
The essential role of non-steroidal anti-inflammatory drugs in pain control following robotic thoracoscopic lung resections
Highlight box
Key findings
• The role of non-steroidal analgesic drugs (NSAIDs) in reducing post operative opioid requirements (especially schedule II) is only apparent in opioid sparing Enhanced recovery after thoracic surgery (ERATS) protocols.
What is known and what is new?
• Thoracic surgical incisions are among the most painful surgical incisions historically requiring large opioid requirements to provide adequate analgesia. ERATS multimodal pain control has significantly diminished opioid requirements following Thoracic Surgery. We present our data highlighting the role of NSAIDs in reducing opioid requirements in an opioid-sparing ERATS regimen.
What is the implication, and what should change now?
• NSAIDs play a critical role in reducing opioid consumption in optimized ERATS protocols.
Introduction
The goal of enhanced recovery after surgery (ERAS) protocols is to optimize postoperative outcomes by reducing postoperative acute pain and complications, shortening length of stay (LOS), cost containment and most importantly increasing patient satisfaction (1). Enhanced recovery after thoracic surgery (ERATS) protocol have been developed for thoracic surgical patients incorporating the nuances of caring for patients undergoing intrathoracic procedures, either by thoracotomy or by minimally invasive thoracoscopic surgery (video-assisted or robotic thoracoscopy) (2,3). Postoperative pain is intrinsic to thoracic surgical procedures, and pulmonary impairment following lung resections together with underlying co-morbidities have a strong impact on post-operative outcomes (4). Effective opioid-sparing multimodal pain management strategy incorporating regional analgesia with intercostal nerve blocks using local anesthetics and non-opioid analgesics is an essential component of successful ERATS protocols (5,6).
Our ERATS protocol, first implemented at our institution on 2/1/2018 and following a 5-month transition period, has since been our standard peri-operative care protocol for all thoracic surgical patients. The most important component of ERATS is the opioid-sparing postoperative pain management strategy consisting of pre-incision skin infiltration and intra-cavitary intercostal nerve block with long-acting local anesthetic agent liposomal bupivacaine (LipoB-Exparel®, Pacira Pharmaceuticals Inc., Parsippany, NJ, USA) was well as scheduled non-opioid analgesics (acetaminophen, gabapentin and ibuprofen) and tramadol (a schedule IV opioid) together with pro-re-nata (PRN) oxycodone (a schedule IV opioid) for break-through pain). Significant reduction of postoperative pain and opioid requirements was observed in patients undergoing thoracic surgical procedures following ERATS implementation compared to those of the pre-ERATS era (7). We subsequently optimized our initial ERATS protocol by diluting LipoB with 0.25% bupivacaine and switching tramadol to PRN dosing while keeping all other care components unchanged (Table S1). We observed further reduction of opioid use compared to the initial ERATS protocol and actually achieved schedule II opioid-free pain management in the postoperative period without affecting subjective acute pain levels (6,8). Non-steroidal anti-inflammatory drugs (NSAIDs) such as ketorolac, celecoxib or ibuprofen are parts of many ERAS protocols and prescribed together with gabapentin and acetaminophen as non-opioid analgesics to reduce postoperative opioid needs. Both ketorolac and ibuprofen are NSAIDs in our protocol with the latter being the class of NSAIDs given at the time of hospital discharge. We routinely avoid NSAIDs in patients with elevated baseline serum creatinine, NSAIDs allergy, thrombocytopenia and those taking other forms of anti-coagulation and/or antiplatelet agents. NSAIDs, based on their direct inhibitory effect on the inflammation pathways, are thought to contribute significantly to pain reduction, hence their opioid-sparing effects. We therefore hypothesized that patients who had a contraindication to NSAIDs would require more opioids to achieve immediate post-operative pain control. Given the ongoing opioid epidemic in the United States strategies to decrease opioid utilization are of national interest, and elucidating the role of NSAIDs in reducing post operative pain is important (9). The primary objective of this retrospective study is to determine the impact of NSAIDs use on postoperative pain and opioid use in patients undergoing robotic-assisted thoracic surgery (RATS). The secondary objective is to define the effect of NSAIDs use on postoperative complications and LOS. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-709/rc) (10).
Methods
A retrospective review of our prospectively maintained thoracic surgery database and the electronic medical records EPIC® of all patients undergoing robotic thoracic surgical procedures between 7/1/2018 and 10/31/2021 at the University of Miami Hospital was performed under the IRB Approval [No. 20180827(10/31/2018)], according to the principles of the Declaration of Helsinki (as revised in 2013) with waiver of patient consent requirement given the lack of identifiable information or interventions. All opioid-naïve adult patients, as defined by Brown et al. (11) (>18 years old) undergoing elective RATS for pulmonary resections (non-anatomic wedge resections and anatomic resections) in whom intercostal nerve blocks with LipoB could be successfully performed were included. Patients in whom accurate assessment of postoperative pain and narcotic consumption was not possible (i.e., those remaining on endotracheal intubation/mechanical ventilation) or those converted to open thoracotomies were excluded.
We implemented our original ERATS protocol hereby labeled as ERATS-V1 on 2/1/2018 for all thoracic surgical patients. After a 5-month transition period, it became our established care pathway. Detailed description of protocol development, implementation and clinical results has been previously reported (7). Optimizations were made to the ERATS-V1 protocol and implemented on 1/1/2020 aiming to further reduce postoperative opioid consumption (ERATS-V2) including: switching tramadol from scheduled dosing to as-needed administration and replacing the diluent of the LipoB mixture from normal saline to 0.25% bupivacaine while keeping all other components of the protocol unchanged. Our regional analgesia strategy consisted of local analgesic infiltration of skin and subcutaneous tissue prior to skin incisions and posterior intercostal nerve blocks by intrathoracic infiltration of the LipoB solution (3 mL/space) into the 2nd through 10th subpleural space as previously described (6). Protocol modification was done without knowledge of the care providers and the nursing staff performed pain assessments with the visual analog pain scale and administered opioid analgesics per ERATS protocol. Acetaminophen, gabapentin and NSAIDs (ketorolac and/or ibuprofen) were given as scheduled doses unless clinically contraindicated or withheld at the discretion of the attending physicians. We provided post-discharge prescriptions with the amount and the types of opioids (schedule II oxycodone and/or schedule IV tramadol) based on in-hospital pain levels and opioids requirements at the day of hospital discharge.
The following were extracted from the database and the hospital electronic medical records: patient demographics, operative details, pathologic diagnoses, TNM staging for primary lung cancer, 90-day postoperative complications (Clavien-Dindo classification), LOS, admission daily pain scores (recorded using the visual analog pain numeric scores by nursing staff multiple times per day to administer PRN analgesics as per ERATS protocol; daily pain scores were calculated as averages over a 24-hour period for up to 4 postoperative days), in-hospital analgesics dispensed (schedule II opioids oxycodone, hydromorphone, morphine, fentanyl and schedule IV opioid tramadol; non-opioid analgesics: acetaminophen, gabapentin, ketorolac, ibuprofen). The quantities of opioids dispensed are expressed as per os (p.o.) morphine milligram equivalent (MME). Information regarding post-discharge re-admissions, either to our hospital or to another healthcare facility, were obtained from EPIC® and via post-discharge telephone follow-ups and clinic visits. Post-discharge analgesics, including type and dosage of opioids prescribed, were collected from the discharge summary. The filling and refilling (within 30-day after discharge) of all types of opioids were monitored by reviewing EPIC® records that contain patients’ history of narcotic use per Florida’s prescription drug monitoring program (PDMP) and by routine patient surveys during telephone follow-ups by our staff and by the attending surgeons at postoperative clinic visits. The patients were stratified to either NSAIDs or no-NSAIDs cohort based on NSAIDs administration in the immediate in-hospital postoperative period.
Statistical analysis
Demographic and operative characteristics, perioperative clinical outcomes, and schedule II or IV MME, of the two cohorts were compared using Fisher exact test for categorical variables, and Mann-Whitney U test for nonparametric continuous variables where appropriate. For postoperative pain, mixed linear model test was used to analyze the pain scores up to day 3 postoperatively. We assumed linear time trends, giving rise to the intercept (initial pain at day 0) and the slope (rate of change in pain per day on study) estimates. Statistical analysis was performed with SAS software, version 9.4 (SAS Institute Inc., Cary, CA, USA).
Results
ERATS-V2 was associated with significant reduction in opiate use
A total of 491 patients met inclusion criteria and were included in this study, 147 patients in ERATS-V1 and 344 in ERATS-V2 (Consort Diagram). Overall, 181 patients (37%) did not receive NSAID [61 (41.5%) in the ERATS-V1 and 120 (35.1%) in the ERATS-V2, P=0.18] (Figure 1). There was no difference in the patient demographics, body mass index (BMI), lung function parameters, duration of operating time, type of lung resections, incidence of malignancy, postoperative complications. All patients of both groups received acetaminophen and gabapentin while in hospital and after discharge as per protocol. However, compared to ERATS-V1 cohort, patients of ERATS-V2 group required significantly lower in-hospital, post-discharge and total (in-hospital + post-discharge) postoperative opioids (Table 1). A slight reduction of mean hospital LOS of 0.5 days was observed following ERATS optimization (ERATS-V1: 2.6 days vs. ERATS-V2: 2.1 days, P=0.03). Protocol optimization was associated with significantly decreased postoperative opioid requirement (both in-hospital, post-discharge and total MMEs) (Table 2). The profound reduction of postoperative MME was attributable to the decrease of schedule IV (in-hospital opioid utilization) and both schedules II and IV (total post-operative opioid utilization) as shown in Figure 2. Moreover, 60% of ERATS-V2 patients were opioid-free post-discharge compared to only 16% of ERATS-V1 patients, correlating with a very low post-discharge MME [ERATS-V2: 0.0 (0.0–60.0) vs. ERATS-V1: 150.0 (60.0–150.0), P<0.00001] and total postoperative MME [ERATS-V2: 28.6 (6.0–96.7) vs. ERATS-V1: 172.5 (100.4–260.0), P<0.00001].
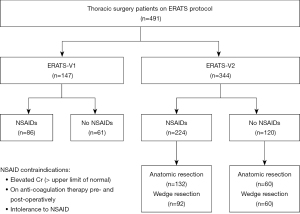
Table 1
| Variables | ERATS-V1 (n=147) | ERATS-V2 (n=344) | P value |
|---|---|---|---|
| Age (years) | 69.0 [59.0–73.0] | 65.0 [58.0–73.0] | 0.25 |
| Gender (female/male) | 78/69 | 202/142 | 0.33 |
| ASA | 3 [3–3] | 3 [3–3] | 0.87 |
| BMI (kg/m2) | 26.8 [23.5–31.1] | 26.9 [23.3–31.3] | 0.66 |
| FEV1 %N | 89.5 [77.0–97.0] | 89.0 [78.0–100.0] | 0.56 |
| DLCO %N | 83.0 [69.0–96.0] | 78.5 [68.7–94] | 0.33 |
| Operating time (min) | 140.0 [95.0–212.5] | 123.5 [79.0–180.0] | |
| Anatomic/wedge | 79/68 | 192/152 | 0.15 |
| Malignant/benign | 126/21 | 284/60 | |
| Complications (Clavian-Dindo) | |||
| 0 | 129 (87.5) | 321 (93.9) | 0.07 |
| 1–2 | 12 (8.2) | 13 (3.8) | |
| 3–4 | 6 (4.1) | 8 (2.3) | |
| LOS (days) | 2.0 [1.0–3.0], mean 2.6 | 2.0 [1.0–2.0], mean 2.1 | 0.03*** |
| In-hospital MME | 38.6 [21.1–67.0] | 14.1 [3.9–33.7] | <0.00001*** |
| Post-discharge MME | 150.0 [60.0–150.0] | 0.0 [0.0–60.0] | <0.00001*** |
| Total postop MME | 172.5 [100.4–260.0] | 28.2 [6.0–96.7] | <0.00001*** |
Data are shown as number, number (percentages) or median [interquartile range]. ***, statistical significance. ERATS, enhanced recovery after thoracic surgery; ASA, American Society of Anesthesiologists patient status classification; BMI, body mass index; FEV1, forced expiratory volume 1; DLCO, diffusion capacity of lungs for carbon monoxide; LOS, length of stay; MME, morphine milligram equivalents.
Table 2
| Variables | NSAIDs (n=86) | No NSAIDs (n=61) | P value |
|---|---|---|---|
| Age (years) | 69.0 [61.2–74.7] | 67.0 [56.0–73.0] | 0.18 |
| Gender (female/male) | 45/41 | 32/29 | 0.86 |
| ASA | 3 [3–3] | 3 [3–3] | 0.45 |
| BMI (kg/m2) | 26.1 [23.5–30.7] | 28.2 [23.5–31.3] | 0.27 |
| FEV1 %N | 88.0 [76.5–96.0] | 91.0 [77.0–97.0] | 0.45 |
| DLCO %N | 82.5 [71.0–97.7] | 83.5 [84–92.7] | 0.31 |
| Operating time (min) | 122 [88.2–186.5] | 151 [95.0–209.0] | 0.46 |
| Anatomic/wedge | 44/42 | 35/26 | 0.61 |
| Malignant/benign | 76/10 | 50/11 | 0.34 |
| Complication (Clavian-Dindo) | 0.43 | ||
| 0 | 78 (90.7) | 53 (86.9) | |
| 1–2 | 6 (7.0) | 5 (8.2) | |
| 3–4 | 2 (2.3) | 3 (4.9) | |
| LOS (days) | 2 [1–3] | 2 [1–3] | 0.88 |
| In-hospital MME | 40.0 [22.5–76.0] | 36.0 [20.5–54.5] | 0.11 |
| Post-discharge MME | 140.0 [60.0–150.0] | 150.0 [60.0–150.0] | 0.52 |
| Total postop MME | 173.6 [108.2–269.3] | 165 [97.5–215.5] | 0.36 |
Data are shown as number, number (percentages) or median [interquartile range]. NSAID, non-steroidal anti-inflammatory drugs; ERATS, enhanced recovery after thoracic surgery; ASA, American Society of Anesthesiologists patient status classification; BMI, body mass index; FEV1, forced expiratory volume 1; DLCO, diffusion capacity of lungs for carbon monoxide; LOS, length of stay; MME, morphine milligram equivalents.

NSAIDs did not have significant effects in reducing opiate use in non-opiate sparing ERATS-V1 protocol
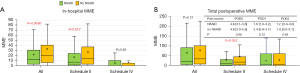
We next analyzed the effect of no NSAIDs use on subjective pain levels and postoperative opioid consumption in ERATS-V1 cohort, the one with high levels of postoperative opioid consumption. There was no difference in patient demographics, complication rate, LOS, or postoperative opioid requirements between NSAIDs and non-NSAIDs subgroups (Table 2). There was no difference in subjective pain levels between NSAIDs and non-NSAIDs subgroup (Figure 3). Similar in-hospital, post-discharge and total postoperative MME opioid utilization was observed between the two subgroups of the ERATS-V1 cohort (Table 2, Figure 3). Stratification by MME schedule type did not reveal a significant difference in pain scores by NSAIDs usage. In-hospital MME usage did not differ (P=0.11) even when examined by schedule type (schedule II, P=0.21 and schedule IV, P=0.19) (Figure 3A). This observation held true in total post operative MME use (P=0.36), and again in schedule II distribution (P=0.21) and schedule IV (P=0.40) (Figure 3B).

NSAIDs use was associated with significant reduction of in-hospital MME use in opiate-sparing ERATS-V2 protocol
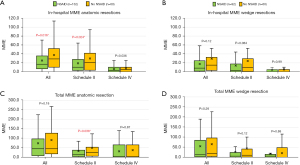
We observed that patient receiving NSAIDs were younger (65 vs. 69, P=0.04) and had lower BMI (26.3 vs. 27.52, P=0.01) compared to the non-NSAIDs receiving patients. No differences were observed in other clinical, operative, pathologic parameters, complication rate or LOS (Table 3). In-hospital MME use was lower in the NSAIDs subgroup [12.0 (2.0–30.2) vs. 20.5 (6.8–40.5), P=0.0096]. Pain scores did not differ by NSAIDs use in the ERATS-V2 group (Figure 4A). Patients who received NSAIDs required less in-hospital opioids, driven by decreased schedule II MME [6.8 (1.4–24.0) vs. 14.2 (3.0–36.4), P=0.012]. We observed lower total post-operative schedule II MME usage [8.8 (1.5–30.0) vs. 17.8 (3.0–43.5), P=0.032] and a trend towards decreased total post operative MME, but this failed to achieve statistical significance (Figure 4B). The impact of NSAIDs administration on statistically significant reduction of postoperative opioid use, particularly the schedule II opioid subclass, was only observed on those undergoing pulmonary anatomic resections and not wedge resections (Figure 5). There was a trend of lower postoperative in-hospital opioid use in patients undergoing wedge resection but this did not reach statistical significance [all: 8.0 (1.4–22.8) vs. 15.0 (3.4–29.0), P=0.12 and schedule II: 3.0 (0.0–16.5) vs. 9.5 (1.1–28.5), P=0.084].
Table 3
| Variables | NSAIDs (n=224) | No NSAIDs (n=120) | P value |
|---|---|---|---|
| Age (years) | 65.0 [57.0–72.0] | 69.0 [59.7–73.0] | 0.04 |
| Gender (female/male) | 141/83 | 61/59 | |
| ASA | 3 [3–3] | 3 [3–3] | 0.48 |
| BMI (kg/m2) | 26.3 [23.0–30.7] | 27.5 [24.8–31.4] | 0.01 |
| FEV1 %N | 89.0 [78.0–99.5] | 89.0 [78.5–100.0] | 0.59 |
| DLCO %N | 78.0 [68.0–89.0] | 77.0 [64.2–93.7] | 0.61 |
| Operating time (min) | 120.0 [79.7–180.0] | 140.5 [79.0–185.2] | 0.32 |
| Anatomic/wedge | 132/92 | 60/60 | 0.14 |
| Malignant/benign | 190/34 | 94/26 | 0.14 |
| Complication (Clavian-Dindo) | 0.76 | ||
| 0 | 211 (94.2) | 112 (93.3) | |
| 1–2 | 9 (4.0) | 4 (3.3) | |
| 3–4 | 4 (1.8) | 4 (3.3) | |
| LOS (days) | 2.0 [1.0–2.0], mean: 2.0 | 1.0 [1.0–2.0], mean 2.0 | 0.19 |
| In-hospital MME | 12.0 [2.0–30.2] | 20.5 [6.8–40.5] | 0.0096*** |
| Post-discharge MME | 0.0 [0.0–60.0] | 0.0 [0.0–60.0] | 0.67 |
| Opioid filled/refilled | 87 (38.8)/17 (7.6) | 45 (37.5)/10 (8.3) | 0.66/0.54 |
| Total postop MME | 20.7 [5.0–90.2] | 34.0 [8.3–100.5] | 0.12 |
Data are shown as number, number (percentages) or median [interquartile range]. ***, statistical significance. NSAID, non-steroidal anti-inflammatory drug; ERATS, enhanced recovery after thoracic surgery; ASA, American Society of Anesthesiologists patient status classification; BMI, body mass index; FEV1, forced expiratory volume 1; DLCO, diffusion capacity of lungs for carbon monoxide; LOS, length of stay; MME, morphine milligram equivalents.
Nearly half (48%) of no-NSAIDs patients did not receive NSAIDs secondary to surgeon discretion with perceived increased risk of peri-operative bleeding. Further analysis demonstrated that 71.7% of the patients who did not receive NSAIDs in the initial ERATS-V1 protocol was due to surgeon discretion without an objective patient risk factor as opposed 35.8% in the subsequent ERATS-V2 protocol (P<0.00001). Elevated creatinine was our next most frequent contraindication (30.2%). A reported allergy (7.8%), or use of antiplatelet or anticoagulant was the exclusion criteria for 5.0% of patients. Finally, a combination of the above risk factors excluded the use of NSAIDs for the remaining 9.0% of patients.
Discussion
We exploited a protocol optimization process to maximize opioid-sparing effects of our ERATS to identify two protocols with distinct opioid requirements but with similar pain control. We further identified a sufficiently large population of non-NSAID patients to conduct this study to define the role of NSAIDs in mitigating opioid needs following robotic thoracoscopic pulmonary resections. Overall, our ERATS patients had excellent postoperative pain control with maximal median pain scores less than 5 (i.e., moderate pain). The optimized ERATS-V2 is truly an opioid-sparing protocol with minimal to no opioid (particularly schedule II subclass) utilization compared to the initial protocol ERATS-V1. Such low opioid utilization enabled us to unmask the analgesic effect of NSAIDs by demonstrating increased opioid consumption in non-NSAID patients of ERATS-V2 and not ERATS-V1 protocol. The quantity and the potency (schedule II versus schedule IV) of opioid utilization is a good metric of postoperative analgesia by the multimodal strategy. A statistically significant reduction of opioid requirements for patients who received NSAIDs in ERATS-V2 indicated the contribution of NSAIDs to pain control and thus less opioid consumption, especially in the immediate postoperative period. There was no difference in postoperative complications and LOS between the two cohorts.
Thoracic surgical procedures, particularly those performed by open thoracotomy, are associated with significant postoperative pain (12). The intensity and duration of acute postoperative pain following thoracic surgical procedures not only is a major source of anxiety to patients but also contributes to postoperative respiratory complications due to decreased ambulation and ineffective chest physiotherapy (13). A pain management protocol relying mainly on potent opioids, either by PCA or TEA, can be hampered by untoward side effects including but not limited to drowsiness, nausea/emesis or in case of epidural analgesia sympathetic blockade, catheter malfunction or misplacement. Enhanced recovery protocols adapted for thoracic surgical patents strongly emphasize on effective postoperative pain control using a multi-modal strategy with pre-emptive regional blockade (14), together with non-opioid analgesics such as acetaminophen (15) and NSAIDs (6,16) and gabapentin (17) to further reduce the reliance on potent opioids. Acetaminophen and NSAIDs used concurrently has been shown to be more effective in controlling post-operative pain than either drug alone (18). Overall reduction of reliance on opioid for postoperative pain management following thoracic surgery minimizes its availability and misuse by the public and therefore directly contributes to the fight against opioid abuse epidemic. Despite the consistent evidence regarding the improved pain control and reduction in opiate use with NSAIDs relative contraindications exist (19). A history of NSAIDs intolerance, allergy vs history of GI bleed while using NSAIDs (18), patients on concurrent anti-platelet or anticoagulation, based on the increased risk of bleeding associated with concurrent NSAIDs use (20). Baseline renal dysfunction is a relative contraindication to NSAIDs use (creatinine high range of normal). Toradol has a black box warning following cardiac surgery but nonetheless based on multiple studies non-selective COX inhibitors are routinely used following cardiac surgery (21). Acetaminophen is very well tolerated and the most consistently used non-opioid analgesic, followed by gabapentin starting at low dosage of 100 mg every 8 hours to minimize intolerance and titrating to higher doses to desired effects. Contra-indications to NSAIDs administration include: a history of NSAIDs intolerance, allergy or history of GI bleed while using NSAIDs (18), concurrent anti-platelet or anticoagulation based on the increased risk of bleeding associated with concurrent NSAID use (20) and baseline renal dysfunction as indicated by elevated serum creatinine higher than the upper limit of normal) (22). Another barrier to NSAIDs use, in addition clinical contraindications mentioned above, is attributable to the attending surgeons. Nearly 75% of the no-NSAIDs patients in the initial ERATS-V1 did not receive this class of analgesic as a clinical decision by the attending surgeons out of concern of postoperative bleeding following difficult procedures. This most likely reflects the initial “learning curve” of having scheduled NSAIDs as part of ERATS. As clinical experience accumulates over time that scheduled NSAIDs are safe and much less NSAIDs withholding by the same attendings (DMN, NV) was observed (35% in ERATS-V2 vs. 75% in ERATS-V1, P<0.00001) yet there was no change in neither the case complexity nor bleeding complications (excessive sanguineous chest tube drainage or reoperation) in ERATS-V2 patients. To address patients with baseline renal dysfunction, history of gastric ulcers and anti-platelet effects, selective COX-2 inhibitors such as celecoxib have been proposed as a safe alternative (23). In addition to their safety profile, selective COX-2 inhibitors have demonstrated equivalence in pain control scores compared to non-selective NSAIDs in patients with severe osteoarthritis and following knee arthroscopy (23). Selective COX-2 inhibitors have also demonstrated improved patient satisfaction, pain scores when administered in the pre and postoperative period when combined with patient controlled thoracic epidurals (24). We replaced ibuprofen with celecoxib on 2/2022 and follow pharmacy’s guidelines for celecoxib withholding based on drug allergy and levels of kidney dysfunction indicated by estimated glomerular filtration rate.
The strengths of our study include our uniform and robust data collection and standardized ERATS protocols for pain scoring and MME administration. Furthermore, we were able to independently verify post operative opioid prescriptions with the state monitoring program. Our study benefited from a long longitudinal period and a continued purposeful optimization; unmasking the effects of NSAIDs in reducing opioid usage. The weakness of our study includes its retrospective nature with uncontrollable intrinsic biases, a single institution study, small sample size precluding propensity-score matching analysis and multi-variable analysis. Our study also failed to replicate the reduction in pain scores seen in other multi-institutional studies, possibly due to our studies single site nature and its relatively small number of patients. Furthermore, we did not differentiate between intravenous and oral NSAID administration, or which post operative day NSAIDs were initiated.
Conclusions
In conclusion, our observational study demonstrates the value of protocol optimization to achieve opioid-sparing property of ERATS and identifies the nuances of the “learning curve” of NSAIDs prescription in the early days of ERATS. More importantly, this retrospectively analysis highlights contribution of NSAIDs in the multi-modality analgesic property—mitigating opioid use while maintaining similar pain control—of an opioid-sparing ERATS protocol.
Acknowledgments
We thank the efforts of our nurse practitioners in collecting the information.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-709/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-709/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-709/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-709/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the University of Miami [No. 20180827(10/31/2018)] with waiver of patient consent requirement given the lack of identifiable information or interventions.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ljungqvist O, Scott M, Fearon KC. Enhanced Recovery After Surgery: A Review. JAMA Surg 2017;152:292-8. [Crossref] [PubMed]
- Cerfolio RJ, Pickens A, Bass C, et al. Fast-tracking pulmonary resections. J Thorac Cardiovasc Surg 2001;122:318-24. [Crossref] [PubMed]
- Salati M, Brunelli A, Xiumè F, et al. Does fast-tracking increase the readmission rate after pulmonary resection? A case-matched study. Eur J Cardiothorac Surg 2012;41:1083-7; discussion 1087. [Crossref] [PubMed]
- Bendixen M, Jørgensen OD, Kronborg C, et al. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol 2016;17:836-44. [Crossref] [PubMed]
- Mehran RJ, Martin LW, Baker CM, et al. Pain Management in an Enhanced Recovery Pathway After Thoracic Surgical Procedures. Ann Thorac Surg 2016;102:e595-6. [Crossref] [PubMed]
- Kodia K, Alnajar A, Szewczyk J, et al. Optimization of an Enhanced Recovery After Surgery protocol for opioid-free pain management following robotic thoracic surgery. JTCVS Open 2022;9:317-28. [Crossref] [PubMed]
- Razi SS, Stephens-McDonnough JA, Haq S, et al. Significant reduction of postoperative pain and opioid analgesics requirement with an Enhanced Recovery After Thoracic Surgery protocol. J Thorac Cardiovasc Surg 2021;161:1689-701. [Crossref] [PubMed]
- Bohnert ASB, Guy GP Jr, Losby JL. Opioid Prescribing in the United States Before and After the Centers for Disease Control and Prevention's 2016 Opioid Guideline. Ann Intern Med 2018;169:367-75. [Crossref] [PubMed]
- Weiner SG. Addressing the ignored complication: chronic opioid use after surgery. BMJ Qual Saf 2021;30:180-2. [Crossref] [PubMed]
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008;61:344-9. [Crossref] [PubMed]
- Brown LM, Kratz A, Verba S, et al. Pain and Opioid Use After Thoracic Surgery: Where We Are and Where We Need To Go. Ann Thorac Surg 2020;109:1638-45. [Crossref] [PubMed]
- Martin LW. Discussion. JTCVS Open 2022;9:326-7. [Crossref] [PubMed]
- Gerner P. Postthoracotomy pain management problems. Anesthesiol Clin 2008;26:355-67. vii. [Crossref] [PubMed]
- Luketich JD, Land SR, Sullivan EA, et al. Thoracic epidural versus intercostal nerve catheter plus patient-controlled analgesia: a randomized study. Ann Thorac Surg 2005;79:1845-9; discussion 1849-50. [Crossref] [PubMed]
- Remy C, Marret E, Bonnet F. Effects of acetaminophen on morphine side-effects and consumption after major surgery: meta-analysis of randomized controlled trials. Br J Anaesth 2005;94:505-13. [Crossref] [PubMed]
- Batchelor TJP, Rasburn NJ, Abdelnour-Berchtold E, et al. Guidelines for enhanced recovery after lung surgery: recommendations of the Enhanced Recovery After Surgery (ERAS®) Society and the European Society of Thoracic Surgeons (ESTS). Eur J Cardiothorac Surg 2019;55:91-115. [Crossref] [PubMed]
- Grosen K, Drewes AM, Højsgaard A, et al. Perioperative gabapentin for the prevention of persistent pain after thoracotomy: a randomized controlled trial. Eur J Cardiothorac Surg 2014;46:76-85. [Crossref] [PubMed]
- Gilron I, Milne B, Hong M. Cyclooxygenase-2 inhibitors in postoperative pain management: current evidence and future directions. Anesthesiology 2003;99:1198-208. [Crossref] [PubMed]
- Romero A, Garcia JE, Joshi GP. The state of the art in preventing postthoracotomy pain. Semin Thorac Cardiovasc Surg 2013;25:116-24. [Crossref] [PubMed]
- Chan DK, Parikh SR. Perioperative ketorolac increases post-tonsillectomy hemorrhage in adults but not children. Laryngoscope 2014;124:1789-93. [Crossref] [PubMed]
- Oliveri L, Jerzewski K, Kulik A. Black box warning: is ketorolac safe for use after cardiac surgery? J Cardiothorac Vasc Anesth 2014;28:274-9. [Crossref] [PubMed]
- Qazi SM, Sindby EJ, Nørgaard MA. Ibuprofen - a Safe Analgesic During Cardiac Surgery Recovery? A Randomized Controlled Trial. J Cardiovasc Thorac Res 2015;7:141-8. [Crossref] [PubMed]
- Chan FKL, Ching JYL, Tse YK, et al. Gastrointestinal safety of celecoxib versus naproxen in patients with cardiothrombotic diseases and arthritis after upper gastrointestinal bleeding (CONCERN): an industry-independent, double-blind, double-dummy, randomised trial. Lancet 2017;389:2375-82. [Crossref] [PubMed]
- Senard M, Deflandre EP, Ledoux D, et al. Effect of celecoxib combined with thoracic epidural analgesia on pain after thoracotomy. Br J Anaesth 2010;105:196-200. [Crossref] [PubMed]


