the operating room, the moment when the value of HCT was the lowest during CPB, and the moment when CPB was stopped), and other relevant indicators (including PF, HCT, and DO2) were significantly higher (P<0.05). Statistical differences could not be observed with regard to other indicators (Table 1).
Table 1
| Baseline and intraoperative indicator | DO2 ≥280 mL/(min·m2) (n=155) | DO2 <280 mL/(min·m2) (n=23) | P value |
|---|---|---|---|
| Male | 138 (89.0) | 20 (87.0) | 0.77 |
| Age (year) | 50.9±12.5 | 51.0±13.1 | 0.98 |
| Height (cm) | 168.7±6.6 | 168.7±6.1 | 0.99 |
| Weight (kg) | 71.0 [65.0, 80.0] | 72.0 [61.0, 89.0] | 0.90 |
| Body surface area (m2) | 1.8 [1.7, 1.9] | 1.8 [1.6, 2.1] | 0.90 |
| Preoperative left ventricular ejection fraction (%) | 65.0±5.2 | 67.1±5.7 | 0.11 |
| EuroScore II (score) | 8.0 [8.0, 10.0] | 8.0 [8.0, 11.0] | 0.80 |
| Concurrent CABG | 59 (38.1) | 7 (30.4) | 0.48 |
| Concurrent Bentall | 54 (34.8) | 7 (30.4) | 0.68 |
| CPB time (min) | 247.0 [215.0, 279.0] | 230.0 [217.0, 296.0] | 0.73 |
| ACC time (min) | 146.0 [127.0, 171.0] | 150.0 [114.0, 169.0] | 0.65 |
| DHCA time (min) | 25.0 [22.0, 29.0] | 28.0 [23.0, 31.0] | 0.08 |
| Indicators during CPB | |||
| Modified ultrafiltration | 89 (57.4) | 7 (30.4) | 0.02 |
| Total liquid balance (mL) | −2,515.0 [−4,100.0, −1,347.5] | −2,680.0 [−3,410.0, −1,380.0] | 0.92 |
| Intraoperative urine volume (mL) | 900.0 [450.0, 1,550.0] | 1,100.0 [560.0, 1,500.0] | 0.81 |
| DelNido cardioplegic solution | 37 (23.9) | 2 (8.7) | 0.10 |
| Cardiac rebeat automatically | 105 (67.7) | 16 (69.6) | 0.86 |
| Red blood cell transfusions volume (mL) | 400.0 [0.0, 600.0] | 400.0 [200.0, 800.0] | 0.53 |
| Mean arterial pressure (mmHg) | 56.3 [50.4, 60.0] | 59.1 [55.5, 60.8] | 0.28 |
| Minimum nasopharyngeal temperature (℃) | 23.1 [21.9, 24.3] | 22.4 [21.6, 23.9] | 0.45 |
| Minimum bladder temperature (℃) | 25.2 [23.8, 26.4] | 24.8 [23.6, 25.9] | 0.35 |
| Average flow [L/(min·m2)] | 2.7±0.2 | 2.3±0.2 | <0.001 |
| Average HCT (%) | 25.5 [24.4, 27.0] | 23.8 [22.8, 24.6] | <0.001 |
| Maximum perfusion pressure (mmHg) | 243.4±28.4 | 259.6±31.4 | 0.01 |
| Average perfusion pressure (mmHg) | 168.9 [156.3, 178.9] | 179.3 [164.4, 193.7] | 0.01 |
| Average SvO2 (%) | 76.8±5.5 | 75.4±7.5 | 0.42 |
| Average DO2 [mL/(min·m2)] | 328.1±28.4 | 257.5±18.4 | <0.001 |
| Oxygen delivery/oxygen consumption ratio | 3.9 [3.5, 4.4] | 3.5 [3.0, 3.9] | 0.05 |
| Immediate indicators when CPB stopped | |||
| Systolic blood pressure (mmHg) | 109.0 [102.0, 117.0] | 106.0 [100.0, 115.0] | 0.29 |
| Diastolic blood pressure (mmHg) | 61.3±10.8 | 59.8±9.5 | 0.55 |
| Central venous pressure (mmHg) | 7.0 [6.0, 9.0] | 7.0 [6.0, 10.0] | 0.61 |
| HCT when patients entered the operating room (%) | 33.65±6.16 | 30.78±7.21 | 0.05 |
| Minimum HCT during CPB (%) | 21 [19, 23] | 19 [17, 21] | 0.03 |
| HCT when CPB stopped (%) | 32.53±4.28 | 30.17±4.82 | 0.02 |
| Blood lactic acid when patients entered the operating room (mmol/L) | 0.90 [0.70, 1.40] | 0.90 [0.80, 1.20] | 0.93 |
| Maximum blood lactic acid during the bypass (mmol/L) | 4.10 [3.30, 5.80] | 4.50 [3.90, 5.60] | 0.32 |
| Blood lactic acid when CPB stopped (mmol/L) | 3.30 [2.10, 5.00] | 3.90 [3.00, 5.60] | 0.28 |
Normally distributed variables were presented as mean ± standard deviation. Non-normally distributed variables were expressed as a median (interquartile range). Categorical variables were presented as frequency (percentage). DO2, oxygen delivery; EuroScore II, European cardiac operative risk evaluation; Bentall surgery, aortic valve replacement + ascending aorta replacement + coronary artery bypass grafting; CABG, coronary artery bypass grafting; CPB, cardiopulmonary bypass; ACC, aortic cross-clamping; DHCA, deep hypothermia circulation arrest; HCT, hematocrit; SvO2, mixed venous oxygen saturation.
Postoperative and survival data characteristics
In the present study, a total of 23 patients (12.9%) died 90 days postoperatively. Compared with the hypoxic group, the 90-day postoperative mortality [16 (10.3%) vs. 7 (30.4%), χ2=7.2, P=0.007] and the application rate of CRRT [12 (7.7%) vs. 7 (30.4%), χ2=10.8, P=0.001] were significantly lower, while the postoperative length of stay in hospital was significantly longer in the normoxic group. No statistical differences could be observed with regard to other indicators (Table 2). The median follow-up time was 4 months (1–38 months). The survival analysis results indicated that the overall cumulative postoperative survival rate of ATAAD patients was 87.7%. In addition, compared with the hypoxic group, the survival rate of ATAAD patients was significantly higher (Z=9.201, log-rank P=0.0024), while the incidence of the postoperative application of CRRT in the normoxic group was significantly lower (Z=12.501, log-rank P=0.0004) (Figures 2,3). Univariate Cox regression demonstrated that the 90-day postoperative mortality in the normoxic group was reduced by 72.1% (HR =0.279, 95% CI: 0.114–0.681, P<0.01). Multivariate Cox regression showed that the prolonged CPB time and the postoperative application of CRRT were the risk factors for the 90-day postoperative mortality of ATAAD patients (Table 3).
Table 2
| Postoperative indicator | DO2 ≥280 mL/(min·m2) (n=155) | DO2 <280 mL/(min·m2) (n=23) | P value |
|---|---|---|---|
| ICU immediate HCT (%) | 31.00 (28.00, 34.00) | 29.00 (27.00, 32.00) | 0.15 |
| ICU 24 h HCT (%) | 29.51±6.28 | 27.13±5.47 | 0.09 |
| ICU immediate blood lactic acid (mmol/L) | 2.20 (1.60, 3.80) | 2.10 (1.50, 6.00) | 0.96 |
| ICU 24 h blood lactic acid (mmol/L) | 2.21±1.43 | 3.28±3.81 | 0.21 |
| Sternum closing time (min) | 202.4±64.0 | 192.6±72.2 | 0.51 |
| 24-h thoracic fluid content (mL) | 320.0 (220.0, 480.0) | 270.0 (220.0, 390.0) | 0.44 |
| Rethoracotomy hemostasis surgery | 10 (6.5) | 0 (0.0) | 0.36 |
| Postoperative blood transfusion (case) | 10 (6.452) | 3 (13.043) | 0.26 |
| Awake time (h) | 7.0 (4.0, 17.0) | 7.0 (6.0, 15.0) | 0.53 |
| Respirator time (h) | 45.0 (22.0, 82.0) | 46.0 (23.0, 67.0) | 0.98 |
| Intra-aortic balloon pump | 5 (3.2) | 1 (4.3) | 0.78 |
| Extracorporeal membrane oxygenation | 3 (1.9) | 1 (4.3) | 0.47 |
| Pulmonary complications | 61 (39.4) | 6 (26.1) | 0.22 |
| Continuous renal replacement therapy | 12 (7.7) | 7 (30.4) | 0.001 |
| Cerebral stroke/hemorrhage | 15 (9.7) | 3 (13.0) | 0.62 |
| Surgical wound bad healing | 8 (5.2) | 2 (8.7) | 0.49 |
| Over 30-day hospitalization | 22 (14.2) | 2 (8.7) | 0.47 |
| 90-day postoperative death | 16 (10.3) | 7 (30.4) | 0.007 |
| Postoperative hospitalization days (d) | 18.0 (14.0, 25.0) | 15.0 (10.0, 22.0) | 0.05 |
Normally distributed variables were presented as mean ± standard deviation. Non-normally distributed variables were expressed as a median (interquartile range). Categorical variables were presented as frequency (percentage). DO2, oxygen delivery; ICU, intensive care unit; HCT, hematocrit.
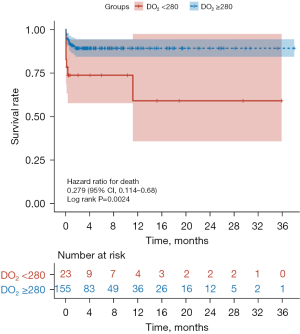
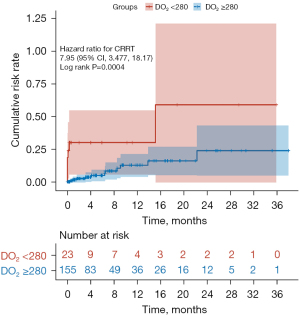
Table 3
| Possible risk factors | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Time of CPB | 1.018 (1.012–1.023) | <0.01 | 1.015 (1.008–1.022) | <0.01 | |
| Grouping (normoxic group) | 0.279 (0.114–0.681) | <0.01 | – | – | |
| Continuous renal replacement therapy | 7.95 (3.477–18.178) | <0.01 | 3.959 (1.201–13.051) | 0.02 | |
| Maximum blood lactic acid during the bypass | 1.235 (1.133–1.345) | <0.01 | – | – | |
| Red blood cell transfusions volume | 1.001 (0.998–1.002) | 0.08 | – | – | |
| EuroScore II | 1.313 (1.111–1.553) | <0.01 | – | – | |
| Modified ultrafiltration | 0.556 (0.241–1.291) | 0.17 | – | – | |
HR, hazard ratio; CI, confidence interval; CPB, cardiopulmonary bypass; EuroScore II, European cardiac operative risk evaluation.
Discussion
There are several significant findings in the present study. First, the application of GDP strategy to maintain DO2 ≥280 mL/(min·m2) during CPB can significantly decrease the 90-day postoperative mortality of ATAAD patients. Furthermore, multivariate Cox regression indicated that prolonged CPB time and higher postoperative application rate of CRRT were the risk factors for the 90-day postoperative mortality of ATAAD patients.
In ATAAD patients, the false cavity in the renal artery can compress the true cavity, which can result in renal blood supply insufficiency. The decrease in glomerular filtration rate leads to water-sodium retention and dilution anemia. In addition, hypoxia-ischemia can intensify inflammatory reactions in the body and result in heart failure. Moreover, high central venous pressure caused by heart failure can increase renal venous hydrostatic pressure. This vicious cycle can further reduce the glomerular filtration rate and lead to the occurrence of AKI (12). Previous studies indicated that the minimum DO2 <280 mL/(min·m2) during CPB was independently associated with the prolonged postoperative mechanical ventilation time, the increased incidence of AKI, and the increased mortality (9,13). Rasmussen et al. found that the postoperative peak creatinine, the incidence of AKI, and the application rate of CRRT were significantly higher when DO2 <272 mL/(min·m2) duration was longer than 30 min (14). Additionally, Mukaida et al. demonstrated that avoiding DO2 <300 mL/(min·m2) could significantly reduce the postoperative incidence of AKI, especially in patients with an estimated minimum HCT <23% after blood dilution or body surface <1.4 m2 (15). In the present study, the average DO2 [328.1±28.4 mL/(min·m2)] in the normoxic group was higher than the minimum threshold in the above studies. Moreover, in line with the above researches, the postoperative application rate of CRRT in the normoxic group was significantly lower than that in the hypoxic group.
Anastasiadis et al. (16) found that compared with monitoring the conventional indicators (PF, HCT, SvO2, blood lactic acid, and dynamic urine volume, etc.) intraoperatively, GDP strategy could significantly reduce the incidence of organ malperfusion, thereby achieving more “physiological” perfusion. Lukaszewski et al. used the linear regression model to draw the curves composed of PF and HCT as core indicators when DO2 was 280, 330 and 380 mL/(min·m2), respectively, thereby simplifying the clinical application of GDP strategy (17). Importantly, the calculation of DO2 in our present study was the same as that of Lukaszewski’s study. For ATAAD patients, only the axillary artery or femoral artery cannulation was performed before the end of DHCA during CPB. The compressed true lumen of peripheral vessels or cannula size-weight mismatch can lead to the increase of perfusion pressure and limit the increase of PF. In the present study, compared with the normoxic group, the maximum perfusion pressure was significantly higher, while the average PF was significantly lower in the hypoxic group. Therefore, we ought to decrease perfusion pressure by increasing the number of perfusion cannulas or shortening the CPB period before the end of DHCA to transit to the high PF phase as early as possible.
Besides, it is equally important to maintain HCT at a high level during CPB so as to increase the level of DO2. Recent studies indicated (18) that every 1% decrease in the nadir HCT during CPB could increase the incidence of postoperative AKI by 7%. In addition, the nadir HCT during CPB caused by hemodilution was independently correlated with postoperative hyperlactatemia and the mortality (19,20). Furthermore, the minimum HCT <25% and DO2 <300 mL/(min·m2) were independent risk factors for the occurrence of postoperative delirium (21). In our present study, the HCT in the normoxic group during CPB were significantly higher than that in the hypoxic group. Moreover, a higher application rate of MUF led to a higher HCT value when CPB was terminated in the normoxic group compared with the hypoxic group. Therefore, we can carry out the following measures to maintain a high level of HCT during CPB, including treating preoperative anemia, adopting an appropriate blood transfusion strategy during CPB, using mini CPB cannulas to reduce priming volume and alleviate hemodilution, and applying MUF when CPB is terminated, to reduce the exposure time of low DO2.
Gao et al. (22) showed that GDP strategy could reduce the overall incidence of postoperative AKI by decreasing the incidence of AKI stage 1. It did not make a difference to severe AKI (stages 2 or 3) and mortality. However, this study included ATAAD patients. In the present study, the Kaplan-Meier curve indicated that the 90-day postoperative mortality in the normoxic group was significantly lower than that in the hypoxic group. In line with Newland’s study (23), the Cox regression results in our study demonstrated that maintaining DO2 ≥280 mL/(min·m2) during CPB among ATAAD patients was associated with the decreased incidence of serious postoperative AKI events (AKI stage 2 or 3). In addition, some prediction models have proved that serum creatinine level and the application of CRRT were independent risk factors for the mortality of ATAAD patients (14,24). Hayward et al. (25) reported that only by maintaining DO2 ≥350 mL/(min·m2) during CPB could decrease the incidence of postoperative AKI in child patients. Therefore, the minimum DO2 threshold should be increased among the patients with higher potential DO2 requirements. Unlike other types of heart surgery, the oxygen debt accumulated in ATAAD patients during DHCA should be repaid in rewarming phase of CPB. In theory, maintaining a higher level of DO2 without prolonging the CPB time can offset the “high level of oxygen consumption” state of the body (26).
There are several limitations to our present studies. Firstly, there were not a few ATAAD patients who died before being operated or even transferred to the hospital. Secondly, this is a retrospective single-center study with a relatively small sample size which may weaken the evidence concluded from the present study and decrease the generalisability. Thirdly, a more sensitive indicator, the area under the curve of DO2 <280 mL/(min·m2) accumulation time, was not compared between both groups. Finally, the follow-up time was relatively short. Therefore, multicenter prospective studies with larger sample sizes and longer follow-up periods should be conducted in the future to prove the validity of the results in the present study.
Conclusions
The application of a GDP strategy to maintain DO2 ≥280 mL/(min·m2) during CPB might be effective in reducing the 90-day mortality of ATAAD patients. Consequently, it is suggested to decrease perfusion pressure as well as shorten the CPB period before the end of DHCA to increase PF to a high level as early as possible in the clinical setting. In addition, some measures aiming at maintaining a high level of HCT are equally of vital importance, including treating preoperative anemia, adopting appropriate blood transfusion strategy during CPB, using mini CPB cannula to reduce priming volume and alleviate hemodilution, and applying MUF when CPB is terminated.
Acknowledgments
Funding: This research was supported by grants from
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-561/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-561/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-561/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-561/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Biomedical Research Ethics Committee of Nanfang Hospital of Southern Medical University (No. NFEC-2022-521) and was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All patients provided written informed consent to participate before enrolling in the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zhu Y, Lingala B, Baiocchi M, et al. Type A Aortic Dissection-Experience Over 5 Decades: JACC Historical Breakthroughs in Perspective. J Am Coll Cardiol 2020;76:1703-13. [Crossref] [PubMed]
- Zhang CH, Ge YP, Zhong YL, et al. Massive Bleeding After Surgical Repair in Acute Type A Aortic Dissection Patients: Risk Factors, Outcomes, and the Predicting Model. Front Cardiovasc Med 2022;9:892696. [Crossref] [PubMed]
- Liu Z, Pang X, Zhang X, et al. Incidence and Risk Factors of Delirium in Patients After Type-A Aortic Dissection Surgery. J Cardiothorac Vasc Anesth 2017;31:1996-9. [Crossref] [PubMed]
- Wang S, Wang D, Huang X, et al. Risk factors and in-hospital mortality of postoperative hyperlactatemia in patients after acute type A aortic dissection surgery. BMC Cardiovasc Disord 2021;21:431. [Crossref] [PubMed]
- Zhang C, Bai H, Zhang L, et al. Differential expression profile of plasma exosomal microRNAs in acute type A aortic dissection with acute lung injury. Sci Rep 2022;12:11667. [Crossref] [PubMed]
- Jiao R, Liu M, Lu X, et al. A nomogram for reduced cardiac function in postoperative acute type A aortic dissection patients with acute kidney injury undergoing continuous renal replacement therapy. Front Cardiovasc Med 2022;9:874715. [Crossref] [PubMed]
- Awad H, Essandoh M. Goal-Directed Oxygen Delivery During Cardiopulmonary Bypass: Can This Perfusion Strategy Improve Biochemical and Clinical Neurologic Outcomes? J Cardiothorac Vasc Anesth 2018;32:2493-4. [Crossref] [PubMed]
- Ranucci M, Johnson I, Willcox T, et al. Goal-directed perfusion to reduce acute kidney injury: A randomized trial. J Thorac Cardiovasc Surg 2018;156:1918-1927.e2. [Crossref] [PubMed]
- Magruder JT, Weiss SJ, DeAngelis KG, et al. Correlating oxygen delivery on cardiopulmonary bypass with Society of Thoracic Surgeons outcomes following cardiac surgery. J Thorac Cardiovasc Surg 2022;164:997-1007. [Crossref] [PubMed]
- Isselbacher EM, Preventza O, Hamilton Black J 3rd, et al. 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2022;146:e334-482. [Crossref] [PubMed]
- Czerny M, Schoenhoff F, Etz C, et al. The Impact of Pre-Operative Malperfusion on Outcome in Acute Type A Aortic Dissection: Results From the GERAADA Registry. J Am Coll Cardiol 2015;65:2628-35. [Crossref] [PubMed]
- Sarafidis P, Martens S, Saratzis A, et al. Diseases of the Aorta and Kidney Disease: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Cardiovasc Res 2022;118:2582-95. [Crossref] [PubMed]
- Magruder JT, Crawford TC, Harness HL, et al. A pilot goal-directed perfusion initiative is associated with less acute kidney injury after cardiac surgery. J Thorac Cardiovasc Surg 2017;153:118-125.e1. [Crossref] [PubMed]
- Rasmussen SR, Kandler K, Nielsen RV, et al. Duration of critically low oxygen delivery is associated with acute kidney injury after cardiac surgery. Acta Anaesthesiol Scand 2019;63:1290-7. [Crossref] [PubMed]
- Mukaida H, Matsushita S, Yamamoto T, et al. Oxygen delivery-guided perfusion for the prevention of acute kidney injury: A randomized controlled trial. J Thorac Cardiovasc Surg 2023;165:750-760.e5. [Crossref] [PubMed]
- Anastasiadis K, Antonitsis P, Deliopoulos A, et al. A multidisciplinary perioperative strategy for attaining "more physiologic" cardiac surgery. Perfusion 2017;32:446-53. [Crossref] [PubMed]
- Lukaszewski M, Lukaszewski R, Kosiorowska K, et al. The use of data science to analyse physiology of oxygen delivery in the extracorporeal circulation. BMC Cardiovasc Disord 2019;19:292. [Crossref] [PubMed]
- Ranucci M, Aloisio T, Carboni G, et al. Acute Kidney Injury and Hemodilution During Cardiopulmonary Bypass: A Changing Scenario. Ann Thorac Surg 2015;100:95-100. [Crossref] [PubMed]
- Ranucci M, Carboni G, Cotza M, et al. Hemodilution on cardiopulmonary bypass as a determinant of early postoperative hyperlactatemia. PLoS One 2015;10:e0126939. [Crossref] [PubMed]
- Wang S, Wang D, Huang X, et al. Risk factors and in-hospital mortality of postoperative hyperlactatemia in patients after acute type A aortic dissection surgery. BMC Cardiovasc Disord 2021;21:431. [Crossref] [PubMed]
- Mukaida H, Matsushita S, Minami Y, et al. Risk factors for postoperative delirium on oxygen delivery-guided perfusion. J Cardiothorac Surg 2022;17:193. [Crossref] [PubMed]
- Gao P, Liu J, Zhang P, et al. Goal-directed perfusion for reducing acute kidney injury in cardiac surgery: A systematic review and meta-analysis. Perfusion 2023;38:591-9. [Crossref] [PubMed]
- Newland RF, Baker RA, Woodman RJ, et al. Predictive Capacity of Oxygen Delivery During Cardiopulmonary Bypass on Acute Kidney Injury. Ann Thorac Surg 2019;108:1807-14. [Crossref] [PubMed]
- Zhang Y, Chen T, Chen Q, et al. Development and evaluation of an early death risk prediction model after acute type A aortic dissection. Ann Transl Med 2021;9:1442. [Crossref] [PubMed]
- Hayward A, Robertson A, Thiruchelvam T, et al. Oxygen delivery in pediatric cardiac surgery and its association with acute kidney injury using machine learning. J Thorac Cardiovasc Surg 2023;165:1505-16. [Crossref] [PubMed]
- Ganushchak YM, Kurniawati ER, van der Horst ICC, et al. Patterns of oxygen debt repayment in cardiogenic shock patients sustained with extracorporeal life support: A retrospective study. J Crit Care 2022;71:154044. [Crossref] [PubMed]



