
Robotic chest wall resection for primary benign chest wall tumors and locally advanced lung cancer: an institutional case series and national report
Highlight box
Key findings
• This study reports excellent outcomes for robotic chest wall resection and demonstrates growing trend of this technique in America via National Cancer Database review.
What is known and what is new?
• Chest wall resections have been traditionally performed open with large morbidity and postoperative pain. Video-assisted thoracic surgery techniques have been used to reduce the pain and morbidity. However, there is a dearth on how to apply robotic techniques to chest wall tumors in the literature.
• This manuscript adds the largest case series on resection of robotic chest wall resections in the literature to date along with descriptive technique on how to perform them.
What is the implication, and what should change now?
• Robotic chest wall resection is a good option for chest wall resection which minimizes postoperative pain with excellent functional outcomes. This technique should be explored more for use in chest wall resections.
Introduction
Resection is the mainstay of treatment for chest wall tumors. Traditionally, open thoracotomy is used for chest wall resection. However, it is frequently associated with postoperative pain and significant morbidity. Based on the location and size of the defect, chest wall reconstruction is usually required in order to protect underlying structures, maintain respiratory mechanics, and reduce respiratory complications (1). Video-assisted thoracic surgery (VATS) and robotic thoracic surgery are minimally invasive approaches that confer potential benefits over an open thoracotomy in the reduction of postoperative morbidity as well as improved visualization. These established benefits are limited incision size, avoiding rib spreading, preservation of uninvolved overlying major muscles, minimizing tissue trauma, reduced inflammatory response and postoperative pain, shortened hospital stay, and faster recovery (2).
However, the use of the robotic approach in chest wall resection is not well described in the literature. Some surgeons believe that postoperative pain is mainly from rib spreading rather than rib resection (3). The robotic approach can facilitate chest wall resection from the inside of the chest cavity with direct visualization of ribs without dividing any overlying muscles or spreading ribs. Prior studies are limited to single case reports highlighting the use of the robot for en-bloc lung resection and management of superior sulcus tumors or first rib resection for thoracic outlet syndrome (3-5). A recent technique paper describing robotic techniques for management of thoracic outlet syndrome briefly mentioned robotic primary chest wall tumor resection is feasible (6). However, two other recently published studies describing state-of-the-art operative technique and single-institution experience for primary chest wall resection do not describe use of robotic technology (7,8).
In this context, we report our initial experience of 6 patients undergoing robotic chest wall resection at our institution and 96 patients found in the National Cancer Database (NCDB). To our knowledge, this study is the largest case series describing the use of robotic technology in chest wall resection for a spectrum of tumors involving the chest wall. We report clinical, demographic, and pathologic features and describe our preoperative workup, operative technique, and postoperative care for our institutional patients and demographic and operative data for the database patients. We present this article in accordance with the STROBE and AME Case Series reporting checklists (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-532/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of Loyola University (LU# 214246; 10/22/2020) and individual consent for this retrospective analysis was waived. We retrospectively reviewed the charts of all adult patients undergoing a robotic chest wall resection at Loyola University Medical Center between September 2016 and September 2021. At our institution, the robotic approach is the preferred technique for our surgeons. We favor the robotic approach because we believe the technology affords several advantages. The three-dimensional magnification and wristed instruments allow for precise dissection, maximizing hemostasis and minimizing intraoperative blood loss. The overlying chest musculature is preserved and provides adequate strength to the chest wall which negates the need for a formal chest wall reconstruction, keeping rib spreading to a minimum and significantly reducing postoperative pain. However, patients with tumors involving major vascular structures, involving the sternum, or are over 10 cm in largest dimension are generally excluded from a robotic approach. Although not described in our experience, chest wall reconstruction is potentially also doable robotically (9). We searched the NCDB for robotic chest wall resections from 2012 to 2017. For our institutional patients, we collected patients’ demographic data, diagnostic findings, operative details, administered pain medications, pathological diagnoses, and follow-ups from the electronic health records. For the NCDB patients we collected demographic data, diagnostic findings, operative details, and follow-up data.
Dependent and independent variables
For our institutional patients, demographic data collected included age, sex, body mass index (BMI), forced expiratory volume in 1 second (FEV1), diffusion capacity of the lung for carbon monoxide (DLCO), Eastern Cooperative Oncology Group (ECOG), performance status, and laterality. Operative details and pathological findings included a method of rib division, blood loss, operative time, operation performed, and need for concurrent lung resection. Tumor characteristics include tumor size, location, and histology. The follow-up information included adjuvant/neoadjuvant therapies used, length of hospital stays, complications, evidence of lung parenchyma herniation, paradoxical chest wall motion, and follow-up periods. For NCDB patients demographic data included age, sex, and laterality. Operative details and pathologic findings included conversion to open rate and concurrent lung resection. Tumor characteristics included tumor size and histology. The follow-up information included length of stay and survival.
Statistical analysis
Age in years was a continuous variable along with tumor size (cm), length of stay (days), and time to last patient follow-up (months). Sex was a binary categorical variable along with conversion to open surgery, 30-day mortality, and 90-day mortality. Histology was a categorical variable consisting of four classifications (adenocarcinoma, squamous cell carcinoma, neuroendocrine, and other). Results are descriptively reported due to the inability to draw statistically significant conclusions comparing a series of 6 patients to a series of 96 patients.
Operative technique
All patients at our institution underwent general endotracheal anesthesia with double-lumen tubes for respective lung isolation. In general, at our institution tumors smaller than 4 cm with benign imaging characteristics can proceed for excisional biopsy. Tumors greater than 4 cm or tumors with any concerning features on cross-sectional imaging will undergo biopsy of the lesion to establish a diagnosis for surgical planning. For hard to visualize or palpate tumors due to patient habitus or location, we use methylene blue to mark the affected rib/ribs and margins utilizing the Veran Medical’s SPiN Thoracic Electromagnetic Navigation System (VERAN Medical Technologies, St. Louis, MO, USA) using the inspiration/expiration computed tomography (CT) scan protocol for transthoracic percutaneous needle localization. We used the Intuitive Surgical Da Vinci Si or Xi robotic surgical system for resection. The patient is placed in the lateral decubitus position. Our preferred port placement consists of four robotic ports (three 8 mm ports and one 12 mm port) all in line in the 8th intercostal space. However, we are flexible with port placement to optimize triangulation to the lesion after the insertion of the first camera port to allow triangulation to tumor. We opt for the four in line ports because we believe when a lung resection is necessary the hilar dissection is the most important part of the operation. The chest wall portion is performed at the end after the hilar dissection is completed. After port placement and initial chest exploration, we dissect the affected ribs free from the surrounding intercostal muscle. We ligate the neurovascular bundle with robotic clips. The proximal and distal aspects of the affected rib(s) are divided, taking into account an adequate margin. Multiple techniques or instruments can be used for this portion, including the Gigli saw, Kerrison rongeurs, Dennis rib cutter, or Chisel rib shear via a thoracic port or separate stab skin incision depending on the access to the location of the bony division. For the Gigli saw, we make a stab wound above and below the rib. The Gigli saw is inserted percutaneously and operated manually, external to the thoracic cavity. To operate the Kerrison rongeur, one arm of the robot is undocked, and the instrument is inserted through the port site using the robotic camera as a guide. All the rib shearing techniques are manually operated and do not use the robot except for the camera and graspers as needed. The specimen is then placed in a specimen bag and retrieved with care, ensuring that the sharp edges of the bone are oriented to allow for easy extraction and prevent tearing the bag or injuring viscera. In cases where a concomitant en-bloc lung resection was required, we prefer first to perform the hilar lung dissection, complete the fissure, and then perform the chest wall resection en-bloc. For tumors arising from the bone, gross margin assessment is utilized as it is not feasible to decalcify bone and obtain microscopic frozen section margin analysis within the duration of the operation. We follow standard chest wall resection guidelines for primary chest wall tumors of obtaining 4 cm margin grossly (10). In all cases, we were able to preserve the overlying extra-thoracic musculatures. These muscles provide adequate coverage to prevent pulmonary herniation. Thus, prosthetic reconstruction of the chest wall was not required in this series. See Video 1 for a demonstration of our operative techniques taken from Case 3 in this series. All procedures were conducted by experienced robotic thoracic surgeons who perform over 100 robotic thoracic operations per year.
Results
During the study period at our institution, 6 patients underwent robotic chest wall resection. There were 4 males and 2 females with a median age of 70.0 (range, 39–91) years. The clinical and demographic details are summarized in Table 1.
Table 1
| Demographic and clinical information | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 |
|---|---|---|---|---|---|---|
| Age (years) | 71 | 69 | 39 | 91 | 73 | 67 |
| Sex (M/F) | F | M | M | M | M | F |
| BMI (kg/m2) | 31.3 | 31.0 | 35.8 | 22.0 | 25.3 | 26.6 |
| FEV1 (% predicted) | N/A | 121% | 123% | 103% | 40% | 85% |
| DLCO (% predicted) | N/A | 88% | 100% | 81% | 51% | 49% |
| ECOG class | 0 | 0 | 0 | 1 | 1 | 0 |
| Laterality | Right | Right | Left | Left | Left | Left |
| Chest wall tumor location | 3rd rib and 10th rib | 4th rib and 5th rib | 4th rib | 2nd rib and 3rd rib | 3rd rib and 4th rib | 2nd rib |
M, male; F, female; BMI, body mass index; FEV1, forced expiratory volume in 1 second; DLCO, diffusion capacity of the lung for carbon monoxide; N/A, not available; ECOG, Eastern Cooperative Oncology Group.
The tumor affected 2 or more ribs in 4 patients, while it affected 1 rib in the remaining 2 patients. None of the patients required chest wall reconstruction. The median operative time was 215 (range, 134–299) min. The median estimated blood loss was 75 (range, 10–500) mL. None of the included patients required a blood transfusion. The median hospital length of stay was 3 (range, 1–6) days. Final pathology demonstrated 3 patients had benign primary chest wall tumors, and 3 patients had locally advanced lung cancer invading the chest wall. The operative and pathological details are highlighted in Table 2. None of the patients in this series had any limitation in their upper limb function in the immediate postoperative period or their last follow-up.
Table 2
| Operative and pathologic findings | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 |
|---|---|---|---|---|---|---|
| Number of ribs resected | 2 | 2 | 1 | 2 | 2 | 1 |
| Estimated blood loss (mL) | 100 | 50 | 20 | 500 | 150 | 10 |
| Method of rib division | Rib shears | Gigli saw | Gigli saw | Rib shears Chisel | Kerrison rongeur | Kerrison rongeur |
| Concurrent en-bloc lung resection | No | No | No | Yes | Yes | Yes |
| Pathology | Fibrous dysplasia | Fibrous tumor with myxoid changes | Langerhans cell histiocytosis | Moderately differentiated Squamous cell carcinoma | Poorly differentiated adenosquamous carcinoma | Squamous cell carcinoma |
| Specimen size (cm3) | 12.2×3.3×2.2 (10th rib); 8.4×5.3×3.1 (3rd rib) |
10.5×6.2×4.2 | 14.1×3.1×2.8 | 11.4×7.0×2.7 (2nd and 3rd rib); 2.2×12.1×4.8 (lung tissue) |
7.5×5.1×3.8 (chest wall); 8.4×4.9×3.0 (lung tissue) |
2.5×1.8×1.8 |
| Hospital length of stay (days) | 1 | 3 | 2 | 5 | 6 | 4 |
In the NCDB, there were 96 patients who underwent robotic chest wall resection, 53 male (55.2%) with a median age of 68.5 (range, 30–89) years. All patients had primary lung cancer invading the chest wall and underwent en-bloc lung and chest wall resection. Of these patients, 92 (95.8%) had adenocarcinoma or squamous cell carcinoma while 4 (4.2%) had malignant neuroendocrine histology. Median tumor size was somewhat larger in our institutional series compared to the NCDB, 5.25 (range, 2.3–8.3) vs. 3.90 (range, 2.4–6.0) cm. There were no conversions to open in our case series, whereas 18 (18.8%) were converted in the NCDB series. There were no unplanned readmissions or mortalities in our institutional case series compared to the NCDB series with 3.1% unplanned readmission in 30 days, 4.2% had a 30-day mortality, and 8.3% had a 90-day mortality. The histologic breakdown of the NCDB tumors and comparison to our institutional series can be seen in Table 3. Use of the robotic approach has increased in more recent years (Figure 1).
Table 3
| Demographic and outcome measures | Institutional series (n=6) | NCDB series (n=96) |
|---|---|---|
| Age (years), median | 70.0 | 68.5 |
| Sex (male), n (%) | 4 (66.7) | 53 (55.2) |
| Tumor size (cm), median | 5.25 | 3.90 |
| Histology, n (%) | ||
| Squamous | 2 (33.3) | 45 (46.9) |
| Adenocarcinoma | 1 (16.7) | 44 (45.8) |
| Neuroendocrine | 0 (0.0) | 6 (6.3) |
| Other | 3 (50.0) | 1 (1.0) |
| Conversion to open, n (%) | 0 (0.0) | 18 (18.8) |
| Length of stay (days), median | 3 | 7 |
| 30-day mortality, n (%) | 0 (0.0) | 4 (4.2) |
| 90-day mortality, n (%) | 0 (0.0) | 8 (8.3) |
| Follow-up (months), median | 18.0 | 49.6 |
NCDB, National Cancer Database.

Case presentation
Case 1
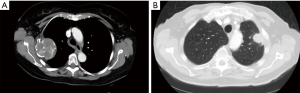
A 68-year-old female with a history of renal cell carcinoma (post left partial nephrectomy status) and polyostotic fibrous dysplasia (ribs, spine, and skull) presented with persistent right lower chest wall pain. A biopsy performed of the rib lesion 6 months prior at another hospital confirmed polycystic fibrous dysplasia. A chest CT showed two masses involving the third and tenth right ribs measuring 5.8×5.7×6.3 and 4.3×2.6×2.5 cm3, respectively (Figure 2). The Da Vinci Si robotic surgical system was used for resection. The rib shears method was used for rib division; two ribs were resected. Postoperative pain was managed with multilevel intercostal nerve block, scheduled acetaminophen [975 mg every 6 hours (q6h)], scheduled gabapentin [100 mg every 8 hours (q8h)], and tramadol (50 mg q6h) as needed. Final pathology revealed fibrous dysplasia with the third rib tumor measuring 7.7×5.7×4.1 cm3 and the tenth rib tumor measuring 4.3×4.1×3.1 cm3. The patient had an uncomplicated postoperative course. No paradoxical chest wall motion on exam 3 months post-surgery. CT scan 12 months post-surgery demonstrated no herniation of lung tissue. Patient was alive and well 24 months post-surgery.
Case 2
A 69-year-old male with a history of hyperlipidemia and tobacco use presented to us after screening CT scan for lung cancer discovered an incidental chest wall mass in the right posterior fourth rib interspace measuring 3.1×2.5×2.6 cm3. Lesion was avid to 4.5 standardized uptake value (SUV) on positron emission tomography (PET)-CT with no other positive lymph nodes or lung lesions. Patient discussed in tumor board and referred for biopsy but was deemed to not be technically feasible due to location anterior to scapula. Therefore, we proceeded for surgical excision. The Da Vinci Xi robotic surgical system was used for the resection. The Gigli saw was used for the division of ribs four and five. Postoperative pain was managed with multilevel intercostal nerve block, scheduled acetaminophen (650 mg q6h), and oxycodone [5 mg every 4 hours (q4h)] as needed. The pathological examination revealed a fibrous tumor with myxoid changes measuring 6.0×4.5×4.2 cm3. The patient had an uncomplicated postoperative course. CT chest 2 years post-surgery demonstrated no herniation of lung tissue. Physical exam 2 years post-surgery was without evidence of paradoxical chest wall motion. Patient alive and well 3.5 years post-surgery.
Case 3
A 39-year-old male with a history of morbid obesity presented with chest wall pain. A chest CT showed a left fourth rib lesion measuring 4.5×2.2×2.8 cm3 behind the scapula. Biopsy of lesion demonstrated Langerhans cell histiocytosis. The patient was discussed in tumor board and deemed appropriate for surgical resection. The Da Vinci Xi robotic surgical system was used for resection. The Gigli saw method was used for rib division; two ribs were resected (Video 1). Postoperative pain was managed with multilevel intercostal nerve block, scheduled acetaminophen (650 mg q6h), and oxycodone (5 mg q4h) as needed. Pathological examination revealed Langerhans cell histiocytosis which measured 2.3×0.8×0.4 cm3. The patient had an uncomplicated postoperative course. CT scan 26 months post-surgery demonstrated no herniation of lung tissue. Patient alive and well with physical exam at 31 months post-surgery without paradoxical chest wall motion.
Case 4
An 88-year-old male with a history of congestive heart failure, coronary artery disease (post-coronary artery bypass grafting status), and abdominal aortic aneurysm (post-repair status) presented with persistent left shoulder pain. A chest CT showed left second and third rib involvement by a mass arising from the lung parenchyma measuring 3.1×3.3×3.4 cm3 (Figure 2). PET-CT demonstrated mass SUV 13.5 with no other fluorodeoxyglucose-avid lesions. A prominent anterior-posterior window lymph node was biopsied and was negative for malignancy. Patient was discussed in thoracic oncology tumor board and deemed appropriate to proceed for resection. The Da Vinci Si robotic surgical system was used for resection. The rib shear was used for rib division; two ribs were resected with en-bloc left upper lobe. Postoperative pain was managed with multilevel intercostal nerve block, scheduled acetaminophen (650 mg q6h) and hydrocodone (5 mg q4h). Pathological examination revealed moderately differentiated squamous cell carcinoma stage pT3N0Mx with the tumor measuring 8.3×5.2×3.0 cm3. The patient had an uneventful postoperative course. Adjuvant therapy was discussed; however, the patient refused. CT scan 4 years post-surgery demonstrated no lung tissue herniation. Exam at 4 years demonstrated no paradoxical chest wall motion. He passed disease-free 4.5 years after resection at age 93 years.
Case 5
A 73-year-old male with a history of diabetes, hypertension, and chronic obstructive pulmonary disease (COPD) was referred to our clinic for management of biopsy proven non-small cell carcinoma of the lung infiltrating the chest wall causing persistent pain. CT showed a pleural-based paraspinal lung lesion measuring 3.7×1.7 cm2. After a complete workup, the patient was deemed a suitable candidate for resection. The Da Vinci Si robotic surgical system was used for resection. Upon exploration a pleural metastasis was identified. However, due to persistent pain, an en-bloc sub-lobar resection was performed consisting of an en-bloc wedge resection of the left upper lobe, superior segment of the lower lobe and the chest wall segment for palliation. The Gigli saw was used for the lateral rib division and the Chisel for the posterior division of rib attachment to the vertebral body. Pathological examination revealed poorly differentiated metastatic adenosquamous carcinoma, stage pT3N0M1b with tumor measuring 4.5×3.7×2.2 cm3. Postoperative pain was managed with multilevel intercostal nerve block, scheduled acetaminophen (650 mg q6h), and oxycodone (5 mg q4h as needed). Adjuvant chemoradiation and immunotherapy were administered. Eight months post-surgery the patient received radiation for a new nodule found to be T1b non-small cell lung cancer. At 42 months post-surgery the patient had no evidence of paradoxical chest wall motion on physical exam and had a CT scan demonstrating neither lung tissue herniation nor evidence of recurrent disease in chest wall or lung parenchyma. The patient was doing well at 42 months post-surgery.
Case 6
A 67-year-old female patient with known, biopsy proven cT3(chest wall invasion) N2 squamous cell lung cancer presented after the completion of neoadjuvant chemoradiation. The left upper lobe lung cancer invaded the left second rib spanning 2.5 cm. After multidisciplinary tumor board discussion, surgical resection was recommended. The Kerrison rongeur was used for rib division. The Da Vinci Xi robotic surgical system was used for en-bloc resection of the left upper lobe. Pathological examination revealed moderately differentiated squamous cell carcinoma, stage ypT1aN0Mx. Postoperative pain was managed with multilevel intercostal nerve block, scheduled acetaminophen (650 mg q6h), and oxycodone (5 mg q4h). The patient had an uneventful postoperative course. At 6 months follow-up, the patient was alive and well without evidence of disease.
Discussion
In this single institution case series enriched with national data, we demonstrate the feasibility for using the robot for chest wall resection for a multitude of different pathologies and locations and buttress our experience by comparing it to outcomes in a national data set. We found that the robotic approach is increasingly utilized and allows for short hospital length of stay without increased morbidity or mortality.
Although thoracotomy had traditionally been the preferred surgical approach for both benign and malignant thoracic tumors requiring resection, the trend recently changed toward minimally invasive approaches due to multiple benefits such as decreased postoperative pain and shorter hospital stays (11). These minimally invasive approaches include both VATS and robotic resections.
Previously published cases of VATS resection for aggressive chest wall tumors have demonstrated satisfactory results with minimal impact on lung function, less reliance on analgesics, decreased length of stay and chest tube drainage and less neurogenic complications than traditional open thoracotomy (12). However, many technical challenges prevent VATS adoption for all chest wall malignancies. VATS requires distinctly different view angles compared to open thoracotomy, has limited accessibility to large central tumors, limits depth perception, and places more demand on surgeons during challenging or ergonomically awkward dissections. Additionally, the straight and rigid VATS instruments do not really allow for smooth motion along the contour and inner curvature of the chest (13,14). The robotic approach has since been introduced as a reliable option for thoracic oncologic resection. Since then, the number of robotic surgeries has been increasing rapidly, not limited to pulmonary resections but also tracheal surgery and locally advanced lung cancer (15-18). However, its application to chest wall resection has been limited in the literature to single case reports or experience with first rib resection for thoracic outlet syndrome.
In our institutional experience, the robotic approach has become the preferred approach for lung and chest wall resection. We prefer to resect all chest wall tumors robotically, so in the single-institution portion of this study, we report our experience with robotic chest wall resection for various benign, symptomatic chest wall tumors and locally advanced lung cancers. The results presented in this case series support the findings from isolated case reports (19). We describe multiple options for rib division and demonstrate the feasibility of this approach to otherwise ergonomically awkward or technically challenging locations. The selection of the best technique for bone division is largely based on the tumor’s location and the individual surgeon’s preference. For example, in one locally advanced left upper lobe lung cancer involving the anterior second rib, the Kerrison rongeur was used as the robotic port placement allowed for easy division of that rib. In other cases, we used the Gigli saw introduced via separate stab incision and operated by the bedside assistant. Other surgeons have reported using bone drills or a modified Gigli saw operated with robotic graspers (20). In general, the technique should be adapted depending on the location, operative access, and surgeon preference.
Although this report demonstrates the feasibility of the robotic approach in various tumor locations, it is particularly useful for chest wall resections located in difficult anatomic regions such as tumors deep to the scapula or in the apex of the chest. The improved three-dimensional magnification provides better visualization allowing for precise dissection, and the wristed instruments prove invaluable. In our opinion, it also allows for better hemostasis, which minimizes intraoperative blood loss. In addition, preserving overlying chest wall musculature, less extensive retraction, and avoiding rib spreading minimizes postoperative pain. As described in this series, patients can recover rapidly with minimal limitations and have an early return to full activity.
Our operative methods demonstrate excellent outcomes when compared to the 96 patients we identified in the NCDB. We had somewhat larger median tumor size at 5.25 vs. 3.90 cm nationally. This highlights our operative technique engenders a quick recovery with an average hospital length of stay of 3 days over national data.
The overall readmission rate, 30- and 90-day mortality rates in the NCDB were 3.1%, 4.2%, and 8.3% which are comparable to average outcome rates for traditional chest wall resections (21).
This study has several limitations. First and foremost, it is a retrospective observational study subject to inherent selection bias. However, the aim of this study is to demonstrate the feasibility and general outcomes profile of adopting the robot for surgical resection at a single institution and nationally to provide a general idea of what is currently being performed and what the outcomes are. Second, this study is limited by its small sample size. Nonetheless, this is the largest report of robotic chest wall resection for neoplasia as most prior studies are limited to case reports or experience with first rib resection for thoracic outlet syndrome. However, in this series we did not resect any primary malignant chest wall tumors so we cannot comment on using our technique for a primary chest wall malignancy. We believe if sound oncologic surgical principles are followed, then it is certainly possible to resect malignant chest wall tumors robotically. Finally, the NCDB does not contain data on complications or functional outcomes, although we do report the outcomes of our individual 6 patients for enrichment. Notwithstanding these limitations, the data presented are relevant to all robotic surgeons as we continue to push the envelope and adopt the robot for more complex and advanced resections.
In conclusion, robotic chest wall resection for primary chest wall tumors or lung cancer invading the chest wall is safe and feasible. We observed minimal morbidity, rapid recovery, and excellent functional outcomes in this case series. Nationally, the robot is increasingly being utilized for lung cancer with chest wall resections with good short-term outcomes. Further multi-institutional studies would be needed to determine the effectiveness of this approach, but our results are promising, and this is our institution’s preferred approach in managing this patient population.
Conclusions
Robotic chest wall resection is feasible and is performed nationally with acceptable short- and long-term outcomes. Our institutional experience reports our technique, resultant short hospital stay, and excellent functional outcomes.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE and AME Case Series reporting checklists. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-532/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-532/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-532/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-532/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of Loyola University (LU# 214246; 10/22/2020) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Leuzzi G, Nachira D, Cesario A, et al. Chest wall tumors and prosthetic reconstruction: A comparative analysis on functional outcome. Thorac Cancer 2015;6:247-54. [Crossref] [PubMed]
- Dal Agnol G, Oliveira R, Ugalde PA. Video-assisted thoracoscopic surgery lobectomy with chest wall resection. J Thorac Dis 2018;10:S2656-63. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Minnich DJ. Minimally invasive chest wall resection: sparing the overlying, uninvolved extrathoracic musculature of the chest. Ann Thorac Surg 2012;94:1744-7. [Crossref] [PubMed]
- Mariolo AV, Casiraghi M, Galetta D, et al. Robotic Hybrid Approach for an Anterior Pancoast Tumor in a Severely Obese Patient. Ann Thorac Surg 2018;106:e115-6. [Crossref] [PubMed]
- Berry MF, Onaitis MW, Tong BC, et al. Feasibility of hybrid thoracoscopic lobectomy and en-bloc chest wall resection. Eur J Cardiothorac Surg 2012;41:888-92. [Crossref] [PubMed]
- Egyud MRL, Burt BM. Robotic First Rib Resection and Robotic Chest Wall Resection. Thorac Surg Clin 2023;33:71-9. [Crossref] [PubMed]
- Gonfiotti A, Salvicchi A, Voltolini L. Chest-Wall Tumors and Surgical Techniques: State-of-the-Art and Our Institutional Experience. J Clin Med 2022;11:5516. [Crossref] [PubMed]
- Lo Iacono G, Mazzella A, Mohamed S, et al. The Role of Surgery in Primary Chest Wall Tumors: Over 20 Years' Experience in Resection and Reconstruction. Cancers (Basel) 2023;15:2153. [Crossref] [PubMed]
- Demmy TL, Yendamuri S, Hennon MW, et al. Thoracoscopic maneuvers for chest wall resection and reconstruction. J Thorac Cardiovasc Surg 2012;144:S52-7. [Crossref] [PubMed]
- King RM, Pairolero PC, Trastek VF, et al. Primary chest wall tumors: factors affecting survival. Ann Thorac Surg 1986;41:597-601. [Crossref] [PubMed]
- Yendamuri S, Nwogu CE, Demmy TL. Thoracoscopic lobectomy with chest wall resection after neoadjuvant therapy. Innovations (Phila) 2009;4:36-8. [Crossref] [PubMed]
- Wu Y, Guan J, Zhang K, et al. Rare chondroblastoma of the 6th left rib, video-assisted thoracoscopy resected: one case report and literature review. J Cardiothorac Surg 2021;16:192. [Crossref] [PubMed]
- McKenna RJ Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg 2006;81:421-5; discussion 425-6. [Crossref] [PubMed]
- Melfi FM, Menconi GF, Mariani AM, et al. Early experience with robotic technology for thoracoscopic surgery. Eur J Cardiothorac Surg 2002;21:864-8. [Crossref] [PubMed]
- Cerfolio RJ. Robotic sleeve lobectomy: technical details and early results. J Thorac Dis 2016;8:S223-6. [PubMed]
- Cerfolio RJ, Bryant AS, Skylizard L, et al. Initial consecutive experience of completely portal robotic pulmonary resection with 4 arms. J Thorac Cardiovasc Surg 2011;142:740-6. [Crossref] [PubMed]
- Abbas AE. Surgical Management of Lung Cancer: History, Evolution, and Modern Advances. Curr Oncol Rep 2018;20:98. [Crossref] [PubMed]
- Mazzei M, Abbas AE. Why comprehensive adoption of robotic assisted thoracic surgery is ideal for both simple and complex lung resections. J Thorac Dis 2020;12:70-81. [Crossref] [PubMed]
- Nguyen DC, Garagozlo C, Moslemi M, et al. Robotic resection of a superior sulcus neurogenic tumor. Innovations (Phila) 2015;10:142-5. [Crossref] [PubMed]
- Burt BM, Palivela N, Karimian A, et al. Transthoracic robotic first rib resection: Twelve steps. JTCVS Tech 2020;1:104-9. [Crossref] [PubMed]
- Mansour KA, Thourani VH, Losken A, et al. Chest wall resections and reconstruction: a 25-year experience. Ann Thorac Surg 2002;73:1720-5; discussion 1725-6. [Crossref] [PubMed]


